Abstract
Advanced maternal age is known to be a risk factor for placental dysfunctions. The most common obstetric complications among older women would be considered as follows: gestational diabetes; preeclampsia; placenta praevia; preterm premature rupture of membranes and the risk of preterm delivery. The aims of research were to determine the impact of maternal age on the structure of terminal villi. The study was conducted on 60 human placentae of term pregnancy divided into two groups: the control group (30 placentae in pregnant women of age between 20 and 34) and the experimental group (30 placentae in pregnant women of 35 years of age and older). Stereological methods were applied to determine the volume density, surface density, total volume and total capillary surface area in terminal villi of placenta. The mean value of volume density of capillaries in terminal villi of placentae in older pregnant women is: Vvkks = (0,376 ± 0,033) mm°, and the mean value of total volume is: Vkks = (157,047 ± 25,022) cm3. The mean value of surface density is: Svkks = (64,783 ± 2,543) mm-1, and the mean value of total surface area is: Skks = (29,959 ± 7,873) m2. Volume density of capillaries in terminal villi of placentae is significantly lower in older pregnant women (p<0,001) in comparison to the younger pregnant women. The total volume, surface density and total capillary surface area in terminal villi of placentae are also significantly lower in older pregnant women (p<0,005) in comparison to the younger pregnant women. Statistically significant lower values of volume density, total volume, surface density and total capillary surface area indicate that there is a decreased metabolic transfer between mother and foetus.
Keywords: older pregnant women, placenta, terminal villi, capillaries, stereology
INTRODUCTION
Optimal age of woman for pregnancy and delivery is between 20 and 29. Pregnancies of women older than 35 are considered at risk (1). The number of deliveries has decreased whereas the number of older pregnant women has progressively increased over the past decade in the world (1). Older maternal age is the cause of early miscarriages and increased frequency of foetal chromosomal abnormalities (2). Advanced maternal age is known as a risk factor for various types of placental dysfunction. The most common obstetric complications among older women are gestational diabetes, pre-eclampsia, placenta praevia, premature rupture of membranes, vaginal bleeding after the 28th gestational week due to pregnancy-induced hypertension, miscarriage risk and preterm delivery and placental abruption (3, 4, 5, 6, 7). The main functional components of the placenta are terminal villi that are very important in fetomaternal transfer of substances. The capillaries are often the only content of terminal villi (8). The structure of the villi alters during the pregnancy to satisfy the increased demands of foetus for its undisturbed growth and development (8). The changes in terminal villous capillaries as a part of placental membrane have a direct effect on fetomaternal transfusion, foetal growth and development (9). The changes in placental membrane can lead to structural placental changes and affect the course and outcome of pregnancy. Placental insufficiency may contribute to the intrauterine growth retardation and disturbed development of foetus. These changes can result in birth to hypotrophic and premature infants, appearance of respiratory distress, acute or chronic hypoxia, and increased perinatal mortality and morbidity (10, 11, 12). Current findings on the impact of mature maternal age on foetus refer to foetal chromosomal defects, frequency of preterm newborns, low birth weight, intrauterine growth retardation and development of foetus, and early neonatal complications (1, 2, 13). Stereological analysis of terminal villi and intravillous space of mature human placentae did not show significant morphological and quantitative differences in comparison to the control group (14). Due to the great importance given in the world to the impact of advanced maternal age on the course and outcome of pregnancy and the lack of mentioned parameters, the quantitative study was undertaken to analyse the parts of placenta that have an effect on the volume of fetomaternal transfer of substances. Since the risk to the foetus is increased with maternal age, the knowledge of the volume of the surface area available for transport of substances is essential for estimating the quantity of nutrients that can be transferred to foetus for its normal growth and development. In our research we assess the volume, surface density and the total volume and total capillary surface area in terminal villi of placentae in older women and compare them to the young ones.
MATERIALS AND METHODS
The research was performed on 60 human placentae of term pregnancy. The samples consisted of pregnant women divided into two groups according to maternal age at delivery: the control group (pregnant women age between 20 and 34) and the experimental group (pregnant women age over 35). Thirty placentae were examined in each group. Placental samples were collected in Gynaecology and Obstetric Department at University Clinical Center Tuzla. Gestational age was calculated from the first day of the last period of pregnant women with regular menstrual cycle, which was confirmed by the ultrasound in the first tri-mester of pregnancy. Membranes and umbilical cord were first removed from each placental sample. Placental weight was measured and expressed in grams. Using the fluid obtained by squeezing of the placenta, placental volume was measured indirectly and expressed in mm3. The tissue samples were taken from various parts of placenta for histological analysis. They were fixed in 10% neutral buffered formalin, embedded in paraffin and cut into 8 mm thick sections. Deparaffinized sections were stained by hemalaun and eosin. Terminal villi of placenta were the reference space for quantitative analysis by stereological methods. The sample size was determined according to procedure introduced by De Hoff (15). Quantitative analysis was performed at 40 x magnification using the multipurpose M-42 test system. Quantitative analysis included calculation of relative values (volume density and surface density of capillaries in terminal villi) and absolute values (total volume and total surface of capillaries in terminal villi).
Mean value (x), standard deviation (s) and standard error (SE) were calculated. Significance of the difference between mature placentae and controls was determined by Student’s t-test.
RESULTS
Relative values
Frequency of volume density of capillaries in terminal villi (Svkk) of placentae in younger and older pregnant women has been shown on the Chart 1. The mean value of volume density of capillaries in terminal villi of placentae in younger pregnant women (control group) is: Vvkkm = (0,429 ± 0,080) mm0 in comparison to Vvkks = (0,376 ± 0,033) mm0 in the older pregnant women placentae (experimental group) (Chart 2).
CHART 1.
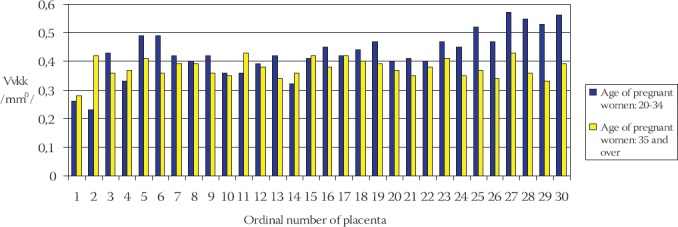
Frequency of volume density of capillaries (Vvkk) in terminal villi of placentae in younger and older pregnant women
CHART 2.
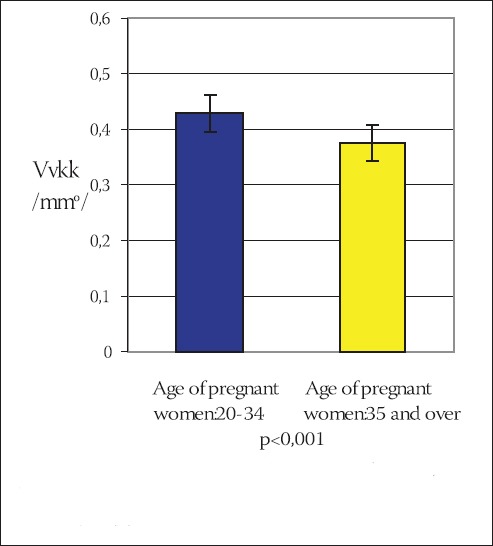
Mean value of volume density of capillaries (Vvkk) in terminal villi of placentae in younger and older pregnant women (x±ls) (mmO)
Statistical analysis of the results using the Student’s t-test indicates that the volume density of capillaries in terminal villi of placentae in older pregnant women is significantly lower in comparison to the younger pregnant women (t=3,539, df=29, p<0,001). Frequency of surface density of capillaries in terminal villi (Svkk) of placentae in older and younger pregnant women has been shown on the Chart 3.
CHART 3.
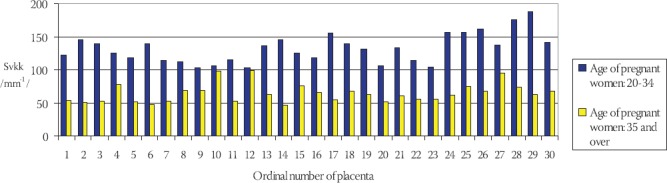
Frequency of surface density of blood capillaries (Svkk) in terminal villi of placentae in younger and older pregnant women
The mean value of surface density of capillaries in terminal villi of placentae in younger pregnant women (control group) is: Svkkm= (132,402 ± 4,022) mm-1 in comparison to Svkks = (64,783 ± 2,543) mm-1 in older pregnant women (experimental group) (Chart 4).
CHART 4.
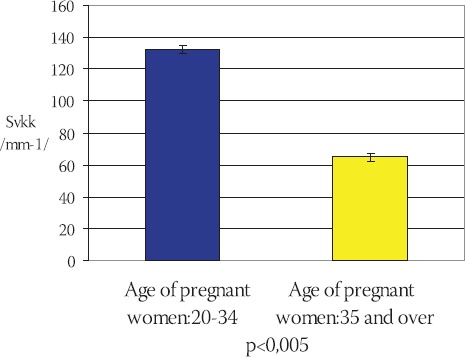
Mean value of surface density of capillaries (Svkk) in terminal villi of placentae in younger and older pregnant women (x±ls) (mm-l)
Statistical analysis of the results using Student’s t-test (t=-13,643, df=29, p<0,005) shows that the surface density of capillaries in terminal villi of placentae in older pregnant women is significantly lower in comparison to the placentae in younger pregnant women.
Absolute values
Frequency of total capillary volume (Vkk) in terminal villi of placentae in young and older pregnant women has been shown on the Chart 5. The mean value of total capillary volume in terminal villi of placentae in younger pregnant women (control group) is: Vkkm= (197,012 ± 47,789) cm3, in comparison to Vkks = (157,047 ± 25,022) cm3 in older pregnant women (experimental group) (Chart 6). By using T-test (t=4,437, df=29, p<0,005) it has been determined that the total capillary volume in terminal villi of placentae in older pregnant women is significantly lower in comparison to the placentae in younger pregnant women. Frequency of total capillary surface area (Skk) in terminal villi of placentae in younger and older pregnant women has been shown on the Chart 7. The mean value of total capillary surface are in terminal villi of placentae in younger pregnant women (control group) is: Skkm= (56,119 ± 11,991) m2, in comparison to Skks= (29,959 ± 7,873) m2 in older pregnant women (experimental group): (Chart 8).
CHART 5.
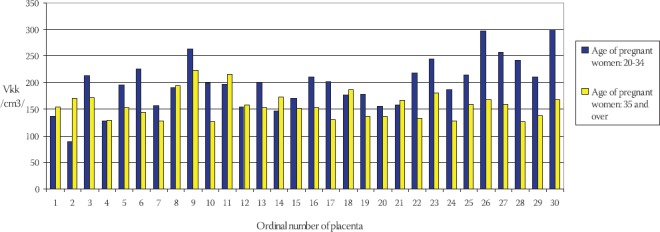
Frequency of total capillary volume (Vkk) in terminal villi of placentae in younger and older pregnant women
CHART 6.
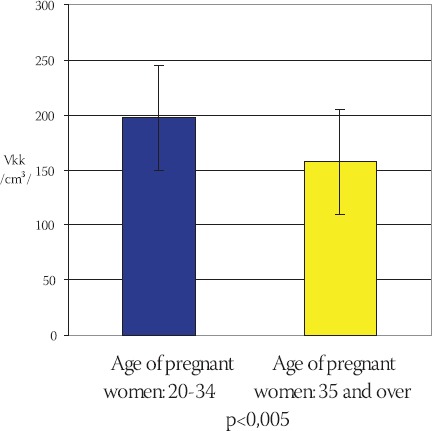
Mean value of total capillary volume (Vkk) in terminal villi of placentae in younger and older pregnant womer (x±ls) (cm3)
CHART 7.
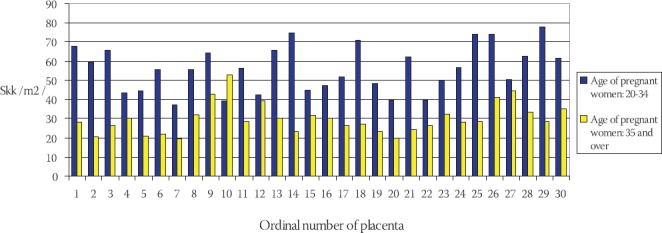
Frequency of total capillary surface area (Skk) in terminal villi of placentae in younger and older pregnant women
CHART 8.
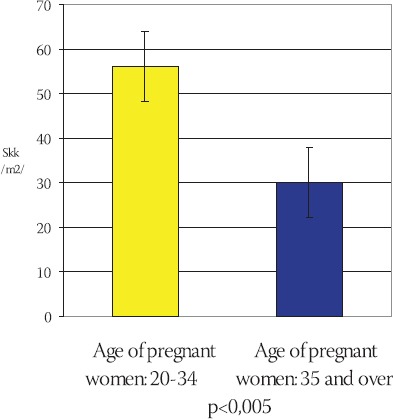
Mean value of total capillary surface area (Skk) in terminal villi of placentae in older and younger pregnant women (x±ls) (m2)
Total capillary surface area in terminal villi of placentae in older pregnant women is significantly lower in comparison to the placentae in younger pregnant women (t=-9,951 df=29, p<0,005).
DISCUSSION
In pregnancy, major physiological changes occur in morphology of the blood vessels in order to meet the demands of the developing foetus. Blood-filled terminal villi are essential for fetomaternal transfer of substances. They consist of inherent sinusoid capillaries. The analysis of structural components of placental terminal villi (capillaries, stroma, and trophoblast) indicates that the placentae undergo extensive morphological alterations during the tenth lunar month. This particularly refers to vascularisation of terminal villi with appearance of sinusoidal capillaries resulting in reduction of the stroma (16). Total capillary volume in terminal villi of mature placentae has not yet been quantitatively determined. Our research shows that the total capillary volume in terminal villi of mature placentae is 157,047 cm3 compared with 197,012 cm3 in the control group. The difference is statistically significant (p<0,005). Our research confirms the research from the literature according to which the decreased volume density and total capillary volume in terminal villi of older pregnant women leads to decreased number of vasculo-syncytial membranes and hypoxia, which can be the cause of growth retardation and disturbed development of infants (17). Capillaries in terminal villi, as a part of placental membrane, are important for fetomaternal transfer of substances. The results from our research suggest that the availability of nutrients in foetus is decreased, which affects foetal growth and development. We did not find data on the surface density of terminal villi capillaries of younger and older pregnant women in the literature. Our research shows that mean value of surface density of capillaries in terminal villi of placentae in older pregnant women is 64,783 mm-1, whereas the mean value of surface density of capillaries in terminal villi of placentae in younger pregnant women is 132,402 mm-1. Statistically significant lower surface density of blood vessels in terminal villi is in mature placentae in comparison to the control group (p°<0,005). Data on total capillary surface area in villi found in the literature vary as they follow: 7,5 m2 (18); 16,8 m2 (19); 15,24 m2 (20). Our research shows that mean value of total capillary surface area of terminal villi of mature placentae is 29,959 m2 compared with 56,119 m2 in the control group. The difference is statistically significant. Our results confirm reported findings (17, 7, 21) that the activation of the functional reserve capacity occurs in older pregnant women, which implies that the transfer of substances across placenta is facilitated by compensatory mechanisms, thus enabling mature placentae to meet the functional demands of the foetus for its normal growth and development.
CONCLUSION
Volume density, total volume, surface density and total capillary surface area in terminal villi of placentae in older pregnant women are significantly lower in comparison to the placentae in younger pregnant women.
Quantitative analysis of capillaries in terminal villi of placentae in older pregnant women represents a contribution to the evaluation of functional capacity of placenta, its compensatory mechanisms and pathological changes.
LIST OF ABBREVIATIONS
Vvkks - Mean value of volume density of capillaries in terminal villi of placentae in older pregnant women
Vvkkm - Mean value of volume density of capillaries in terminal villi of placentae in younger pregnant women
Svkks - Mean value of surface density
Skks - Mean value of total surface
Svkk - Frequency of surface density of capillaries in terminal villi of placentae in older and younger pregnant women
Svkkm - Mean value of surface density of capillaries in terminal villi of placentae in younger pregnant women
Svkks - Mean value of surface density of capillaries in terminal villi of placentae in older pregnant women
Vkk - Frequency of total capillary volume
Vkkm - Mean value of total capillary volume in terminal villi of placentae in younger pregnant women
Vkks - Mean value of total capillary volume in terminal villi of placentae in older pregnant women
Skk - Frequency of total capillary surface area in terminal villi of placentae in younger and older pregnant women
Skkm - Mean value of total capillary surface area in terminal villi of placentae in younger pregnant women
Skks - Mean value of total capillary surface area in terminal villi of placentae in older pregnant women
REFERENCES
- 1.Miletić T, Aberle N, Mikulandra F. Perinatal outcome of pregnancies in women aged 40 and over. Coll Antropol. 2002;26:251–258. [PubMed] [Google Scholar]
- 2.Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265:30–33. doi: 10.1007/s004040000122. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Heija AT, Jallad MF, Abukteish F. Maternal and perinatal outcome of pregnancies after the age of 45. J Obstet Gynaecol Res. 2000;26:27–30. doi: 10.1111/j.1447-0756.2000.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Ha Z, Teng Q. Clinical study on morphological characteristics of placenta on severe pregnancy induced hypertension. Zhonghua Fu Chen Ke Ze Zhi. 2000;35(11):651–653. [PubMed] [Google Scholar]
- 5.Sheiner E, Shoham-Vardi I, Hallak M. Placental abruption in term pregnancies: Clinical significance and obstetric risk factors. J. Matern. Fetal Neonatal Med. 2003;13:45–49. doi: 10.1080/jmf.13.1.45.49. [DOI] [PubMed] [Google Scholar]
- 6.Vogt Isaksen C. Maternal smoking, intrauterine growth restriction, and placental apoptosis. Pediatr. Dev. Pathol. 2004;7(5):433–442. doi: 10.1007/s10024-004-0105-1. [DOI] [PubMed] [Google Scholar]
- 7.Neale DM, Mor G. The role of Fas mediated apoptosis in pre-eklampsia. J. Perinat. Med. 2005;33(6):471–7. doi: 10.1515/JPM.2005.085. [DOI] [PubMed] [Google Scholar]
- 8.Nikolić I, Rančić G, Radenković G, Lačković V, Todorović V, Mitić D. Ekstraembrionalne strukture. In: Nikolić I, editor. Embriologija čoveka. Beograd: Data status; 2006. p. str. 41. [Google Scholar]
- 9.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequence fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000;92(1):35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 10.Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Z, Kitagawa M, Takemura T, Hirokawa K. Effect of maternal age on incidences of apoptotic and proliferative cells in trophoblasts of full-term human placenta. Mol. Hum. Reprod. 2001;7:1779–1785. doi: 10.1093/molehr/7.12.1179. [DOI] [PubMed] [Google Scholar]
- 13.Battistelli M, Burattini S, Pomimi F. Ultrastructural study on human placenta from intrauterine growth retardation cases. Microsc. Res. Tech. 2004;65(3):150–158. doi: 10.1002/jemt.20120. [DOI] [PubMed] [Google Scholar]
- 14.Ramić S, Žigić Z, Alečković M. Stereological analysis of mature human placenta of pregnant women of different age. Bosn. J. Basic Med. Sci. 2006;6(2):7–10. doi: 10.17305/bjbms.2006.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kališník M. Temelji stereologije. Vol. 14. Ljubljana: Društvo za stereologijo in kvantitativno analizo slike (DSKAS); 2002. p. 47. [Google Scholar]
- 16.Grbeša Đ, Durst-Živković B.D. Does the human placenta differentiate structurally during the 10th lunar month. Acta Med. Croat. 1994;48(3):117–121. [PubMed] [Google Scholar]
- 17.Mayhew TM. Stereological studies on fetal vascular development in human placental villi. Placenta. 2004;25(3-5):226–233. [Google Scholar]
- 18.Teasdale F, Jean Jacques G. Morphometric evaluation of the microvillous surface enlargement factor in the human placenta from midgestation to term. Placenta. 1985;6:375–381. doi: 10.1016/s0143-4004(85)80014-x. [DOI] [PubMed] [Google Scholar]
- 19.Boyd PA. Quantitative structure of the normal human placenta from 10 weeks of gestation to term. Early Hum. Dev. 1984;9:297–307. doi: 10.1016/0378-3782(84)90074-4. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanović G. Uticaj pušenja u trudnoći na morfometrijska svojstva posteljice i stanje novorođenčeta. Tuzla: Doktorska disertacija, Medicinski fakultet Univerziteta u Tuzli; 2005. pp. 14–24. [Google Scholar]
- 21.Huppertz B, Burton G, Cross C, Kingdom JCP. Placental morphology: From molecule to mother. Placenta. 2006;27(7):754–760. doi: 10.1016/j.placenta.2006.01.007. [DOI] [PubMed] [Google Scholar]


