Abstract
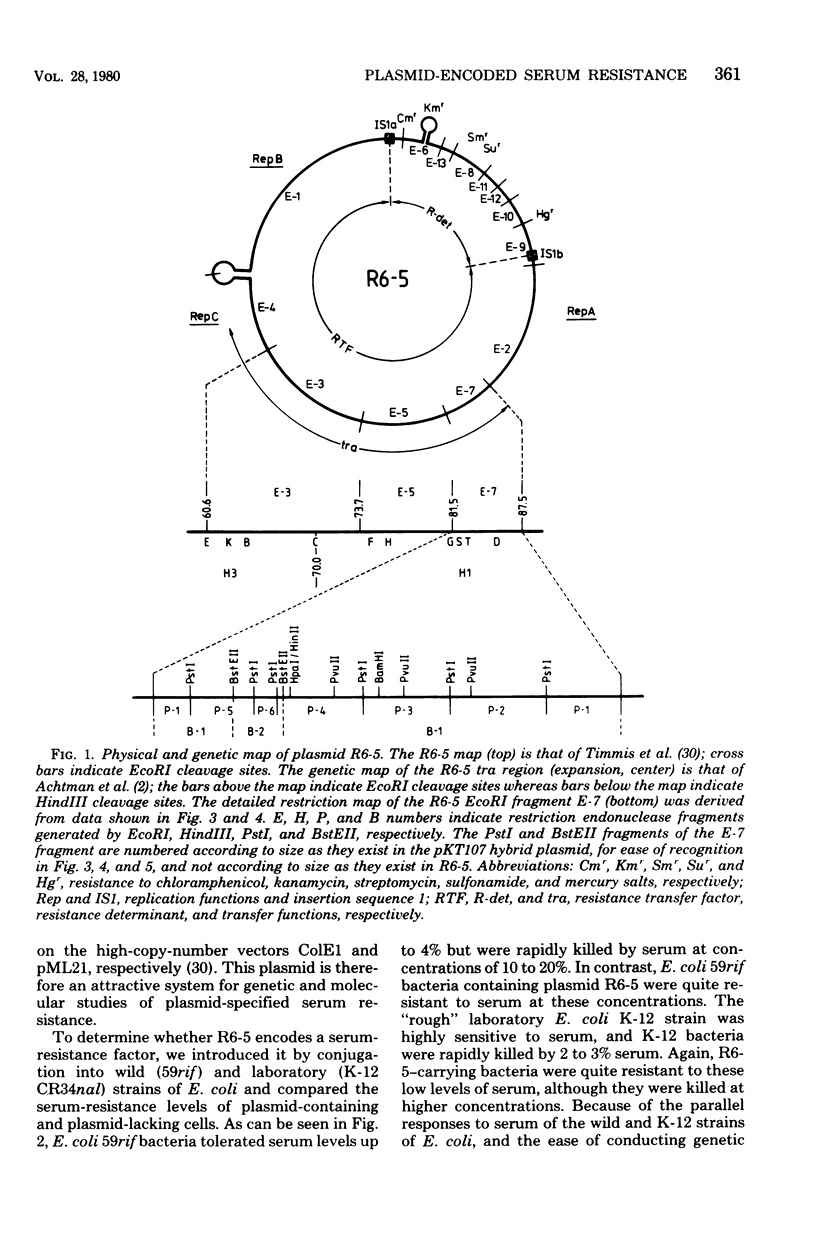
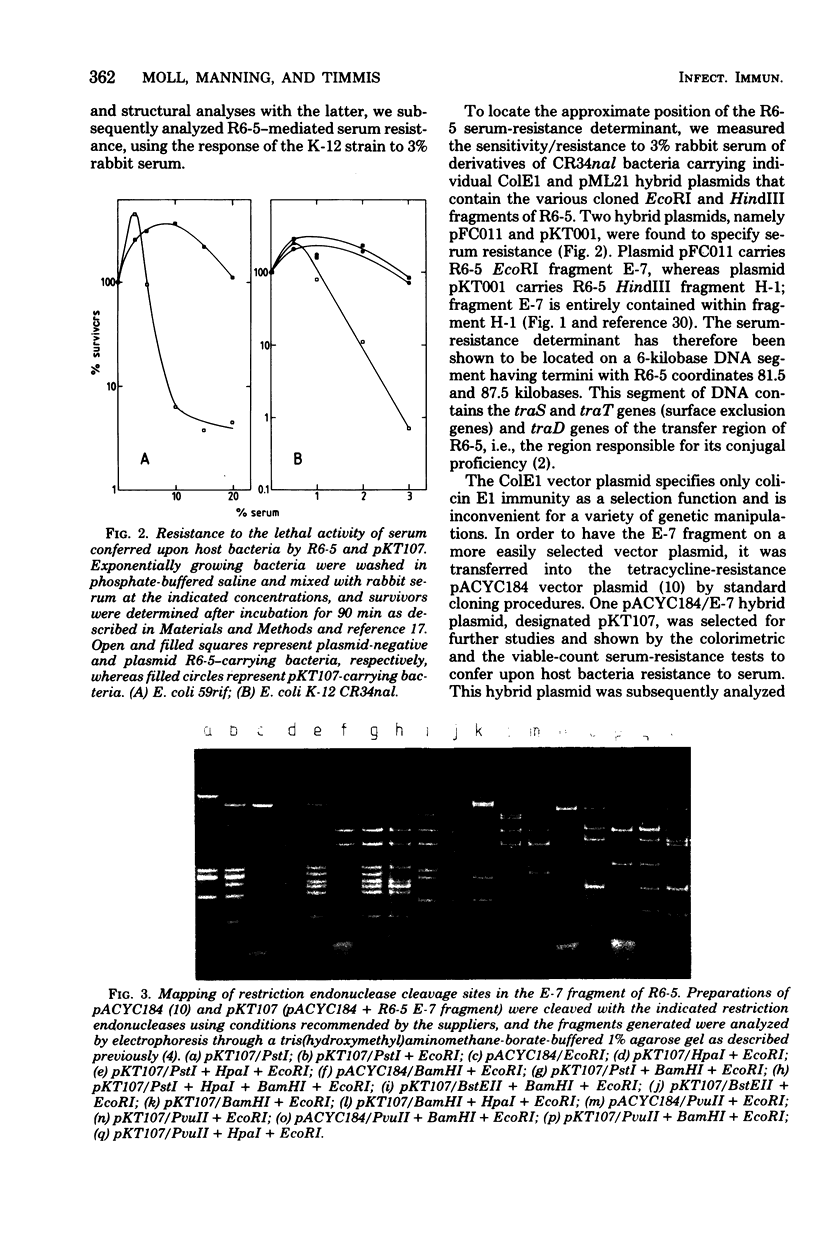
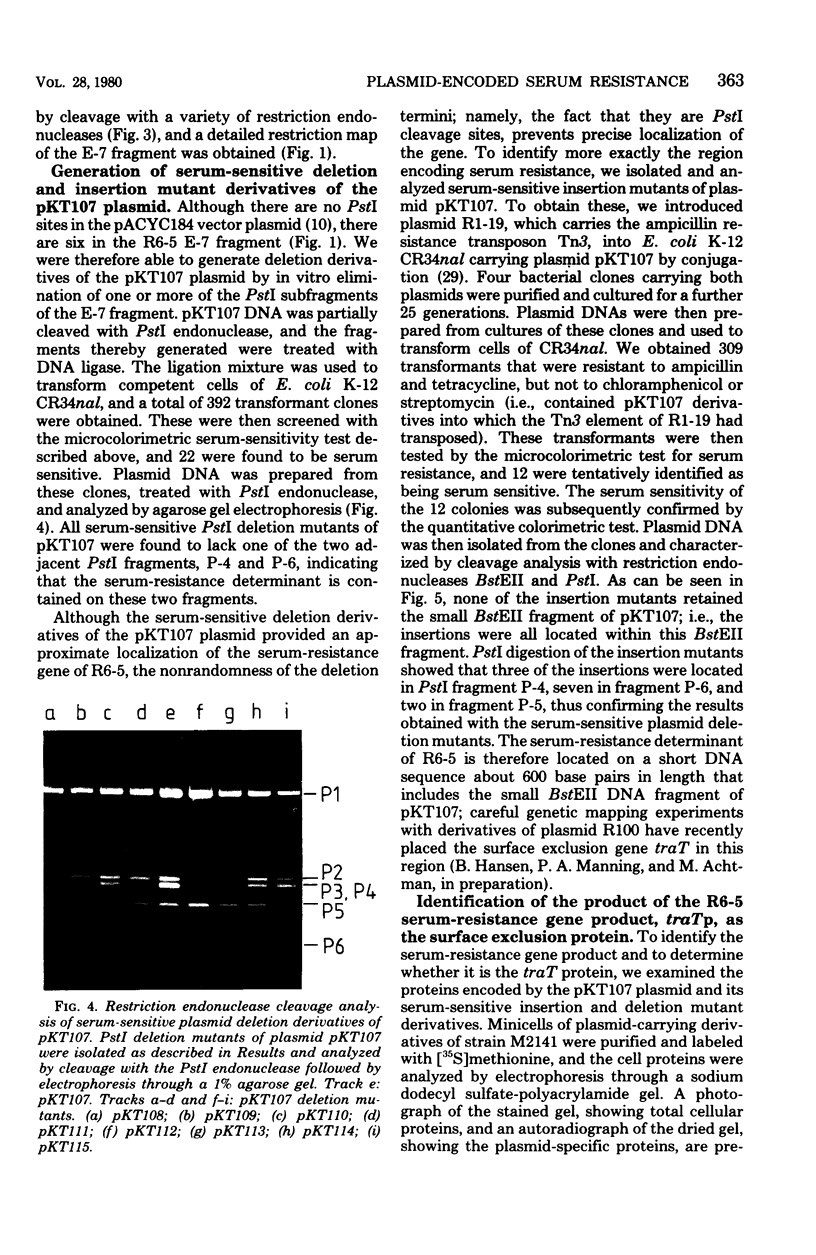
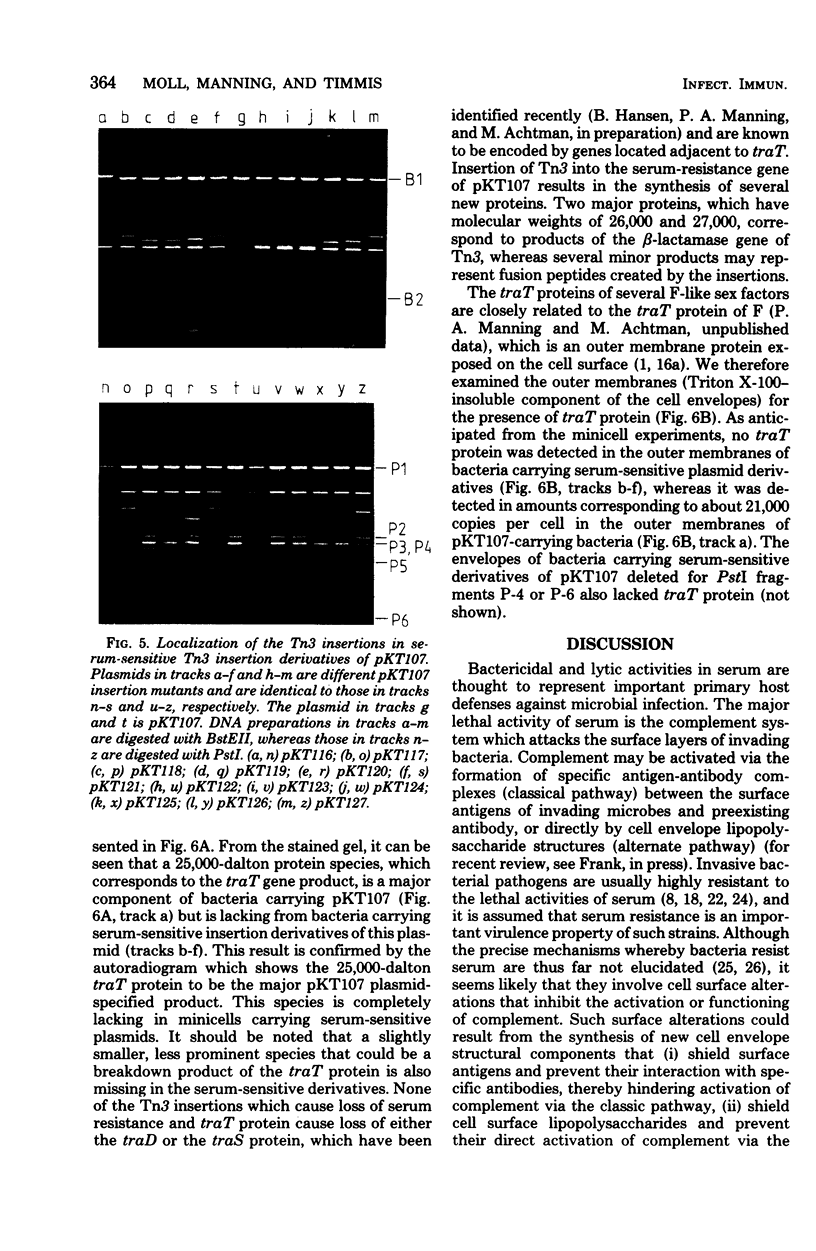
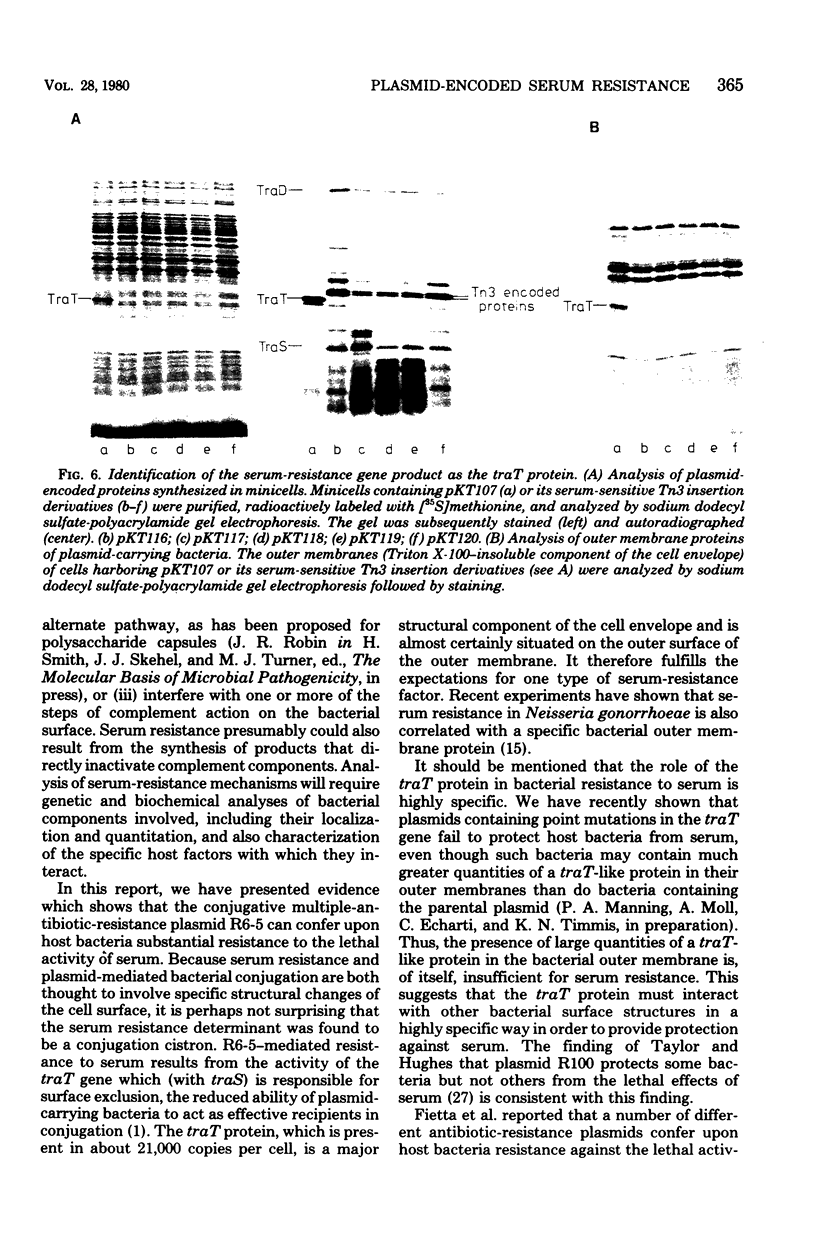
Resistance to the bactericidal activity of serum appears to be an important virulence property of invasive bacteria. The conjugative multiple-antibiotic-resistance plasmid R6-5 was found to confer upon Escherichia coli host bacteria increased resistance against rabbit serum. Gene-cloning techniques were used to localize the serum resistance determinant of R6-5 to a segment of the plasmid that encodes conjugal transfer functions, and a pACYC184 hybrid plasmid, designated pKT107, that contains this segment was constructed. The generation and analysis of deletion and insertion mutant derivatives of the pKT107 plasmid that no longer specify serum resistance permitted precise localization of the serum-resistance cistron on the R6-5 map and demonstrated that this locus is coincident with that of traT, one of the two surface exclusion genes of R6-5. Examination of the proteins synthesized in E. coli minicells of pKT107 and its serum-sensitive mutant derivative plasmids confirmed that the serum-resistance gene product of R6-5 is the traT protein and showed that this protein is a major structural component (about 21,000 copies per cell) of the bacterial outer membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Kusećek B., Timmis K. N. Tra cistrons and proteins encoded by the Escherichia coli antibiotic resistance plasmid R6-5. Mol Gen Genet. 1978 Jul 11;163(2):169–179. doi: 10.1007/BF00267407. [DOI] [PubMed] [Google Scholar]
- Andrés I., Slocombe P. M., Cabello F., Timmis J. K., Lurz R., Burkardt H. J., Timmis K. N. Plasmid replication functions. II. Cloning analysis of the repA replication region of antibiotic resistance plasmid R6-5. Mol Gen Genet. 1979 Jan 5;168(1):1–25. doi: 10.1007/BF00267929. [DOI] [PubMed] [Google Scholar]
- Archer G., Fekety F. R. Experimental endocarditis due to Pseudomonas aeruginosa. I. Description of a model. J Infect Dis. 1976 Jul;134(1):1–7. doi: 10.1093/infdis/134.1.1. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns M. M., Davies D. L., Hardy K. G. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature. 1979 Jun 28;279(5716):778–781. doi: 10.1038/279778a0. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Kaijser B. Interaction of human serum and neutrophils with Escherichia coli strains: differences between strains isolated from urine of patients with pyelonephritis or asymptomatic bacteriuria. Infect Immun. 1978 Nov;22(2):308–311. doi: 10.1128/iai.22.2.308-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Protective role of complement in experimental Escherichia coli endocarditis. Infect Immun. 1977 Apr;16(1):213–217. doi: 10.1128/iai.16.1.213-217.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher B. S., Reynard A. M., Beck M. E., Cunningham R. K. Potentiation of antibiotic bactericidal activity by normal human serum. Antimicrob Agents Chemother. 1978 May;13(5):820–826. doi: 10.1128/aac.13.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietta A., Romero E., Siccardi A. G. Effect of some R factors on the sensitivity of rough Enterobacteriaceae to human serum. Infect Immun. 1977 Nov;18(2):278–282. doi: 10.1128/iai.18.2.278-282.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarosa E. J., Bennett J. V., Wyatt C., Pierce P. E., Olarte J., Hernandes P. M., Vázquez V., Bessudo D. An epidemic-associated episome? J Infect Dis. 1972 Aug;126(2):215–218. doi: 10.1093/infdis/126.2.215. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olling S. Sensitivity of gram-negative bacilli to the serum bactericidal activity: a marker of the host-parasite relationship in acute and persisting infections. Scand J Infect Dis Suppl. 1977;(10):1–40. doi: 10.3109/inf.1977.9.suppl-10.01. [DOI] [PubMed] [Google Scholar]
- Reynard A. M., Beck M. E., Cunningham R. K. Effects of antibiotic resistance plasmids on the bactericidal activity of normal rabbit serum. Infect Immun. 1978 Mar;19(3):861–866. doi: 10.1128/iai.19.3.861-866.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynard A. M., Beck M. E. Plasmid-mediated resistance to the bactericidal effects of normal rabbit serum. Infect Immun. 1976 Sep;14(3):848–850. doi: 10.1128/iai.14.3.848-850.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Simberkoff M. S., Ricupero I., Rahal J. J., Jr Host resistance to Serratia marcescens infection: serum bactericidal activity and phagocytosis by normal blood leukocytes. J Lab Clin Med. 1976 Feb;87(2):206–217. [PubMed] [Google Scholar]
- Taylor P. W. Genetical studies of serum resistance in Escherichia coli. J Gen Microbiol. 1975 Jul;89(1):57–66. doi: 10.1099/00221287-89-1-57. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Hughes C. Plasmid carriage and the serum sensitivity of enterobacteria. Infect Immun. 1978 Oct;22(1):10–17. doi: 10.1128/iai.22.1.10-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Immunochemical investigations on lipopolysaccharides and acidic polysaccharides from serum-sensitive and serum-resistant strains of Escherichia coli isolated from urinary-tract infections. J Med Microbiol. 1976 Nov;9(4):405–421. doi: 10.1099/00222615-9-4-405. [DOI] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Timmis K. Purification and characterization of colicin D. J Bacteriol. 1972 Jan;109(1):12–20. doi: 10.1128/jb.109.1.12-20.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]