Abstract
Tricellular contacts are the places where three cells meet. In vertebrate epithelial cells, specialized structures called tricellular tight junctions (tTJs) and tricellular adherens junctions (tAJs) have been identified. tTJs are important for the maintenance of barrier function, and disruption of tTJ proteins contributes to familial deafness. tAJs have recently been attracting the attention of mechanobiologists because these sites are hot spots of epithelial tension. Although the molecular components, regulation, and function of tTJs and tAJs, as well as of invertebrate tricellular junctions, are beginning to be characterized, many questions remain. Here we broadly cover what is known about tricellular junctions, propose a new model for tension transmission at tAJs, and discuss key open questions.
INTRODUCTION
Epithelial sheets cover the surfaces of many organs in the bodies of multicellular organisms and function as a barrier by regulating translocation of fluids, solutes, and cells between compartments. Epithelial cells are polarized and form apical cell–cell junctions between adjacent cells, which are composed of tight junctions (TJs), adherens junctions (AJs), and desmosomes in vertebrates and AJs and septate junctions (SJs) in most invertebrates. In epithelial sheets, where cells are packed two dimensionally, there are many points where three cells meet (Figure 1, A and B). These points are called tricellular contacts. At tricellular contacts, cell–cell junctions take on specialized organizations generally referred to as tricellular junctions, including tricellular TJs (tTJs), tricellular AJs (tAJs), and tricellular SJs (tSJs). Here we discuss what is known about tricellular junctions and the mechanisms that regulate their formation and maintenance, the functional importance of tricellular junctions in development and disease, and unanswered questions.
FIGURE 1:
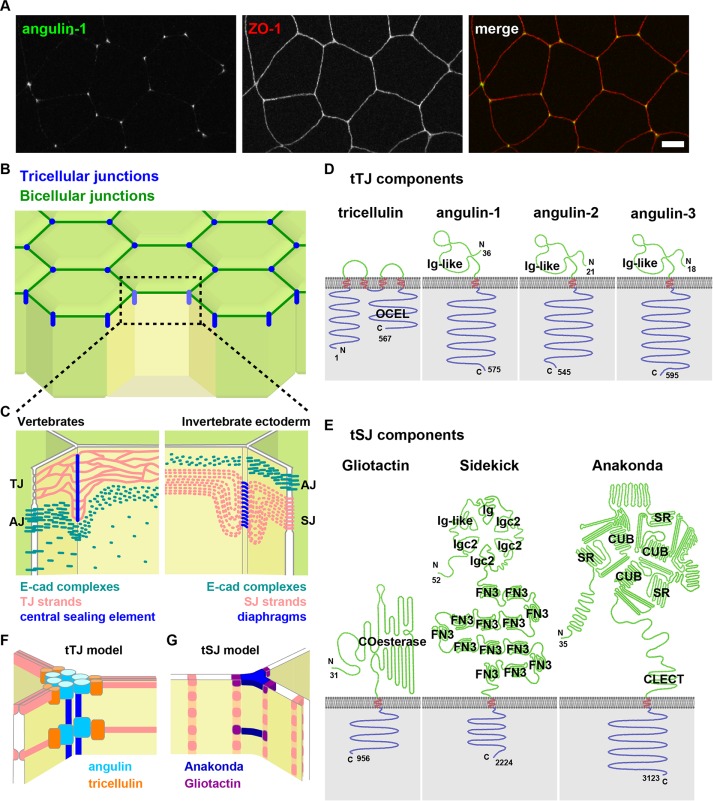
Tricellular tight junctions and tricellular septate junctions. (A) Immunofluorescence staining of X. laevis gastrula-stage embryo using anti–angulin-1 (tTJ marker; green) and anti–ZO-1 (bicellular TJ marker; red). Bar, 10 µm. (B) Epithelial organization. Tricellular junctions (blue) and bicellular cell–cell junctions (dark green). (C) Structure of tricellular junctions. Vertebrate epithelium (left) has TJs, AJs, and desmosomes (not depicted here). TJ strands (pink) turn in the basolateral direction and make connections to central sealing elements (blue). AJs, consisting of E-cadherin complexes (green), are deepened at the tricellular region. Invertebrate ectoderm epithelium (right) has AJs and SJs. SJ strands (pink) run parallel to the Z-axis at the tricellular region and make lateral connections to the diaphragms (blue). (D) Molecular components of tTJs. Top, extracellular; bottom, intracellular. OCEL, occludin ELL-like domain; Ig-like, Ig-like domain. (E) Molecular components of tSJs. COesterase, carboxylesterase domain; Igc2, Ig domain C2-set type; Ig-like, Ig-like domain; Ig, Ig domain; FN3, fibronectin type III domain; SR, scavenger receptor cysteine-rich domain; CUB, complement C1r/C1s/Uegf/Bmp1 domain; CLECT, C-type lectin domain. (F) Model of tTJ organization. Angulins (cyan) help make central sealing elements (blue) and recruit tricellulin (orange)-associated TJ strands (pink). (G) Model of tSJ organization. Anakonda (dark blue) makes diaphragms and recruits Gliotactin (purple)-associated SJ strands (pink).
TRICELLULAR TIGHT JUNCTIONS
Structure of tTJs
TJs, or zonula occludens (ZO), were originally observed by transmission electron microscopy (TEM) as apical structures in epithelial cells where the plasma membranes are tightly apposed, thus serving as a paracellular barrier (Farquhar and Palade, 1963; for review, see Krug et al., 2014; Van Itallie and Anderson, 2014; Zihni et al., 2016). By freeze-fracture replica electron microscopy, TJs appear as multiple sealing strands (Staehelin et al., 1969), which are primarily composed of claudins (Furuse et al., 1998). At tricellular contacts, the apical-most TJ strands from two neighboring cells meet at tricellular contacts and turn basally (Figure 1C). These TJ strands are closely attached, forming a structure called the central sealing element (Staehelin, 1973), which seals the space among the three cells. Other TJ strands from the two neighboring bicellular junctions make many connections with the central sealing element at roughly right angles (Friend and Gilula, 1972; Staehelin, 1973; Wade and Karnovsky, 1974; Walker et al., 1985; Figure 1C). This specialized TJ structure at tricellular contacts, including the central sealing elements and connected TJ strands, is referred to as the tTJ (Ikenouchi et al., 2005). Because the three paired strands of central sealing elements cannot eliminate the space among three cells, it has been speculated that there is a narrow channel, called the central tube (Staehelin, 1973; Walker et al., 1985; Ikenouchi et al., 2005).
Molecular components of tTJs: tricellulin and angulins
Two protein components are known to specifically localize to vertebrate tTJs—tricellulin and angulins (Figure 1D). Tricellulin was the first molecular component of tTJs to be identified (Ikenouchi et al., 2005). Tricellulin (also known as marveld2), occludin, and marveld3 form the TJ-associated MARVEL protein (TAMP) family, which is conserved among vertebrates (Steed et al., 2009; Raleigh et al., 2010). Tricellulin is localized strongly at tTJs and very weakly at bicellular junctions in almost all types of epithelial cells (Ikenouchi et al., 2005). In immunoreplica electron microscopy, tricellulin signal was detected along the central sealing element (Ikenouchi et al., 2005). Tricellulin has four transmembrane domains and large N- and C-terminal cytoplasmic tails. In spite of sequence homology with occludin, the extracellular region of tricellulin does not support homophilic trans-interaction between tricellulin-transfected HEK293 cells (Cording et al., 2013). The latter half of the C-terminal tail is highly homologous to the occludin ELL domain, which binds to ZO-1 (Riazuddin et al., 2006), a TJ plaque protein. Tricellulin knockdown causes altered TJ morphology and decreased transepithelial electrical resistance (TER) in the mammary epithelial cell line EpH4 (Ikenouchi et al., 2005), indicating that tricellulin is required for the maintenance of morphology and barrier function of TJs. In addition, removal of tricellulin disrupts the characteristic structure of TJ strands connected to the central sealing element at tTJs in the inner ear (Nayak et al., 2013), suggesting that tricellulin is required for the formation of proper tTJ structure.
Named for the Latin word angulus, meaning corner, the angulins are the other proteins known to localize at vertebrate tTJs. The angulin family proteins, composed of angulin-1 (also known as lipolysis-stimulated lipoprotein receptor [LSR]), angulin-2 (also known as immunoglobulin [Ig]-like domain–containing receptor [ILDR] 1), and angulin-3 (also known as ILDR2, LISCH-like, or C1orf32), are single-pass transmembrane proteins (Masuda et al., 2011; Higashi et al., 2013). Angulin family proteins have an extracellular Ig-like domain, which in other proteins, such as junctional adhesion molecule (JAM), is known to mediate cell recognition and adhesion, although the adhesion activity of this domain has not been directly tested in angulins. The angulins are differentially expressed in various epithelial tissues (Higashi et al., 2013). All angulin proteins have multiple splice isoforms (Higashi et al., 2013; Reaves et al., 2017), and at least one isoform of each angulin is localized at tricellular junctions and weakly at bicellular junctions in each epithelial tissue examined (Masuda et al., 2011; Higashi et al., 2013). Angulin-1 is detected at central sealing elements of tTJs by immunoreplica electron microscopy (Masuda et al., 2011). EpH4 cells express primarily angulin-1 (vs. the other angulins), and angulin-1–knockdown EpH4 cells exhibit reduced TER and increased macromolecule flux, indicating that angulin-1 is required for maintenance of barrier function (Masuda et al., 2011; Higashi et al., 2013). Expression of angulin-2 in angulin-1–knockdown EpH4 cells rescued the TER and macromolecule flux phenotypes, suggesting that angulin-2 has barrier-supporting properties similar to angulin-1. In contrast, angulin-3 expression in the angulin-1–knockdown cells resulted in limited restoration of barrier function (Higashi et al., 2013).
Angulins recruit tricellulin to tTJs
In angulin-1–knockdown cells, tricellulin does not show specific localization at tTJs and is instead uniformly localized at the bicellular membrane (Masuda et al., 2011). Conversely, angulin-1 is still clearly localized at tTJs in tricellulin-knockdown cells (Masuda et al., 2011). Similarly, in angulin binding–deficient tricellulin–knock-in mice, angulin-2 is still localized at tricellular contacts of hair cells in the inner ear, whereas mutant tricellulin is absent from tTJs (Nayak et al., 2013). Based on these results, it was proposed that angulins establish primitive tTJs at tricellular contacts and recruit tricellulin to help these structures mature. Although conserved regions in the cytoplasmic tail of angulins and the C-terminal cytoplasmic tail of tricellulin are required for the recruitment of tricellulin (Masuda et al., 2011; Higashi et al., 2013), whether the interaction between angulins and tricellulin is direct or indirect is unclear.
Model of tTJ architecture
Neither superresolution nor electron microscopy data are available to distinguish the localization of angulins and tricellulin at tTJs. However, it is possible to speculate about the molecular organization of tTJs, and a model of tTJ architecture was proposed in which angulins comprise the central sealing element of tTJs to form landmarks for nascent tTJs and then recruit tricellulin together with claudin-based TJ strands (Masuda et al., 2011; Figure 1F). This model is supported by the following experimental data: 1) angulins can form a molecular complex without tricellulin (Masuda et al., 2011), 2) angulins can interact with tricellulin (Masuda et al., 2011; Higashi et al., 2013), and 3) freeze-fracture electron microscopy of claudin/tricellulin double-transfected HEK293 cells shows that tricellulin can interact with claudin-based TJ strands and alter their morphology from a rounded shape to more-rectangular meshes (Ikenouchi et al., 2008; Cording et al., 2013). Furthermore, TJ strands in these modified HEK293 cells intersect at roughly right angles in a way that closely resembles the structure of tTJs observed in freeze-fracture replica electron microscopy where TJ strands make end-on connections to central sealing elements (Ikenouchi et al., 2008; Cording et al., 2013). Recently it was found, using superresolution microscopy, that another TAMP family protein, occludin, is concentrated at the ends and intersections of TJ strands (Van Itallie et al., 2017), which suggests that the TAMP family member tricellulin may also be integrated at the intersections of TJ strands, particularly those TJ strands intersecting with the central sealing element.
tTJ biogenesis
To maintain epithelial order, epithelial cells must be continuously renewed (Ragkousi and Gibson, 2014). Old cells undergo apoptosis and are extruded from the epithelial sheet (Eisenhoffer and Rosenblatt, 2013), and new cells are added by cell division. In some situations, specific cell types need to be inserted into or removed from the epithelial sheet (Sedzinski et al., 2016). In addition, cell intercalation within the epithelial plane is important for many developmental processes, such as convergent extension (Walck-Shannon and Hardin, 2014). In all of these processes, cell–cell junctions—including tTJs—are reorganized, while at the same time, the epithelial barrier must be maintained. Recent studies are beginning to shed light on tTJ biogenesis and remodeling during these dynamic processes.
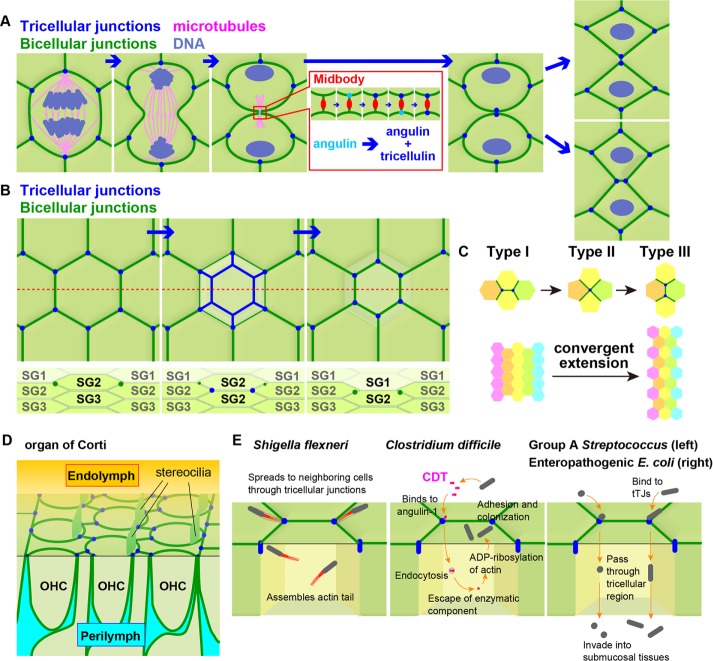
Recently we reported how tTJs are formed during epithelial cytokinesis in Xenopus embryos (Higashi et al., 2016). In the Xenopus gastrula-stage epithelium, most daughter cells are separated by neighboring cells after cytokinesis, and each daughter cell makes a new tricellular contact with two neighboring cells (Figure 2A). This is in clear contrast with the Drosophila epithelium, where daughter cells maintain contact after cytokinesis (Gibson et al., 2006; Founounou et al., 2013; Herszterg et al., 2013; Guillot and Lecuit, 2013a). In the Xenopus epithelium, at the two newly formed tricellular contacts, angulin and then tricellulin are recruited to establish mature tTJs (Figure 2A). In almost half of the divisions observed, the two nascent tricellular junctions merge and then redistribute over the course of 1 h to form two tTJs among two daughter cells and one neighboring cell (Figure 2A).
FIGURE 2:
New tricellular junction formation and functional importance of tricellular junctions. (A) Nascent tTJ formation after cytokinesis. When the cleavage furrow ingresses and the bicellular cell–cell junctions (green) from each side of the furrow meet, a new cell–cell interface between neighboring cells and two new vertices are formed. Angulin-1 (cyan) and then tricellulin are recruited to the newly formed vertices and build mature tTJs (blue). Formation of one tTJ is soon followed by the other. After cytokinesis, new tTJs either separate as the bicellular junctions between them elongate (top) or fuse and reorganize to make tTJs among a different combination of the cells (bottom). (B) tTJ formation during turnover of epidermal cells. TJs exist only at the second layer of stratum granulosum (SG2). The red dotted line in the top, en face view indicates the position of the cross-section view (bottom). The TJ-bearing cells at SG2 turn over sporadically. When a cell at SG2 is going lose its apical cell–cell junctions, new cell–cell junctions are formed at the basal side of the cell. These new cell–cell junctions are formed among three cells, including a neighboring cell in the SG2 layer and an underlying cell in the SG3 layer, and the new junctions are enriched with tTJ components such as angulin-1 and tricellulin. As the upper cell–cell junctions disappear, tTJ components of the new, lower cell–cell junctions gradually become focused at cell vertices. (C) Cell intercalation during convergent extension. In type I/II/III transition, shortening of cell–cell junctions perpendicular to the tissue elongation axis results in fusion of two tricellular junctions and formation of a four-way junction. Then the four-way junction becomes two tricellular junctions along the elongation axis. In some cases, instead of four-way junctions, more than four cells make a multiway junction (also called a rosette; not depicted here). (D) tTJs (blue) are important for barrier function of the sensory epithelium and viability of hair cells in the inner ear. OHC, outer hair cells. (E) Tricellular junctions are involved in pathogenesis of versatile species of bacteria. S. flexneri spreads to neighboring cells via the tricellular junctions by utilizing host actin and penetrating through the corners of infected cells. C. difficile secretes the binary toxin CDT, which binds to angulin-1, a tTJ component. CDT is then incorporated into the cell by endocytosis and modulates actin and microtubules, which induces cell protrusions at the cell surface and contributes to adherence and colonization of the bacteria. Group A Streptococcus and enteropathogenic E. coli preferentially bind to tricellular junctions and invade into submucosal tissues through tricellular junctions.
Another example of tTJ biogenesis occurs in the cornified stratified epithelia of the mammalian epidermis, where cells proliferate in the basal layer and then differentiate and move up to the surface to sequentially form three distinct layers of the stratified epithelia, including the stratum granulosum. Of interest, TJs and tTJs are formed only between cells in the second cell layer of the stratum granulosum, where they serve as a barrier (Furuse et al., 2002; Kubo et al., 2009). A recent study beautifully described how TJs and tTJs are maintained during continuous turnover of cells in the second layer of the stratum granulosum (Yokouchi et al., 2016; Figure 2B). When an epidermal cell sporadically translocates from a lower layer to the second layer of the stratum granulosum, the preexisting upper cell forms additional TJs at its basal side to make a double-edged polygon, and the preexisting TJs at the apical side disappear over time. By transiently making TJs at both the apical and basal sides of cells, this specialized epithelium can maintain barrier function during the continuous turnover of cells. Of note, angulin-1 and tricellulin localize all along the newly forming cell–cell junctions at the basal side, whereas they localize specifically at tricellular vertices at the apical side. As the preexisting TJs disappear at the apical side, both angulin-1 and tricellulin become focused at tricellular contacts in the newly formed junctions. Yokouchi et al. (2016) speculated that newly formed basal junctions are “tricellular junctions” among the preexisting cell, the translocating cell, and surrounding cells, which is consistent with the their cell-packing model, in which the epidermal cells have a flattened tetradecahedron shape (polygon with 14 faces; also known as Kelvin’s tetrakaidecahedron).
A third example of tTJ biogenesis is associated with the penetration of keratinocyte TJs by the dendrites of Langerhans cells—major immune cells responsible for uptake and presentation of antigens in the skin (Kubo et al., 2009). When activated, Langerhans cells extend their dendrites between keratinocytes, and the tips of dendrites penetrate keratinocyte TJs to take up antigens from the extra-TJ environment above the TJs. Intriguingly, when dendrites are inserted between keratinocytes, tricellulin is recruited to the newly formed tricellular contacts among the dendrites and keratinocytes. Similar transepithelial projections from basal cells were observed in the epididymides (Shum et al., 2008). Furthermore, cell insertion into epithelial sheets is observed in many developmental and regenerative processes, including emergence of multiciliated cells in the Xenopus laevis epidermis (Sedzinski et al., 2016) and insertion of olfactory neurons in the olfactory epithelium (Leung et al., 2007; Higashi et al., 2013). Future studies are required to further investigate how new tTJs are established when cells or cell processes penetrate TJs.
Signaling at tTJs
Given the unique structure and position of tTJs in the cell, one would predict that specific signaling pathways might regulate tTJ formation and maintenance and that specific signals might be relayed from tTJs to other parts of the cell (i.e., to other junctions or the nucleus). However, very limited information is available about signaling related to tTJs.
Tricellulin and angulins may be regulated by phosphorylation. In Western blots of epithelial cell lysates, tricellulin is detected as multiple bands, which are phosphorylation dependent (Ikenouchi et al., 2005). Because the function of occludin, another TAMP family protein, is regulated by casein kinase 2–dependent phosphorylation (Raleigh et al., 2011), it is plausible that phosphorylation of tricellulin also modulates its function. The kinase responsible for tricellulin phosphorylation has not been identified, although c-Jun N-terminal kinase (JNK) is implicated in the regulation of tricellulin expression (Kojima et al., 2010). Angulin-1 is also observed as multiple bands in Western blots of epithelial cell lysates (Masuda et al., 2011), which is apparently due to phosphorylation or might be caused by additional posttranslational modifications. It has been shown that JNK1-dependent phosphorylation of angulin-1 at Ser 288 is important for exclusive localization of angulin-1 at tTJs (Nakatsu et al., 2014).
tTJs may be localized sites of Rho family GTPase signaling. The Cdc42 GEF Tuba is reported to bind the N-terminal cytoplasmic tail of tricellulin (Oda et al., 2014). Tuba is localized at bicellular junctions through its binding to the TJ plaque protein ZO-1, and Tuba regulates junctional curvature of epithelial cells by activating Cdc42 and its effector N-WASP (Otani et al., 2006). It is not known whether Tuba localizes to tTJs in epithelial cells and regulates Cdc42 activation at these sites. Given that the effect of tricellulin knockdown on epithelial configuration was limited to the phase of cell–cell junction formation (Oda et al., 2014) and that tricellulin-knockout mice do not exhibit global epithelial defects (Kamitani et al., 2015), the contribution of tricellulin to Tuba- and Cdc42-dependent regulation of epithelial order remains elusive.
TRICELLULAR ADHERENS JUNCTIONS
Structure and molecular composition of tAJs
The structure and core molecular components of AJs have been identified; however, the composition and organization of tAJs is still emerging. The zonula adherens was originally characterized by transmission electron microscopy as a cell–cell junction structure located just basal to the TJ with a characteristic ∼20 nm intercellular space between parallel cell membranes filled with low-density material and accompanied by high-density material in the cytoplasm adjacent to the junctions (Farquhar and Palade, 1963). Later it was appreciated that AJs can be divided into two categories based on their morphology: apical belt-like AJs of the zonula adherens and spot-like AJs (also called punctum adherens; Yonemura, 2011b). The key molecular components of both types of AJs are the cadherin/catenin complex, composed of the transmembrane protein E-cadherin and cytoplasmic catenins. Cadherin/catenin complexes are directly or indirectly linked with the actin cytoskeleton, which was visible in the original electron microscopy images as the high-density cytoplasmic material associated with the junctions. β-Catenin binds to the cytoplasmic tail of E-cadherin and recruits α-catenin, which binds to F-actin. α-Catenin forms a stable interaction with F-actin only under actomyosin-generated tension (Buckley et al., 2014). Of interest, the orientation of the F-actin linkage is different at the belt-like AJs, where actin is aligned parallel to the cell–cell junctions versus the spot-like AJs, where actin makes end-on connections with cell–cell junctions (Yonemura, 2011b). Recent studies using superresolution microscopy showed that the belt-like AJ is composed of clusters of spot-like AJs (Wu et al., 2015). Furthermore, F-actin was implicated in corralling the spot-like E-cadherin clusters (Wu et al., 2015), and actomyosin drives their coalescence and stabilization to form the apical belt-like AJ (Ratheesh and Yap, 2012).
In a review article, Yonemura (2011a) was the first (to our knowledge) to introduce the term tAJ. Many questions remain about the structure and molecular composition of tAJs. The cadherin/catenin complex is present at tAJs, and its localization often appears to be deeper (localized more basally) compared with its localization at belt-like AJs of bicellular junctions. However, the nature of E-cadherin interactions, the cytoplasmic scaffold proteins involved, and the orientation of the actin linkages at tAJs have not been well defined. In addition, we do not know whether there are specific molecular components that are localized strongly to tAJs, as is the case for tTJs. Finally, it has not been determined whether interactions between tTJ and tAJ components are important for proper organization of tricellular contacts.
tAJs are tension hot spots
AJs sense and transmit force generated by actomyosin from one epithelial cell to another. This ability to detect and respond to mechanical stimuli from their neighbors is essential for epithelial cells to maintain and remodel adhesions during cell shape change events such as cell division, cell extrusion, apical constriction, and intercalation. When the actomyosin-mediated tugging force between the two cells is increased, the area of cadherin-mediated adhesion between two cells increases (Liu et al., 2010). Similarly, in focal adhesions, increased mechanical tugging force results in focal adhesion growth. Mechanotransduction—the process by which mechanical forces are converted into biochemical signals—has been well documented for focal adhesions (e.g., force-dependent recruitment of adaptor proteins, kinases, and actin to focal adhesions; Bershadsky et al., 2003). Emerging evidence indicates that AJs can also serve as sites of mechanotransduction. Recent work shows that AJs become reinforced under increased force, and α-catenin plays a central role in mechanotransduction at AJs. α-Catenin senses increased force and responds by changing conformation, revealing a binding site for vinculin (le Duc et al., 2010; Yonemura et al., 2010). Vinculin is also an actin-binding protein; therefore, recruitment of vinculin promotes an increase in junctional F-actin, resulting in strengthening of the AJ (Yonemura, 2011b). In addition, vinculin can recruit Ena/VASP proteins, leading to new linear actin polymerization in response to increased junctional tension (Leerberg et al., 2014).
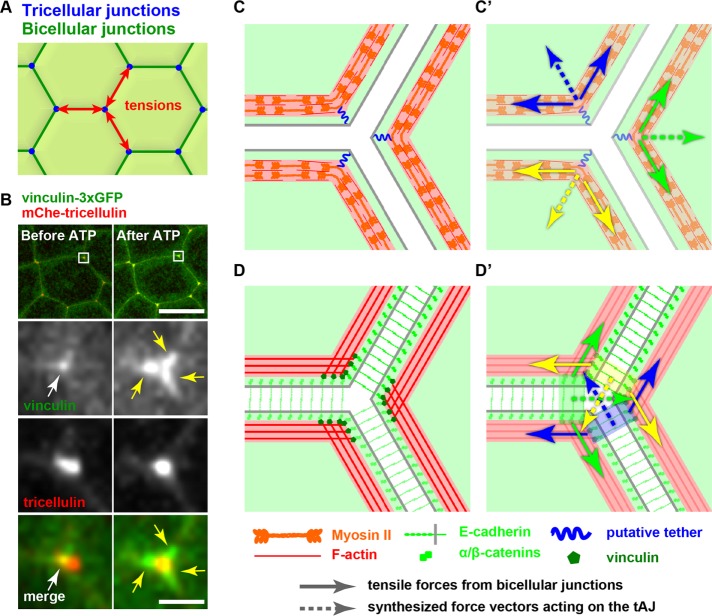
tAJs are potentially important sites for mechanotransduction. Mathematical modeling of cells in epithelial tissues using a vertex model approach in which cells are represented in edges and vertices (Trichas et al., 2012) suggests that tricellular contacts are tension hot spots where increased force may be applied to junctional proteins. Vertices experience high tension due to outward forces generated by actomyosin-dependent line tension acting along each of the cell edges connected to a vertex (Figure 3A). Both in silico and in vivo data show that cell edges and vertices are highly dynamic, as cell rearrangements take place during cell division and morphogenesis, causing changes in actomyosin-dependent line tension along the cell edges (Farhadifar et al., 2007; Rauzi et al., 2008) and putting additional stress on the vertices. Recent experimental data support the conclusion that tAJs are tension hot spots and show that in response to this increased tension, specific proteins are recruited to tAJs. First, vinculin accumulates strongly at tricellular junctions in comparison to bicellular junctions (Higashi et al., 2016). Fluorescently tagged vinculin is recruited to AJs in response to increased tension and can be used to approximate junctional tension in vivo (Hara et al., 2016; Higashi et al., 2016). When junctional tension is acutely increased globally, there is a significant increase in recruitment of vinculin–triple green fluorescent protein (3xGFP) or the tension-reporting anti–α-catenin α18 antibody to tricellular and bicellular junctions, but the signal is particularly elevated at tricellular junctions (Yonemura et al., 2010; Higashi et al., 2016). Of interest, under increased tension, the tricellular vinculin signal is very intense and is observed as three spots (Figure 3B), which may indicate a mechanosensitive response to strengthen the connection to F-actin specifically at tAJs, as we discuss later.
FIGURE 3:
Tension transmission at tAJs. (A) Line tension (red arrows) is applied on the tricellular junctions where three cells meet. (B) Vinculin-3xGFP is localized strongly at tAJs and weakly at bicellular AJs. At 30 min after addition of 50 µM ATP to increase tension, vinculin signal is separated into three spots, suggesting that line tension along cell–cell junctions is transmitted at bicellular AJs adjacent to tAJs. Scale bar, 20 µm (top), 2 µm (bottom). (C) Previously proposed myosin II organization model at tAJs (Ebrahim et al., 2013). Myosin II and F-actin make a quasisarcomeric structure along bicellular cell–cell junctions, and at tAJs, the rod region of a myosin II filament is tethered to the membrane by an unknown molecule, which makes myosin II filaments bend to fit the curvature of tricellular vertices of the cells. Tensile forces (arrows) from bicellular junctions are synthesized (dotted arrows) and applied on the putative tether, generating a “separation force” (C’). (D) Tension transmission model at tAJs based on TEM images from Yonemura (2011a) and Choi et al. (2016). Actomyosin bundles make end-on connections with cadherin–catenin complexes at tAJs, and the force vectors applied on trans-E-cadherin complexes from two adjacent cells (arrows) are synthesized toward the center of the vertex (dotted arrows), generating a “tightening force,” which could help to tighten tricellular junctions (D’).
Second, myosin II may be enriched at tAJs in a tension-sensitive manner. In the Xenopus embryo epithelium, signal for phospho–myosin II light chain is weakly localized along bicellular junctions and intensely localized at tricellular junctions (Reyes et al., 2014). When junctional tension is reduced by knocking down the actomyosin scaffolding protein anillin, junctional phospho–myosin II signal is reduced and no longer enriched at tricellular junctions (Reyes et al., 2014). In addition, Ebrahim et al. (2013) reported periodic assemblies of bipolar myosin II and actin forming a sarcomere-like belt around each epithelial cell; intriguingly, myosin II was localized at the corner of each cell. Furthermore, the fluorescence intensity of myosin II was higher at those sites (Ebrahim et al., 2013). It has been demonstrated that increased tension recruits and stabilizes myosin II along bicellular junctions in intercalating cells of the Drosophila embryo (Fernandez-Gonzalez et al., 2009), which raises the possibility that tricellular recruitment of myosin II could be tension dependent as well.
Third, Canoe/afadin, a scaffolding protein that cross-links AJs and the actin cytoskeleton, appears to play an important role in strengthening the AJ–actin link at tAJs. In Drosophila, Canoe is essential for linking the actin cytoskeleton to AJs during the mechanical stresses involved in morphogenetic movements during development (Choi et al., 2011; Sawyer et al., 2011). In addition, Choi et al. (2016) showed that in ZO-1/ZO-2 double-knockdown Madin–Darby canine kidney cells, which exhibit elevated junctional tension via a RhoA/Shroom3/ROCK pathway, cells respond to the increased junctional tension by recruiting additional afadin to both bicellular and tricellular junctions. Furthermore, they showed that afadin is necessary for maintaining tissue integrity and barrier function under high tension and is particularly important for maintaining adhesion and actomyosin architecture at tAJs (Choi et al., 2016). Afadin is also known to mediate interactions between AJs and TJs, which could be important at tricellular junctions (Ooshio et al., 2010).
Model for tension transmission at tAJ
Recently, conflicting models representing how actin is organized at the tAJ have been proposed. One model, proposed by Ebrahim et al. (2013), is based on their data observing the organization of myosin II, which was double tagged so that they could visualize the head and tail of myosin II, in explant cultures of organ of Corti from P2 mice. Strikingly, they found that myosin II and actin were organized in a periodic sarcomere-like belt around each epithelial cell. Further, they reported that myosin II appeared to be physically linked to the tAJ, and, due to this physical interaction, the myosin II filaments were bent inward at the cell corners (Figure 3C). The protein responsible for this potential linkage of myosin II to the tAJ was not identified. An alternate explanation consistent with the data is that two overlapping orientations of myosin II make up the myosin II accumulation observed at cell corners. This model will require additional superresolution microscopy and/or electron microscopy (EM) to confirm the orientation of F-actin and myosin II. A second group of models is based on EM images of F-actin orientation at tAJs (Gomez et al., 2011; Yonemura, 2011a; Choi et al., 2016). Yonemura (2011a) showed, by EM of MTD-1A cultured epithelial cells, that F-actin is connected to tAJs in a perpendicular end-on manner. Choi et al. (2016) showed similar organization of F-actin at tAJs in MDCK cells exhibiting increased tension due to ZO knockdown. They proposed that each cell edge acts as an independent contractile unit, with F-actin bundles anchored end-on to cadherin/catenin complexes at tAJs (Figure 3D). Therefore, building on these models, we propose that actomyosin bundles make end-on connections with cadherin–catenin complexes at tAJs. Under increased tensile force along cell–cell junctions, it is predicted that “separation forces,” that is, forces that promote separation of cells at the tricellular junctions, are applied (Figure 3C’; Jarvis, 1998). This could lead to disengagement of tricellular junctions and opening of the central tube. However, because additional proteins, including vinculin, are recruited to strengthen the connection to the tAJ under high tension and E-cadherins make extracellular transdimers, the force vectors applied on trans–E-cadherin complexes from two adjacent cells can be synthesized and generate a “tightening force” toward the center of the vertex, which could help maintain tricellular junctions in dynamic epithelial tissues (Figure 3D’).
TRICELLULAR JUNCTIONS IN INVERTEBRATES
Most invertebrates have SJs, which are located basal to AJs and are made up of multiple belt-like strands of septa spanning the intercellular space (Izumi and Furuse, 2014). SJs are regarded as functionally analogous to TJs and form a paracellular permeability barrier, although the protein components of SJs are distinct from those of TJs. At invertebrate tricellular contacts, the intercellular space is connected by regularly spaced, vertically stacked triangular structures called diaphragms (Figure 1, C and G). Each bicellular SJ strand turns either apically or basally near tricellular junctions and makes a loop to become parallel to the axis of vertical tricellular junctions, forming the limiting septa (also called limiting strands). The sides of limiting septa are connected to the diaphragms (Graf et al., 1982; Noirot-Timothee et al., 1982; Figure 1, C and G).
Three protein components of tricellular septate junctions (tSJs) have been identified (Figure 1E). First, Gliotactin, a cholinesterase-like transmembrane protein localized exclusively at tSJs, is required for establishment of proper SJ structure and function (Schulte et al., 2003). Of interest, Gliotactin expression is lost from the midgut of aged flies, which exhibit aging-associated phenotypes, including increased intestinal stem cell proliferation and blocked terminal differentiation of enterocytes (Resnik-Docampo et al., 2017). Loss-of-function analysis showed that Gliotactin maintains barrier function of intestinal cells and contributes to maintaining stem cell homeostasis in a non–cell-autonomous manner (Resnik-Docampo et al., 2017). Second, Sidekick, a transmembrane protein with Ig domains, is also localized at tricellular contacts (Lye et al., 2014) and is required for pattern formation in the eye (Nguyen et al., 1997). The role of Sidekick in SJ assembly and barrier function has not been determined. Third, Anakonda (also known as Bark beetle), a transmembrane protein with a unique tripartite extracellular domain and a C-terminal PDZ-binding motif, is exclusively localized at tSJs and is required for proper assembly and function of tSJs (Byri et al., 2015). In addition, Anakonda is required for the localization of Gliotactin at tSJs. It has been suggested that Gliotactin is integrated with the limiting septa on each side of the tSJ (Schulte et al., 2003) and that the large tripartite extracellular domain of Anakonda self-organizes in the triangular extracellular space and helps make the diaphragms there (Byri et al., 2015; Figure 1G). Gliotactin, Sidekick, and Anakonda have no sequence similarity to tricellulin or angulins, suggesting that the molecular mechanisms regulating the assembly of the tSJ and tTJ may be distinct.
FUNCTIONAL IMPORTANCE OF TRICELLULAR JUNCTIONS
Failure in tTJs causes nonsyndromic familial deafness
Mutations in TJ genes—including tTJ genes—can cause deafness. The organ of Corti, located in the cochlea of the inner ear, is a specialized epithelial tissue (Figure 2D). Mechanosensory hair cells are embedded in the epithelial cells of the organ of Corti and are bathed in endolymph (high electric potential and unique ion composition: high potassium and low sodium) at the apical side and in perilymph (normal ion composition) at the basal side. Hair cells convert the vibration signal detected by stereocilia at the apical side into electric action potential and relay it to auditory neurons. Failure in barrier function of the organ of Corti epithelium results in cell death of hair cells and causes deafness. For example, mutation of claudin-14 causes nonsyndromic familial deafness, DFNB29 (OMIM 614035; Wilcox et al., 2001), and claudin-14–knockout mice exhibit deafness, with severe loss of hair cells (Ben-Yosef et al., 2003).
Truncation of the C-terminal cytoplasmic tail of tricellulin has also been reported as a cause of nonsyndromic familial deafness, DFNB49 (OMIM 610153; Riazuddin et al., 2006). There was no obvious phenotype other than deafness, which is probably because the inner ear epithelium is most susceptible to barrier dysfunction, whereas other proteins may play compensatory functions in other organs. Knock-in mice harboring a tricellulin R497* mutation, which is equivalent to one of the DFNB49 mutations (R500*), also exhibit severe deafness (Nayak et al., 2013). The C-terminus of tricellulin is sufficient for recruitment of tricellulin by angulin-1 (Masuda et al., 2011) and is required for the recruitment by angulin-2 (Higashi et al., 2013). The tricellulin R497* mutation prematurely terminates the protein, resulting in loss of the C-terminal occludin-ELL domain. In the tTJs of tricellulin R497*–knock-in mice, tricellulin R497* was mislocalized, and the linkage between bicellular TJ strands and the central sealing elements was completely lost (Nayak et al., 2013), suggesting that tricellulin serves as a connector of claudin-based TJ strands with tricellular central sealing elements (Figure 1F). The phenotypes of tricellulin-knockout mice and R497* knock-in mice were in fact very similar (Kamitani et al., 2015), indicating that mislocalization of tricellulin caused by C-terminal truncation abolishes the entire function of tricellulin.
Occludin loss-of-function studies also support the conclusion that tricellulin is important for proper hearing. Occludin is an integral membrane protein in bicellular TJs and belongs to the TAMP family along with tricellulin. It has been reported that loss of occludin causes redistribution of tricellulin from tTJs to bicellular TJs in MDCK II cells (Ikenouchi et al., 2008). Occludin-knockout mice exhibit a complex phenotype, including growth retardation and chronic inflammation (Saitou et al., 2000). Occludin-knockout mice also exhibit deafness caused by apoptotic cell death of hair cells (Kitajiri et al., 2014). The localization of tricellulin was perturbed in the organ of Corti, which leads to loss of barrier function (Kitajiri et al., 2014), and may explain the cause of hair cell loss.
Among the three angulins, only angulin-2 is expressed in the organ of Corti (Higashi et al., 2013). Mutation of angulin-2 was reported as a cause of another nonsyndromic familial deafness, DFNB42 (OMIM 609646; Borck et al., 2011). Angulin-2–knockout mice exhibit deafness with postnatal loss of hair cells (Higashi et al., 2015; Morozko et al., 2015; Sang et al., 2015). In angulin-2–knockout mice, tricellulin localization was not lost from tTJs, and the connection between bicellular TJ strands with the central sealing element is maintained (Morozko et al., 2015). This could be explained by compensatory localization of angulin-1 at tTJs of the knockout mice. In contrast, angulin-1 was negligibly detected in wild-type mice (Higashi et al., 2015). In these mice, angulin-1-based tTJs show less prominent central sealing elements compared with angulin-2–based tTJs in freeze-fracture replica EM (Morozko et al., 2015), and the localization of tricellulin is slightly shifted to the basolateral side (Higashi et al., 2015; Morozko et al., 2015), which may result in the loss of strong barrier function required for proper organ of Corti function and maintenance of hair cells.
tTJs in blood–brain and blood–retinal barrier
Although tricellular junctions in endothelial cells are less well characterized than those in epithelial cells, recent studies shed light on their importance in blood–brain barrier (BBB) and blood–retinal barrier (BRB). Maintenance of BBB and BRB is essential for homeostasis of neurons, and extrapolating from epithelial barrier function, it seems likely that not only bicellular TJs but also tTJs are required for endothelial barrier function in the brain and retina. Indeed, tricellulin (Mariano et al., 2013) and angulin-1 (Iwamoto et al., 2014) are expressed and localized at tricellular contacts in brain and retinal endothelial cells but not in peripheral endothelial cells. Of interest, there is no BBB in particular regions of the brain, such as the choroid plexus and circumventricular organs. Of note, tricellulin and angulin-1 were not observed at endothelial tricellular contacts in these tissues (Iwamoto et al., 2014). Recently the functional importance of tTJs in brain endothelium was reported. The expression of angulin-1 in brain endothelial cells correlates with the onset of BBB formation, and the brain endothelial cells in angulin-1–knockout mice become more permeable to small molecules, which may explain the embryonic lethality of angulin-1–knockout mice (Sohet et al., 2015). Furthermore, angulin-1 is down-regulated in experimental models of multiple sclerosis and stroke, which implicates a role for angulin-1 in pathological BBB leakage (Sohet et al., 2015). In the future, freeze-fracture replica EM of tTJs in brain or retinal endothelium will be necessary to provide solid evidence of tTJs in blood vessels in vivo.
tTJs implicated in type 2 diabetes
Little is known about potential connections between tTJ proteins and type 2 diabetes, but several studies provide intriguing clues. Angulin-3 was identified as a candidate modifier of susceptibility to type 2 diabetes in mice (Dokmanovic-Chouinard et al., 2008). Furthermore, human angulin-3 is located in Chr1q23, which has been repeatedly associated with type 2 diabetes (Dokmanovic-Chouinard et al., 2008). In addition, angulin-1 was originally cloned as a lipoprotein receptor (Yen et al., 1999), and liver-specific knockdown was reported to cause hypertriglyceridemia (Narvekar et al., 2009), which is one of major risk factors for type 2 diabetes. In the future, it will be interesting to determine how tricellular junction proteins are mechanistically involved in the pathogenesis of type 2 diabetes.
Tricellular junctions in bacterial pathogenesis
Several pathogenic bacteria exploit tricellular junctions to effectively infect epithelial cells (Figure 2E). For example, Shigella, the cause of bacterial dysentery, invades and proliferates in the colonic epithelium. Shigella flexneri hijacks the cellular actin assembly machinery to generate an actin tail, allowing it to move around within infected cells. Then S. flexneri forms protrusive pseudopodia at tricellular junctions in a tricellulin-dependent manner (Fukumatsu et al., 2012). Neighboring cells engulf the bacterium-containing pseudopodia, which results in spreading of S. flexneri from infected cells to surrounding healthy cells.
Clostridium difficile, a cause of antibiotic-associated diarrhea and pseudomembranous colitis, secretes the binary toxin C. difficile transferase (CDT), which consists of a binding component and an actin-ADP ribosylating enzymatic component. The binding component binds to the cell surface, induces endocytosis, and allows the enzymatic component to translocate into the cytosol. Then actin restructuring within the cell causes cell death and at lower toxin concentration induces formation of microtubule-based protrusions, which enable adherence and colonization of the bacteria. Of interest, the CDT-binding component uses angulin-1 as a receptor (Papatheodorou et al., 2011). Related toxins from Clostridium perfringens and Clostridium spiroforme also use angulin-1 as a receptor (Papatheodorou et al., 2011, 2012). It has not been investigated whether the binding of the toxins occurs at epithelial tricellular junctions.
Group A Streptococcus (GAS) is a cause of various human diseases called GAS infections, ranging from mild, superficial infections, such as strep pharyngitis (strep throat), to life-threatening systemic infection. Recently it was shown that GAS targets tricellular junctions through an interaction with tricellulin using host-derived plasminogen as a ligand to breach the epithelial barrier, which may explain the mechanism of GAS invasion into submucosal tissues (Sumitomo et al., 2016). Similarly, enteropathogenic Escherichia coli was also reported to target tTJs using the bacterial effector protein EspG1 (Morampudi et al., 2017). If novel molecular components of tricellular junctions are identified, it will be interesting to test whether they, like tricellulin and angulin-1, are also exploited by bacterial pathogens.
Tricellular junctions in cell division
Recently it was reported that tricellular junctions regulate the orientation of cell division in Drosophila epithelial cells (Bosveld et al., 2016). During mitosis, when cells round up, the interphase cell shape is no longer maintained, but Gliotactin-based tricellular junctions maintain information regarding the anisotropy of the interphase cell shape. The tricellular junctions recruit the dynein-associated protein Mud. In this way, the distribution of tricellular junctions orchestrates dynein- and Mud-dependent astral microtubule pulling forces, which orient the mitotic spindle along the long axis of the interphase cell, thus assuring generation of equal-sized daughter cells. Mitotic spindle dynamics of vertebrate epithelial cells have recently been analyzed using automated tracking of live images (Larson and Bement, 2017). In this study, the mitotic spindle exhibits rapid oscillatory rotation during late metaphase, and the spindle poles frequently approach and nearly contact to the cell cortex and then rapidly move away from it. Of interest, the cell cortex seems to have specific contact targets for spindle poles. Tricellular junctions could be potential candidates for such cortical targets because tTJs contain specific components including tricellulin and angulins. In addition, NuMA, the vertebrate homologue of Mud, appears to be concentrated at tricellular junctions in vertebrate epithelial cells (see Figure 5b in Gloerich et al., 2017). It would be interesting to test directly whether vertebrate tricellular junctions serve as a spatial landmark to orient cell division.
Tricellular junctions in development and morphogenesis
Epithelial cells play major roles in morphogenetic movements during development. One such morphogenetic movement that occurs as epithelial tissues elongate is cell intercalation (Guillot and Lecuit, 2013b). During intercalation, the cells that formerly resided next to each other become separated by neighboring cells, which squeeze in between and form new cell–cell contacts (Figure 2C). In this situation, two tricellular junctions first fuse to make one four-way junction, which then separates into two new tricellular junctions formed among a different combination of cells. It would be interesting to investigate how this exchange of tricellular junctions is regulated and whether tTJ proteins are involved in this process. After cytokinesis in the Xenopus epithelium, the two daughter cells are separated by neighbors, but they frequently reorganize their tTJs and make a daughter–daughter interface (Higashi et al., 2016; Figure 2A), making this a potential model for studying the cell intercalation process.
OPEN QUESTIONS
How are new tTJs specified?
Although it is likely that angulins and Anakonda make up the primary structure of tTJs and tSJs, respectively, how they recognize the tricellular contact points is unclear. For Anakonda, an attractive model has been proposed in which it makes equal contacts with the surfaces of the three cells at tSJs using extracellular triple repeat regions and, for steric reasons, becomes selectively stabilized by three-way contacts at cell vertices (Byri et al., 2015). For angulin-2, a similar model has been presented (Kim et al., 2015). By constructing a computational three-dimensional structure model, it was suggested that three angulin-2 proteins from each cell make a complex at tTJs where the Ig-like extracellular domains form a trimer.
However, at least for tTJs, angulin trimer formation might not be the main driving force of tTJ specification because angulins are also preferentially localized at four-way junctions, which may not stabilize trimers (Masuda et al., 2011; Figure 1C). In addition, angulin-2 is localized at tTJs between olfactory neurons and supporting cells in the olfactory epithelium, in which three cells meet at angles of 90, 90, and 180° (Higashi et al., 2013). Furthermore, angulin-1 makes a trans-complex at bicellular contacts and is not preferentially localized at tricellular contacts when expressed in L fibroblasts, suggesting that angulin-1 alone cannot recognize tricellular contacts (Masuda et al., 2011).
Another speculative model is that angulins or Anakonda accumulate where the TJ or SJ barrier is not sufficient. Because the components of bicellular TJs cannot efficiently seal the tricellular contacts, the barrier is breached at tricellular regions. If one assumes that angulins or Anakonda are excluded from the region where the barrier is established or that they are preferentially localized at a breach area, then they are necessarily localized at tricellular contacts. This model could explain why angulin-1 is localized at newly forming cell–cell junctions at the basal side in an epidermal cell sheet (Yokouchi et al., 2016). As the old TJ gradually loses its barrier function, angulin-1 accumulates along the entire cell–cell junction in the lower layer. As the barrier function of the new bicellular TJ become mature, angulin-1 is excluded from bicellular TJs. Because there is no direct evidence proving this “leak-detection” model, future studies will be required to test this attractive hypothesis.
Other possibilities for how proteins may recognize the tricellular contact points invite further investigation. One possibility is that angulins or Anakonda can sense a specific feature of the tricellular region, such as negative membrane curvature of cell corners or specific lipid composition. Another possibility is that the molecular machinery of abscission (e.g., endosomal sorting complex required for transport) or the midbody may trigger the formation of tTJs or help transport tTJ components to the cleavage site after cell division. Curiously, when nascent tTJs are formed after cytokinesis, the timing of tTJ establishment is slightly different between the two tTJs (Figure 2A). Because the abscission event also occurs first on one side of the midbody and then later at the other side, it is possible that the abscission machinery and/or midbody are involved in tTJ specification.
Are there other molecular components of tricellular junctions or binding partners for them?
No tAJ-specific component has been identified. In addition, it is unknown whether tAJs undergo tension-dependent changes. For bicellular AJs, loading of a mechanical tugging force positively regulates the size of cell–cell junctions (Liu et al., 2010) and causes recruitment of vinculin (Yonemura et al., 2010). The size and molecular composition of the tAJ are also likely to be regulated by applied tension (Figure 3B). It will be interesting to test whether tension-induced changes at the tAJ are accompanied by recruitment of additional scaffold or signaling molecules (e.g., kinases) that are specific to tAJs or similar to the proteins recruited to bicellular AJs under tension.
For tTJs and tSJs, although specific transmembrane components have been identified, binding partners are still largely unknown. ZO-1 (Riazuddin et al., 2006) and Tuba (Oda et al., 2014) have been reported as tricellulin-binding proteins, and Tjp2iso3 (splicing isoform of ZO-2) colocalizes with tricellulin in Sertoli cells (Chakraborty et al., 2014). Future studies using new techniques such as the BioID approach may reveal additional interacting partners for tTJ and tSJ components.
Are tricellular junctions and bicellular junctions mutually dependent?
tTJ formation is dependent on bicellular TJs. For example, tricellulin is not localized at tricellular contacts in ZO-1/2 double-negative cells, which lack TJs (Ikenouchi et al., 2007), and loss of occludin causes mislocalization of tricellulin (Ikenouchi et al., 2008; Kitajiri et al., 2014). Whether tTJs affect bicellular TJs is controversial. Several groups have reported phenotypes of tricellulin loss, which vary from no obvious change (Van Itallie et al., 2010; Nayak et al., 2013; Kamitani et al., 2015), to abnormal occludin localization near tricellular contacts (Ikenouchi et al., 2005), to disruption of apical actin structure (Oda et al., 2014; Salomon et al., 2017). Because tTJ formation depends on bicellular TJs, and TJ formation is believed to depend on AJs, one would expect that tTJ formation is also affected by AJs. Recently it was reported that knockdown of EpCAM causes unusual expansion of the apical domain at tricellular contacts and basal displacement of tricellulin through abnormal accumulation of myosin II at tricellular contacts (Salomon et al., 2017), suggesting that bicellular AJs may influence tTJ positioning.
Do tTJs have unique selective permeability?
Epithelial tissues have TJs with specialized selectivity for fluid and solutes. For example, the proximal tubules of kidney have high permeability for cations and contribute to reabsorption of sodium ions from primitive urine. It is unclear whether tTJs have specific unique selectivity. There are two distinguishable pathways for paracellular transport (Watson et al., 2001; Guo et al., 2003; Van Itallie et al., 2008), called the pore pathway and the leak pathway (Anderson and Van Itallie, 2009; Shen et al., 2011). The pore pathway allows ions and water to pass through with high charge and size selectivity and is probably formed by intercellular, channel-like structures made up of claudins (Yu et al., 2009; Krug et al., 2014; Suzuki et al., 2015). In contrast, the leak pathway is not size selective and has relatively low capacity compared with the pore pathway. The physical basis of the leak pathway is unclear, but the idea that local transient discontinuities of TJ strands correspond to the leak pathway is widely accepted (Shen et al., 2011). Given that there is a 10-nm-wide “tube”-like central sealing element at the tTJ, tTJs are another candidate for the leak pathway. Overexpression of tricellulin at tTJs in MDCK II cells strengthened the barrier for macromolecules without affecting ion permeability (Krug et al., 2009), which supports the idea that tTJs constitute the leak pathway. Knockdown of tricellulin (Ikenouchi et al., 2005) or angulin-1 (Masuda et al., 2011; Higashi et al., 2013) in EpH4 cells, which normally have high resistance for ions, causes increased permeability of macromolecules as well as ions. It is intriguing that angulin-1–knockdown cells did not show changes in charge and size selectivity, although they exhibit ∼10-times-higher ion permeability (Higashi et al., 2013), which might suggest that there is no “leak” at tTJs. Such a property of selective permeability is reminiscent of the nuclear pore, which is filled with disordered proteins enriched with Phe and Gly residues and allows the passage of macromolecules such as mRNAs, at the same time restricting permeation of other molecules. Of interest, the first extracellular loop of tricellulin (and occludin) contains a Tyr- and Gly-rich sequence. An alternate possibility is that the knockdown of tTJ proteins changes the structure of bicellular TJs, which affects the continuity of TJ strands and opens up the leak pathway.
SUMMARY
The molecular machinery comprising tricellular junctions is starting to be uncovered. Because tricellular junctions are critical for barrier function, mechanohomeostasis of epithelial tissues, development, and several diseases, it will be exciting to further investigate tricellular junction components and their functional roles in both cultured epithelial cells and in vivo models.
Note added in proof. During the review process, a new report about angulin-2 was published (Gong et al., 2017). The authors showed that angulin-2, which was known to be expressed in the kidney, regulates paracellular water transport and urine concentration.
Acknowledgments
We thank members of the Miller lab and Shigenobu Yonemura for stimulating discussions and Rachel Stephenson and Shigenobu Yonemura for critical feedback on the manuscript. Research in the Miller lab is supported by National Institutes of Health Grant R01 GM112794 and National Science Foundation Award 1615338. T.H. was supported by a Postdoctoral Fellowship from the Japanese Society for the Promotion of Science.
Abbreviations used:
- AJ
adherens junction
- SJ
septate junction
- tAJ
tricellular adherens junction
- TER
transepithelial electrical resistance
- TJ
tight junction
- tSJ
tricellular septate junction
- tTJ
tricellular tight junction.
Footnotes
REFERENCES
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Borck G, Ur Rehman A, Lee K, Pogoda HM, Kakar N, von Ameln S, Grillet N, Hildebrand MS, Ahmed ZM, Nurnberg G, et al. Loss-of-function mutations of ILDR1 cause autosomal-recessive hearing impairment DFNB42. Am J Hum Genet. 2011;88:127–137. doi: 10.1016/j.ajhg.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosveld F, Markova O, Guirao B, Martin C, Wang Z, Pierre A, Balakireva M, Gaugue I, Ainslie A, Christophorou N, et al. Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis. Nature. 2016;530:495–498. doi: 10.1038/nature16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byri S, Misra T, Syed ZA, Batz T, Shah J, Boril L, Glashauser J, Aegerter-Wilmsen T, Matzat T, Moussian B, et al. The triple-repeat protein anakonda controls epithelial tricellular junction formation in Drosophila. Dev Cell. 2015;33:535–548. doi: 10.1016/j.devcel.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Chakraborty P, William Buaas F, Sharma M, Smith BE, Greenlee AR, Eacker SM, Braun RE. Androgen-dependent sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol Endocrinol. 2014;28:1055–1072. doi: 10.1210/me.2013-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Acharya BR, Peyret G, Fardin MA, Mege RM, Ladoux B, Yap AS, Fanning AS, Peifer M. Remodeling the zonula adherens in response to tension and the role of afadin in this response. J Cell Biol. 2016;213:243–260. doi: 10.1083/jcb.201506115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Jung KC, Nelson KS, Bhat MA, Beitel GJ, Peifer M, Fanning AS. The single Drosophila ZO-1 protein Polychaetoid regulates embryonic morphogenesis in coordination with Canoe/afadin and Enabled. Mol Biol Cell. 2011;22:2010–2030. doi: 10.1091/mbc.E10-12-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cording J, Berg J, Kading N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Gunzel D, Wolburg H, Piontek J, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126:554–564. doi: 10.1242/jcs.114306. [DOI] [PubMed] [Google Scholar]
- Dokmanovic-Chouinard M, Chung WK, Chevre JC, Watson E, Yonan J, Wiegand B, Bromberg Y, Wakae N, Wright CV, Overton J, et al. Positional cloning of “Lisch-Like,” a candidate modifier of susceptibility to type 2 diabetes in mice. PLoS Genet. 2008;4:e1000137. doi: 10.1371/journal.pgen.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S, Fujita T, Millis BA, Kozin E, Ma X, Kawamoto S, Baird MA, Davidson M, Yonemura S, Hisa Y, et al. NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr Biol. 2013;23:731–736. doi: 10.1016/j.cub.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer GT, Rosenblatt J. Bringing balance by force: live cell extrusion controls epithelial cell numbers. Trends Cell Biol. 2013;23:185–192. doi: 10.1016/j.tcb.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadifar R, Roper JC, Aigouy B, Eaton S, Julicher F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol. 2007;17:2095–2104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Founounou N, Loyer N, Le Borgne R. Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells. Dev Cell. 2013;24:242–255. doi: 10.1016/j.devcel.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972;53:758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumatsu M, Ogawa M, Arakawa S, Suzuki M, Nakayama K, Shimizu S, Kim M, Mimuro H, Sasakawa C. Shigella targets epithelial tricellular junctions and uses a noncanonical clathrin-dependent endocytic pathway to spread between cells. Cell Host Microbe. 2012;11:325–336. doi: 10.1016/j.chom.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MC, Patel AB, Nagpal R, Perrimon N. The emergence of geometric order in proliferating metazoan epithelia. Nature. 2006;442:1038–1041. doi: 10.1038/nature05014. [DOI] [PubMed] [Google Scholar]
- Gloerich M, Bianchini JM, Siemers KA, Cohen DJ, Nelson WJ. Cell division orientation is coupled to cell-cell adhesion by the E-cadherin/LGN complex. Nat Commun. 2017;8:13996. doi: 10.1038/ncomms13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Gong Y, Himmerkus N, Sunq A, Milatz S, Merkel C, Bleich M, Hou J. ILDR1 is important for paracellular water transport and urine concentration mechanism. Proc Natl Acad Sci USA. 2017;114:5271–5276. doi: 10.1073/pnas.1701006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf F, Noirot-Timothee C, Noirot C. The specialization of septate junctions in regions of tricellular junctions. I. Smooth septate junctions (= continuous junctions) J Ultrastruct Res. 1982;78:136–151. doi: 10.1016/s0022-5320(82)80019-1. [DOI] [PubMed] [Google Scholar]
- Guillot C, Lecuit T. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev Cell. 2013a;24:227–241. doi: 10.1016/j.devcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013b;340:1185–1189. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- Guo P, Weinstein AM, Weinbaum S. A dual-pathway ultrastructural model for the tight junction of rat proximal tubule epithelium. Am J Physiol Renal Physiol. 2003;285:F241–F257. doi: 10.1152/ajprenal.00331.2002. [DOI] [PubMed] [Google Scholar]
- Hara Y, Shagirov M, Toyama Y. Cell boundary elongation by non-autonomous contractility in cell oscillation. Curr Biol. 2016;26:2388–2396. doi: 10.1016/j.cub.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Herszterg S, Leibfried A, Bosveld F, Martin C, Bellaiche Y. Interplay between the dividing cell and its neighbors regulates adherens junction formation during cytokinesis in epithelial tissue. Dev Cell. 2013;24:256–270. doi: 10.1016/j.devcel.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Higashi T, Arnold TR, Stephenson RE, Dinshaw KM, Miller AL. Maintenance of the epithelial barrier and remodeling of cell-cell junctions during cytokinesis. Curr Biol. 2016;26:1829–1842. doi: 10.1016/j.cub.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Katsuno T, Kitajiri S, Furuse M. Deficiency of angulin-2/ILDR1, a tricellular tight junction-associated membrane protein, causes deafness with cochlear hair cell degeneration in mice. PLoS One. 2015;10:e0120674. doi: 10.1371/journal.pone.0120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, Furuse M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci. 2013;126:966–977. doi: 10.1242/jcs.116442. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell. 2008;19:4687–4693. doi: 10.1091/mbc.E08-05-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Umeda K, Tsukita S, Furuse M, Tsukita S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto N, Higashi T, Furuse M. Localization of angulin-1/LSR and tricellulin at tricellular contacts of brain and retinal endothelial cells in vivo. Cell Struct Funct. 2014;39:1–8. doi: 10.1247/csf.13015. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Furuse M. Molecular organization and function of invertebrate occluding junctions. Semin Cell Dev Biol. 2014;36:186–193. doi: 10.1016/j.semcdb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Jarvis MC. Intercellular separation forces generated by intracellular pressure. Plant Cell Environ. 1998;21:1307–1310. [Google Scholar]
- Kamitani T, Sakaguchi H, Tamura A, Miyashita T, Yamazaki Y, Tokumasu R, Inamoto R, Matsubara A, Mori N, Hisa Y, Tsukita S. Deletion of tricellulin causes progressive hearing loss associated with degeneration of cochlear hair cells. Sci Rep. 2015;5:18402. doi: 10.1038/srep18402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NK, Higashi T, Lee KY, Kim AR, Kitajiri S, Kim MY, Chang MY, Kim V, Oh SH, Kim D, et al. Downsloping high-frequency hearing loss due to inner ear tricellular tight junction disruption by a novel ILDR1 mutation in the Ig-like domain. PLoS One. 2015;10:e0116931. doi: 10.1371/journal.pone.0116931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Katsuno T, Sasaki H, Ito J, Furuse M, Tsukita S. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biol Open. 2014;3:759–766. doi: 10.1242/bio.20147799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, Kikuchi S, Ogasawara N, Ohkuni T, Masaki T, et al. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol. 2010;225:720–733. doi: 10.1002/jcp.22273. [DOI] [PubMed] [Google Scholar]
- Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206:2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ME, Bement WM. Automated mitotic spindle tracking suggests a link between spindle dynamics, spindle orientation, and anaphase onset in epithelial cells. Mol Biol Cell. 2017;28:746–759. doi: 10.1091/mbc.E16-06-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerberg JM, Gomez GA, Verma S, Moussa EJ, Wu SK, Priya R, Hoffman BD, Grashoff C, Schwartz MA, Yap AS. Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr Biol. 2014;24:1689–1699. doi: 10.1016/j.cub.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye CM, Naylor HW, Sanson B. Subcellular localisations of the CPTI collection of YFP-tagged proteins in Drosophila embryos. Development. 2014;141:4006–4017. doi: 10.1242/dev.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano C, Palmela I, Pereira P, Fernandes A, Falcao AS, Cardoso FL, Vaz AR, Campos AR, Goncalves-Ferreira A, Kim KS, et al. Tricellulin expression in brain endothelial and neural cells. Cell Tissue Res. 2013;351:397–407. doi: 10.1007/s00441-012-1529-y. [DOI] [PubMed] [Google Scholar]
- Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011;124:548–555. doi: 10.1242/jcs.072058. [DOI] [PubMed] [Google Scholar]
- Morampudi V, Graef FA, Stahl M, Dalwadi U, Conlin VS, Huang T, Vallance BA, Yu HB, Jacobson K. Tricellular tight junction protein tricellulin is targeted by the enteropathogenic escherichia coli effector EspG1, leading to epithelial barrier disruption. Infect Immun. 2017;85:00700-16. doi: 10.1128/IAI.00700-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozko EL, Nishio A, Ingham NJ, Chandra R, Fitzgerald T, Martelletti E, Borck G, Wilson E, Riordan GP, Wangemann P, et al. ILDR1 null mice, a model of human deafness DFNB42, show structural aberrations of tricellular tight junctions and degeneration of auditory hair cells. Hum Mol Genet. 2015;24:609–624. doi: 10.1093/hmg/ddu474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu D, Kano F, Taguchi Y, Sugawara T, Nishizono T, Nishikawa K, Oda Y, Furuse M, Murata M. JNK1/2-dependent phosphorylation of angulin-1/LSR is required for the exclusive localization of angulin-1/LSR and tricellulin at tricellular contacts in EpH4 epithelial sheet. Genes Cells. 2014;19:565–581. doi: 10.1111/gtc.12158. [DOI] [PubMed] [Google Scholar]
- Narvekar P, Berriel Diaz M, Krones-Herzig A, Hardeland U, Strzoda D, Stohr S, Frohme M, Herzig S. Liver-specific loss of lipolysis-stimulated lipoprotein receptor triggers systemic hyperlipidemia in mice. Diabetes. 2009;58:1040–1049. doi: 10.2337/db08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak G, Lee SI, Yousaf R, Edelmann SE, Trincot C, Van Itallie CM, Sinha GP, Rafeeq M, Jones SM, Belyantseva IA, et al. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J Clin Invest. 2013;123:4036–4049. doi: 10.1172/JCI69031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DN, Liu Y, Litsky ML, Reinke R. The sidekick gene, a member of the immunoglobulin superfamily, is required for pattern formation in the Drosophila eye. Development. 1997;124:3303–3312. doi: 10.1242/dev.124.17.3303. [DOI] [PubMed] [Google Scholar]
- Noirot-Timothee C, Graf F, Noirot C. The specialization of septate junctions in regions of tricellular junctions. II. Pleated septate junctions. J Ultrastruct Res. 1982;78:152–165. doi: 10.1016/s0022-5320(82)80020-8. [DOI] [PubMed] [Google Scholar]
- Oda Y, Otani T, Ikenouchi J, Furuse M. Tricellulin regulates junctional tension of epithelial cells at tricellular contacts through Cdc42. J Cell Sci. 2014;127:4201–4212. doi: 10.1242/jcs.150607. [DOI] [PubMed] [Google Scholar]
- Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, Ogita H, Takai Y. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem. 2010;285:5003–5012. doi: 10.1074/jbc.M109.043760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodorou P, Carette JE, Bell GW, Schwan C, Guttenberg G, Brummelkamp TR, Aktories K. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT) Proc Natl Acad Sci USA. 2011;108:16422–16427. doi: 10.1073/pnas.1109772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodorou P, Wilczek C, Nolke T, Guttenberg G, Hornuss D, Schwan C, Aktories K. Identification of the cellular receptor of Clostridium spiroforme toxin. Infect Immun. 2012;80:1418–1423. doi: 10.1128/IAI.06378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragkousi K, Gibson MC. Cell division and the maintenance of epithelial order. J Cell Biol. 2014;207:181–188. doi: 10.1083/jcb.201408044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, Turner JR. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–1213. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh A, Yap AS. A bigger picture: classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:673–679. doi: 10.1038/nrm3431. [DOI] [PubMed] [Google Scholar]
- Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- Reaves DK, Hoadley KA, Fagan-Solis KD, Jima DD, Bereman M, Thorpe L, Hicks J, McDonald D, Troester MA, Perou CM, Fleming JM. Nuclear localized LSR: a novel regulator of breast cancer behavior and tumorigenesis. Mol Cancer Res. 2017;15:165–178. doi: 10.1158/1541-7786.MCR-16-0085-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik-Docampo M, Koehler CL, Clark RI, Schinaman JM, Sauer V, Wong DM, Lewis S, D’Alterio C, Walker DW, Jones DL. Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat Cell Biol. 2017;19:52–59. doi: 10.1038/ncb3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes CC, Jin M, Breznau EB, Espino R, Delgado-Gonzalo R, Goryachev AB, Miller AL. Anillin regulates cell-cell junction integrity by organizing junctional accumulation of Rho-GTP and actomyosin. Curr Biol. 2014;24:1263–1270. doi: 10.1016/j.cub.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, Khan SN, Chattaraj P, Friedman PL, Anderson JM, et al. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–1051. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon J, Gaston C, Magescas J, Duvauchelle B, Canioni D, Sengmanivong L, Mayeux A, Michaux G, Campeotto F, Lemale J, et al. Contractile forces at tricellular contacts modulate epithelial organization and monolayer integrity. Nat Commun. 2017;8:13998. doi: 10.1038/ncomms13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q, Li W, Xu Y, Qu R, Xu Z, Feng R, Jin L, He L, Li H, Wang L. ILDR1 deficiency causes degeneration of cochlear outer hair cells and disrupts the structure of the organ of Corti: a mouse model for human DFNB42. Biol Open. 2015;4:411–418. doi: 10.1242/bio.201410876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer JK, Choi W, Jung K-CC, He L, Harris NJ, Peifer M. A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension. Mol Biol Cell. 2011;22:2491–2508. doi: 10.1091/mbc.E11-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol. 2003;161:991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedzinski J, Hannezo E, Tu F, Biro M, Wallingford JB. Emergence of an apical epithelial cell surface in vivo. Dev Cell. 2016;36:24–35. doi: 10.1016/j.devcel.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–1117. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohet F, Lin C, Munji RN, Lee SY, Ruderisch N, Soung A, Arnold TD, Derugin N, Vexler ZS, Yen FT, Daneman R. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J Cell Biol. 2015;208:703–711. doi: 10.1083/jcb.201410131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- Staehelin LA, Mukherjee TM, Williams AW. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma. 1969;67:165–184. doi: 10.1007/BF01248737. [DOI] [PubMed] [Google Scholar]
- Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95. doi: 10.1186/1471-2121-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo T, Nakata M, Higashino M, Yamaguchi M, Kawabata S. Group A Streptococcus exploits human plasminogen for bacterial translocation across epithelial barrier via tricellular tight junctions. Sci Rep. 2016;7:20069. doi: 10.1038/srep20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Tani K, Tamura A, Tsukita S, Fujiyoshi Y. Model for the architecture of claudin-based paracellular ion channels through tight junctions. J Mol Biol. 2015;427:291–297. doi: 10.1016/j.jmb.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Trichas G, Smith AM, White N, Wilkins V, Watanabe T, Moore A, Joyce B, Sugnaseelan J, Rodriguez TA, Kay D, et al. Multi-cellular rosettes in the mouse visceral endoderm facilitate the ordered migration of anterior visceral endoderm cells. PLoS Biol. 2012;10:e1001256. doi: 10.1371/journal.pbio.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157–165. doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Tietgens AJ, Anderson JM. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol Biol Cell. 2017;28:524–534. doi: 10.1091/mbc.E16-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JB, Karnovsky MJ. The structure of the zonula occludens. A single fibril model based on freeze-fracture. J Cell Biol. 1974;60:168–180. doi: 10.1083/jcb.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walck-Shannon E, Hardin J. Cell intercalation from top to bottom. Nat Rev Mol Cell Biol. 2014;15:34–48. doi: 10.1038/nrm3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DC, MacKenzie A, Hulbert WC, Hogg JC. A re-assessment of the tricellular region of epithelial cell tight junctions in trachea of guinea pig. Acta Anat (Basel) 1985;122:35–38. doi: 10.1159/000145982. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol. 2001;281:C388–C397. doi: 10.1152/ajpcell.2001.281.2.C388. [DOI] [PubMed] [Google Scholar]
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kanchanawong P, Zaidel-Bar R. Actin-delimited adhesion-independent clustering of E-cadherin forms the nanoscale building blocks of adherens junctions. Dev Cell. 2015;32:139–154. doi: 10.1016/j.devcel.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Yen FT, Masson M, Clossais-Besnard N, Andre P, Grosset JM, Bougueleret L, Dumas JB, Guerassimenko O, Bihain BE. Molecular cloning of a lipolysis-stimulated remnant receptor expressed in the liver. J Biol Chem. 1999;274:13390–13398. doi: 10.1074/jbc.274.19.13390. [DOI] [PubMed] [Google Scholar]
- Yokouchi M, Atsugi T, Logtestijn MV, Tanaka RJ, Kajimura M, Suematsu M, Furuse M, Amagai M, Kubo A. Epidermal cell turnover across tight junctions based on Kelvin’s tetrakaidecahedron cell shape. Elife. 2016;5:e19593. doi: 10.7554/eLife.19593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol. 2011a;23:515–522. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Yonemura S. A mechanism of mechanotransduction at the cell-cell interface: emergence of alpha-catenin as the center of a force-balancing mechanism for morphogenesis in multicellular organisms. Bioessays. 2011b;33:732–736. doi: 10.1002/bies.201100064. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]