Abstract
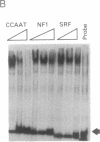
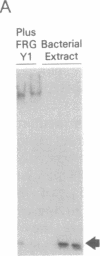
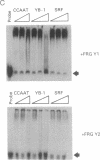
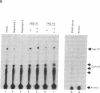
We describe the cloning and characterization of two cDNAs from Xenopus laevis that encode sequence-specific DNA binding proteins called FRG Y1 and FRG Y2 (frog Y-box proteins 1 and 2). During oogenesis and embryogenesis, the genes encoding these proteins are differentially expressed. FRG Y1 mRNA is present in oocytes, embryos, and all adult tissues examined, whereas FRG Y2 mRNA is found only in testis and immature oocytes. The FRG Y1 and FRG Y2 proteins are shown to stimulate transcription from a promoter containing a Y box (CTGATTGGCCAA). This promoter element is found in both mammalian major histocompatibility complex class II and Xenopus germ-cell-specific genes. FRG Y1, FRG Y2, and a human Y-box binding protein are homologous and represent a distinct family of sequence-specific DNA binding proteins. We identify protamine-like regions that are present within this family of transcription factors, suggesting that they use unusual means of binding to DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almouzni G., Méchali M., Wolffe A. P. Competition between transcription complex assembly and chromatin assembly on replicating DNA. EMBO J. 1990 Feb;9(2):573–582. doi: 10.1002/j.1460-2075.1990.tb08145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Bendig M. M., Williams J. G. Fidelity of transcription of Xenopus laevis globin genes injected into Xenopus laevis oocytes and unfertilized eggs. Mol Cell Biol. 1984 Oct;4(10):2109–2119. doi: 10.1128/mcb.4.10.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. A CCAAT box confers cell-type-specific regulation on the Xenopus hsp70 gene in oocytes. Cell. 1986 Sep 26;46(7):1037–1042. doi: 10.1016/0092-8674(86)90703-8. [DOI] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell. 1986 Jun 6;45(5):753–760. doi: 10.1016/0092-8674(86)90789-0. [DOI] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. EMBO J. 1984 Nov;3(11):2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Woulfe S. L., Zacheis M., Schwartz B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg A. M., King B. O., Roeder R. G. Xenopus 5S gene transcription factor, TFIIIA: characterization of a cDNA clone and measurement of RNA levels throughout development. Cell. 1984 Dec;39(3 Pt 2):479–489. doi: 10.1016/0092-8674(84)90455-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Hahn S., Pinkham J., Wei R., Miller R., Guarente L. The HAP3 regulatory locus of Saccharomyces cerevisiae encodes divergent overlapping transcripts. Mol Cell Biol. 1988 Feb;8(2):655–663. doi: 10.1128/mcb.8.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I. W., Lim K., Chae C. B. Characterization of the S-phase-specific transcription regulatory elements in a DNA replication-independent testis-specific H2B (TH2B) histone gene. Mol Cell Biol. 1990 Feb;10(2):585–592. doi: 10.1128/mcb.10.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Peschon J. J., Yelick P. C., Palmiter R. D., Hecht N. B. Sequence homologies in the mouse protamine 1 and 2 genes. Biochim Biophys Acta. 1988 May 6;950(1):45–53. doi: 10.1016/0167-4781(88)90071-1. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Clark S. P., Felsenfeld G., Gould H. An erythrocyte-specific protein that binds to the poly(dG) region of the chicken beta-globin gene promoter. Genes Dev. 1988 Jul;2(7):863–873. doi: 10.1101/gad.2.7.863. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. In vitro synthesis of vertebrate U1 snRNA. EMBO J. 1989 Jan;8(1):287–292. doi: 10.1002/j.1460-2075.1989.tb03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Garrett N. An amphibian cytoskeletal-type actin gene is expressed exclusively in muscle tissue. Development. 1987 Oct;101(2):393–402. doi: 10.1242/dev.101.2.393. [DOI] [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Pinkham J. L., Olesen J. T., Guarente L. P. Sequence and nuclear localization of the Saccharomyces cerevisiae HAP2 protein, a transcriptional activator. Mol Cell Biol. 1987 Feb;7(2):578–585. doi: 10.1128/mcb.7.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C., Tjian R. SV40 T antigen binding site mutations that affect autoregulation. Cell. 1983 Apr;32(4):1227–1240. doi: 10.1016/0092-8674(83)90305-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., Kelly T. J. Purification of nuclear factor I by DNA recognition site affinity chromatography. J Biol Chem. 1986 Jan 25;261(3):1398–1408. [PubMed] [Google Scholar]
- Sakura H., Maekawa T., Imamoto F., Yasuda K., Ishii S. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene. 1988 Dec 20;73(2):499–507. doi: 10.1016/0378-1119(88)90514-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Scotto K. W., Kaulen H., Roeder R. G. Positive and negative regulation of the gene for transcription factor IIIA in Xenopus laevis oocytes. Genes Dev. 1989 May;3(5):651–662. doi: 10.1101/gad.3.5.651. [DOI] [PubMed] [Google Scholar]
- Taylor W., Jackson I. J., Siegel N., Kumar A., Brown D. D. The developmental expression of the gene for TFIIIA in Xenopus laevis. Nucleic Acids Res. 1986 Aug 11;14(15):6185–6195. doi: 10.1093/nar/14.15.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Van Den Berg D. J., Korn L. J. Structure of the gene for Xenopus transcription factor TFIIIA. Nucleic Acids Res. 1986 Mar 11;14(5):2187–2200. doi: 10.1093/nar/14.5.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Vriz S., Taylor M., Méchali M. Differential expression of two Xenopus c-myc proto-oncogenes during development. EMBO J. 1989 Dec 20;8(13):4091–4097. doi: 10.1002/j.1460-2075.1989.tb08593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrant R. W., Kim S. H. alpha-Helix-double helix interaction shown in the structure of a protamine-transfer RNA complex and a nucleoprotamine model. Nature. 1978 Jan 12;271(5641):130–135. doi: 10.1038/271130a0. [DOI] [PubMed] [Google Scholar]
- Wistow G. Cold shock and DNA binding. Nature. 1990 Apr 26;344(6269):823–824. doi: 10.1038/344823c0. [DOI] [PubMed] [Google Scholar]