Inactivation of Sec23b exclusively in the pancreatic acinar cells of adult mice results in loss of pancreatic mass, with evidence of cell loss, degeneration of exocrine cells (with smaller-than-normal zymogen granules and ER dilation), ER stress, and increased pancreatic cell apoptosis.
Abstract
Mice with germline absence of SEC23B die perinatally, exhibiting massive pancreatic degeneration. We generated mice with tamoxifen-inducible, pancreatic acinar cell–specific Sec23b deletion. Inactivation of Sec23b exclusively in the pancreatic acinar cells of adult mice results in decreased overall pancreatic weights from pancreatic cell loss (decreased pancreatic DNA, RNA, and total protein content), as well as degeneration of exocrine cells, decreased zymogen granules, and alterations in the endoplasmic reticulum (ER), ranging from vesicular ER to markedly expanded cisternae with accumulation of moderate-density content or intracisternal granules. Acinar Sec23b deletion results in induction of ER stress and increased apoptosis in the pancreas, potentially explaining the loss of pancreatic cells and decreased pancreatic weight. These findings demonstrate that SEC23B is required for normal function of pancreatic acinar cells in adult mice.
INTRODUCTION
SEC23 is a core component of coat protein complex II (COPII) vesicles, which transport secretory proteins from the endoplasmic reticulum (ER) to the Golgi apparatus. The genes encoding COPII vesicle components are highly evolutionarily conserved; however, in contrast to yeast, the mammalian genome contains two or more paralogues for most of these genes (Bonifacino and Glick, 2004; Khoriaty et al., 2012; Zanetti et al., 2012). The two mammalian paralogues for SEC23—SEC23A and SEC23B—exhibit ∼85% amino acid sequence identity (Bonifacino and Glick, 2004; Khoriaty et al., 2012; Zanetti et al., 2012). In humans, SEC23A mutations result in cranio-lenticulo-sutural dysplasia, an autosomal recessive disease characterized by late closure of fontanelles, skeletal abnormalities, and sutural cataracts (Boyadjiev et al., 2010; Lang et al., 2006), whereas homozygous/compound heterozygous loss-of-function SEC23B mutations result in congenital dyserythropoietic anemia type II (CDAII; Bianchi et al., 2009; Schwarz et al., 2009; Khoriaty et al., 2012), a disease characterized by a defect in erythroid maturation. We previously reported that mice genetically deficient for SEC23A exhibit midembryonic lethality and a cranial developmental defect (Zhu et al., 2015), and mice homozygous for a Sec23b null allele die perinatally with massive pancreatic degeneration (Tao et al., 2012). Mice with embryonic pancreas-specific SEC23B deficiency recapitulated the latter phenotype (Khoriaty et al., 2016), with pancreas tissues harvested from these mice demonstrating abnormal acinar cell histology but normal-appearing islet cells.
The pancreas is a professional secretory organ with two highly active cell types engaged in protein synthesis and secretion—the exocrine acinar cell, which secretes digestive enzymes, and the endocrine beta and alpha cells, which secrete insulin and glucagon, respectively. Acinar cells contain abundant rough endoplasmic reticulum (RER), prominent Golgi, and large secretory granules termed zymogen granules (ZGs; Gorelick and Jamieson, 2012). Approximately 20 different digestive enzymes are synthesized on the RER and traverse the secretory pathway, with the ER to Golgi being a major concentrating step (Oprins et al., 2001). The early steps by which coated vesicles bud from smooth regions of the ER termed transition zones appear similar in acinar cells and other secretory cells. The concentrations of amylase and chymotrypsinogen are similar in the COPII vesicle and the RER lumen, suggesting a bulk flow process for this initial step (Martinez-Menarguez et al., 1999; Klumperman, 2000; Barlowe and Helenius, 2016).
To test whether SEC23B is required for normal pancreatic function in adult mice, we now report the characterization of mice with tamoxifen-inducible, acinar cell–specific SEC23B deficiency. Tamoxifen administration to adult mice results in loss of pancreatic mass with evidence of cell loss, degeneration of exocrine cells (with smaller-than-normal zymogen granules and ER dilation), ER stress, and increased pancreatic cell apoptosis.
RESULTS
Generation of mice with tamoxifen-inducible, pancreatic acinar cell–specific Sec23b deletion
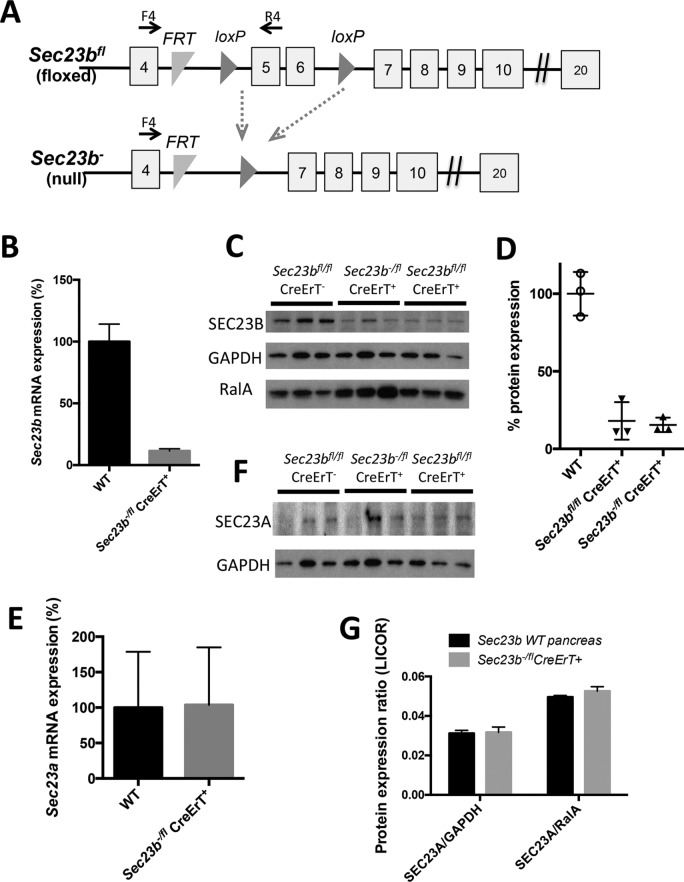
Mice with tamoxifen-inducible, acinar cell–specific deletion of Sec23b were generated by crossing Sec23b+/− CreErT+ mice to Sec23b+/fl mice. This cross yielded the expected number of Sec23b−/fl CreErT+ mice at weaning (Table 1). One week after tamoxifen administration, pancreas tissues were harvested from the latter mice to determine the degree of excision of Sec23b. Sec23b−/fl CreErT+ pancreata exhibited ∼90% lower expression of wild-type (WT) Sec23b mRNA by quantitative RT-PCR (qRT-PCR) than WT pancreata (Figure 1B), with similar decreases in steady-state SEC23B protein by Western blot analysis (Figure 1, C and D). Because CreErT is expressed only in acinar cells (Ji et al., 2008), some or all of the residual SEC23B expression could be derived from islet and other nonacinar cells. Thus these data are consistent with high-level excision of Sec23b in pancreatic acinar cells after tamoxifen administration. Sec23a mRNA and protein levels were not increased in pancreata of mice with acinar cell deletion of Sec23b (Figure 1, E–G).
TABLE 1:
Results of Sec23b+/− CreErT(+) x Sec23b+/fl matings to generate mice with tamoxifen-inducible, acinar cell–specific deletion of Sec23b.
| Genotype | Sec23b+/+ CreErT(+) | Sec23b+/+ CreErT(–) | Sec23b+/fl CreErT(+) | Sec23b+/fl CreErT(−) | Sec23b+/− CreErT(+) | Sec23b+/− CreErT(–) | Sec23b−/fl CreErT(+) | Sec23b−/fl CreErT(–) | p valuea |
|---|---|---|---|---|---|---|---|---|---|
| Expected ratio (%) | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | |
| Percentage observed at weaning (n = 174) | 9 (16) | 13 (23) | 14 (24) | 14 (24) | 13 (22) | 13 (23) | 11 (19) | 13 (23) | >0.8 |
ap calculated for Sec23b−/fl CreErT(+) mice versus all other genotypes.
FIGURE 1:
Sec23b inactivation in pancreatic acinar cells. (A) Sec23b alleles (not drawn to scale; Khoriaty et al., 2014). Each square indicates an exon, and horizontal lines between exons indicate introns. F4 and R4 are primers used for assessing efficiency of Cre-mediated excision. (B) Sec23b excision determined by qPCR (n = 3 for each genotype) and (C) Western blot on pancreas tissues 7 d after administration of tamoxifen. (D) Quantification of the SEC23B band intensities in C relative to average of GAPDH and RalA performed using ImageJ. (E–G) Quantitation of Sec23a expression by (E) qPCR (three controls and four Sec23b−/fl CreErT+ mice), (F) chemiluminescence Western blot detection, and (G) quantitative Western blot (infrared fluorescence detection) in pancreas tissues 7 d after administration of tamoxifen (three mice per genotype).
Depletion of Sec23b in acinar cells of adult mice results in lower pancreatic weight
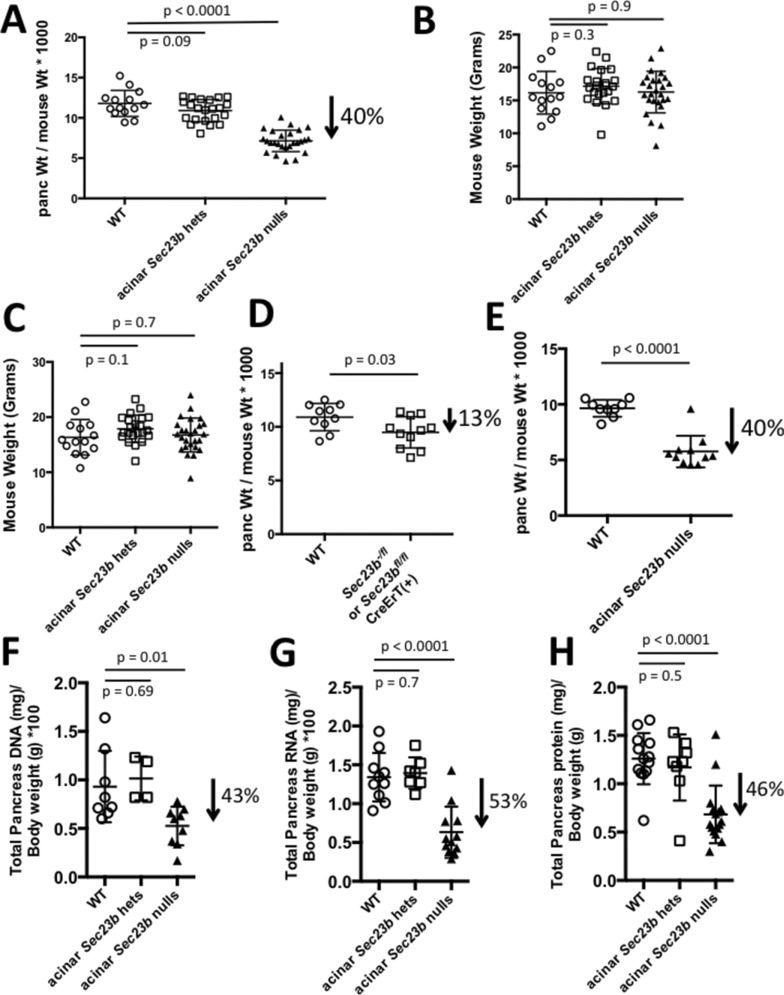
One week after tamoxifen administration, mice were killed and pancreata were dissected and weighed. Mice with acinar cell deletion of Sec23b (Sec23b−/fl CreErT+ or Sec23bfl/fl CreErT+ mice) exhibited ∼40% decrease in pancreatic weight compared with WT control mice (Sec23bfl/fl CreErT−, Sec23b+/fl CreErT−, Sec23b+/+ CreErT+, and Sec23b+/+ CreErT− mice; p < 0.0001), whereas pancreatic weights of mice with heterozygous acinar cell deletion of Sec23b (Sec23b+/− CreErT+, Sec23b+/− CreErT−, and Sec23b+/fl CreErT+ mice) were not significantly different than those of WT mice (p = 0.09; Figure 2A).
FIGURE 2:
Sec23b deletion in acinar cells results in decreased pancreatic weight from cell loss. (A) Ratios of pancreas to total body weight 7 d after administration of tamoxifen indicate substantial loss of pancreas weight resulting from inactivation of Sec23b in pancreas acinar cells. Mouse weights (B) before and (C) 1 wk after tamoxifen administration indicates no loss of total body weight with acinar deletion of Sec23b. (D) Ratios of pancreas to total body weight 7 d after administration of corn oil demonstrate that only a small portion of the pancreatic weight loss is explained by basal CreErT activity in the absence of tamoxifen induction. (E) A cohort of mice were evaluated 14 d after tamoxifen administration, demonstrating no continued drop in pancreatic weight compared with evaluation performed 7 d after tamoxifen in A. The loss of pancreatic weight is associated with a comparable degree of decrease in pancreas (F) DNA, (G) RNA, and (H) total protein, consistent with cell loss. Each data point represents one mouse.
The mouse weights before and after tamoxifen administration were indistinguishable between mice with acinar depletion of SEC23B and WT control mice (Figure 2, B and C), suggesting no effect of acinar Sec23b deletion on food intake or duodenal pathology.
To determine the baseline CreErT activity in the absence of tamoxifen induction, we gavaged a cohort of mice (Sec23b-/fl CreErT+, Sec23bfl/fl CreErT+, and WT controls) with corn oil not containing tamoxifen. Sec23b−/fl CreErT+ and Sec23bfl/fl CreErT+ mice exhibited ∼13% lower pancreatic weights than WT mice (p = 0.03; Figure 2D), explaining only a portion of the decrease in pancreatic weight observed in Sec23b−/fl CreErT+ and Sec23bfl/fl CreErT+ mice after tamoxifen administration.
To determine whether the pancreatic weight after Sec23b acinar cell deletion would drop further with time, we followed a cohort of mice for 2 wk after administration of tamoxifen. The latter mice exhibited a ∼40% decrease in pancreas weight compared with WT control mice (Figure 2 E), which is indistinguishable from the drop observed 1 wk after tamoxifen administration.
The lower pancreatic weight in mice with acinar deletion of Sec23b is due to cell loss
We calculated total pancreatic DNA, RNA, and protein contents 1 wk after tamoxifen administration. Mice with acinar cell deletion of Sec23b exhibited ∼43% decrease in pancreatic DNA content (p = 0.01; Figure 2F), ∼53% decrease in RNA content (p < 0.0001; Figure 2G), and ∼46% decrease in protein content (p < 0.0001; Figure 2H) compared with WT mice. The total DNA, RNA, and protein contents of pancreas tissues decreased proportionately to the decrease in pancreas weights resulting from Sec23b deletion in acinar cells, indicating that the decreased pancreas weight can be fully explained by a reduction in cell number, not cell size.
Acinar Sec23b deletion results in degeneration of exocrine cells, decreased zymogen granules, and ER alterations
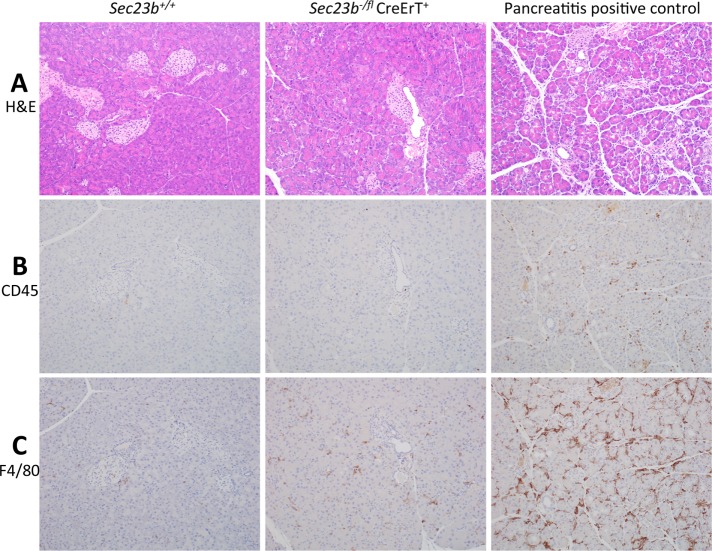
One week after acinar cell–specific deletion of Sec23b, histologic evaluation of pancreas tissues demonstrated smaller-than-normal pancreata with mild to moderate disruption of normal lobular architecture due to multifocal degeneration of exocrine epithelial cells within pancreatic lobules, with shrinkage of lobules and prominence of supporting stroma and periductular fibrosis. Degenerate acinar cells were shrunken, with loss of zymogen granules, condensed nuclei, and cytoplasmic enhancement of the eosin stain (Figure 3A). Histologic evaluation of pancreas tissues performed 2 wk after tamoxifen administration revealed comparable findings (Supplemental Figure S1A).
FIGURE 3:
Histologic evaluation of pancreas tissues 7 d after deletion of acinar Sec23b. The evaluation was performed by an investigator blinded to the genotypes of the mice from which the tissues were derived (four mice of each genotype were evaluated). (A) At 7 d after acinar Sec23b deletion, evaluation of pancreas tissues by hematoxylin and eosin stains demonstrated overall loss of parenchymal size and lobular atrophy due to degeneration of acinar epithelial cells, characterized by cellular shrinkage, cytoplasmic loss with loss of zymogen granules, enhancement of the eosin stain, and nuclear pyknosis (middle) compared with WT controls (left). Right, positive control for pancreatitis 1 d after cerulean treatment. Immunohistochemistry for (B) CD45 and (C) F4/80 demonstrates a small number of white blood cells (mostly macrophages) infiltrating pancreas tissues of mice with acinar Sec23b deletion compared with mice with cerulean-induced pancreatitis (positive control).
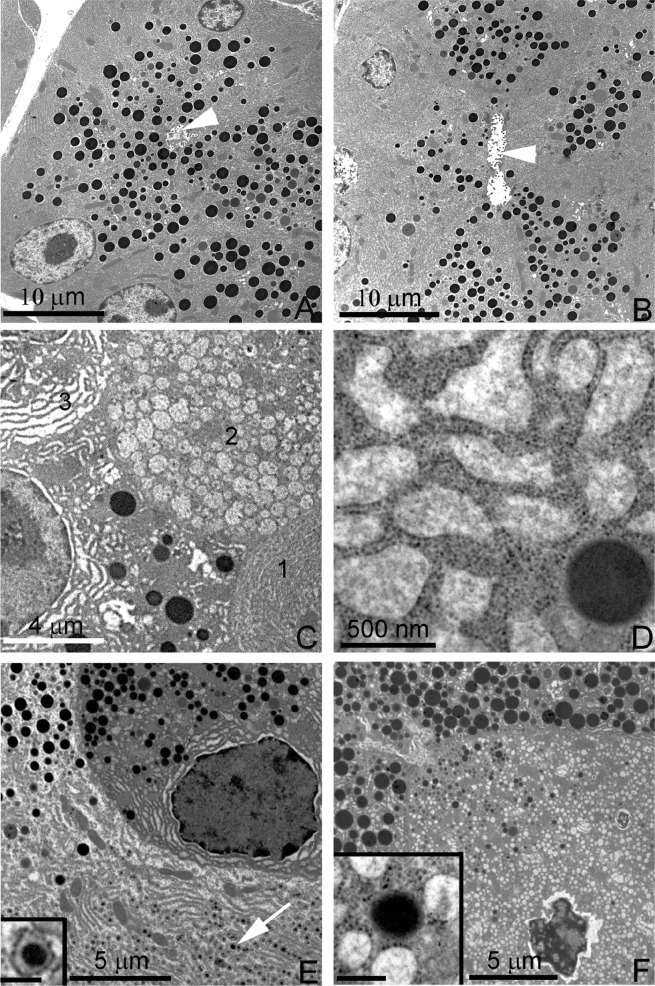
Alteration in acinar morphology after Sec23b excision was also assessed by electron microscopy (Figure 4). Seven days after tamoxifen treatment, acinar cell structure in pancreata of WT mice exhibited the expected concentration of zymogen granules surrounding the acinar lumen in the apical pole of the cell and abundant RER in basolateral regions (Figure 4A). In contrast, the majority of acinar cells in Sec23b−/fl CreErT+ mice exhibited striking abnormalities, including decreased zymogen granules (Figure 4) and alterations in ER morphology ranging from vesicular ER to markedly expanded cisternae with accumulation of moderate-density content or intracisternal granules (Figure 4, B–F). Cells containing vesicular ER often showed ribosomes associated with only portions of the vesicular membrane (Figure 4, F and inset). Many acinar cells showed alteration in their general polarity, with granules accumulating in basal regions of the cell, whereas other cells had greatly reduced granule densities. The acinar cell morphology was also assessed 14 d after administration of tamoxifen, with findings similar to those at the earlier time point (Supplemental Figure S2).
FIGURE 4:
Electron microscopy of pancreas tissues 7 d after deletion of acinar Sec23b. Evaluation of pancreas tissues by electron microscopy demonstrates normal acinar morphology in WT control mice, with numerous granules adjacent to the lumen (arrowhead) and basal RER cisternae (A). Pancreas tissues with acinar deletion of Sec23b exhibited variably decreased zymogen granules (B; arrowhead denotes ER lumen), as well as multiple alterations, including vesicular or expanded RER (C, cells 2 and 3; in contrast to normal cell morphology, C, cell 1), expanded RER cisternae with amorphous content (C, D), small intracisternal granules (E, arrow and higher-magnification inset), crenulated nucleus and vesicular RER (F), with amorphous content and partial studding with ribosomes (F and inset). Scale bars as indicated; 250 nm (insets). Three mice of each genotype were evaluated.
Sec23b deletion in acinar cells fails to produce clear manifestations of acute pancreatitis
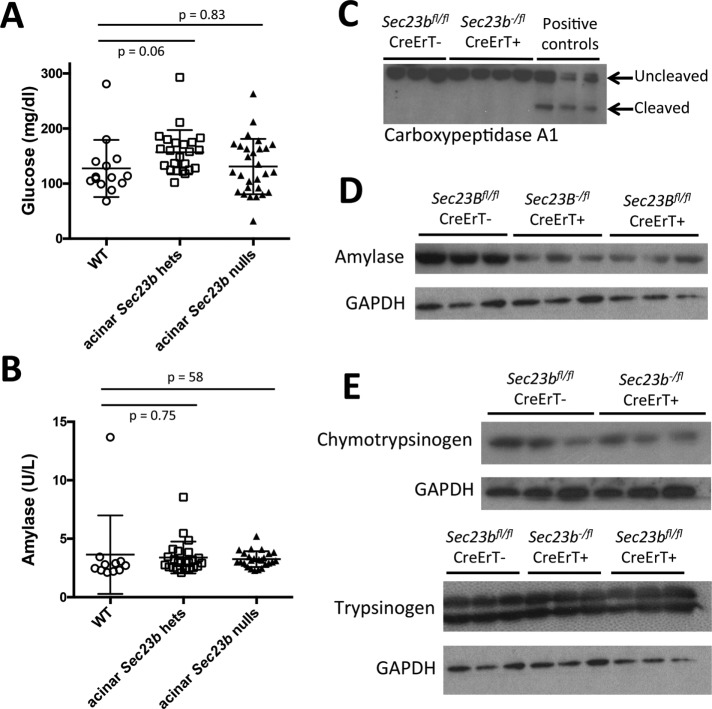
We measured random blood glucose and plasma amylase levels 1 wk after tamoxifen administration. Mice with acinar cell deletion of Sec23b exhibited indistinguishable blood glucose (Figure 5A) and plasma amylase levels (Figure 5B) compared with mice heterozygous for Sec23b in their acinar cells and WT controls. In addition, a small number of white blood cells (mostly resident macrophages and lymphocytes) were observed by immunohistochemistry, but much fewer than in the pancreatitis positive control samples, which contained a significant number of neutrophils in addition to large numbers of other inflammatory cells (Figure 3, B and C, and Supplemental Figure S1, B and C). Carboxypeptidase A1 cleavage, which is seen in pancreatitis (Leach et al., 1991), was not observed in pancreas tissues of mice with acinar Sec23b deletion (Figure 5C). Other pancreas digestive enzymes were variably affected relative to total protein. Amylase was decreased in total pancreatic cell lysates prepared from mice with acinar deletion of Sec23b compared with WT mice (Figure 5D), with a less clear effect on chymotrypsin and trypsin (Figure 5E).
FIGURE 5:
Deletion of Sec23b in mouse acinar cells does not affect plasma glucose or amylase but results in decreased pancreatic amylase in total pancreas cell lysates. (A) Sec23b deletion in acinar cells does not result in increased plasma glucose or (B) increased plasma amylase (each data point represents one mouse). (C) Carboxypeptidase cleavage observed in pancreas tissues of mice given cerulein to induce pancreatitis was not observed in pancreas tissues of mice with acinar Sec23b inactivation. (D) Sec23b deletion in pancreas acinar cells results in decreased pancreatic amylase in total pancreas cell lysates, (E) with a less clear effect on chymotrypsin and trypsin.
Acinar Sec23b deletion results in ER stress and increased apoptosis
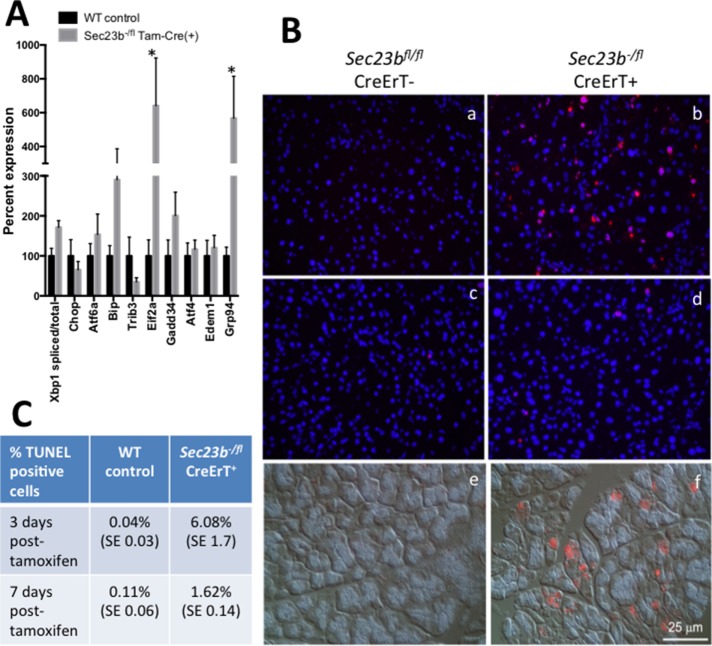
To determine whether the ER dilatation observed after acinar Sec23b deletion is associated with induction of ER stress and the unfolded protein response (UPR), we determined the expression level of a panel of associated genes by qRT-PCR. Expression for many of these genes was increased after acinar Sec23b deletion, with the increase in Eif2a and Grp94 reaching statistical significance (p < 0.05; Figure 6A).
FIGURE 6:
Expression of UPR genes and TUNEL assay in pancreas tissues after acinar Sec23b deletion. (A) Real time RT-PCR expression of select UPR genes was performed on pancreas tissues harvested 1 wk following administration of tamoxifen, with levels normalized to β-actin. Data are represented by mean ± SEM. Asterisks indicate statistically significant difference between WT and Sec23b−/fl CreErT(+) samples. Six mice from each genotype were evaluated. (B) TUNEL assays overlaid on DAPI were performed 3 d (a, b) and 7 d (c, d) after administration of tamoxifen. Sec23b−/fl CreErT+ mice exhibit increased apoptosis at both time points. Immunostaining for active caspase-3 demonstrates increased expression in mice with acinar deletion of Sec23b (f) compared with WT mice (e) on Nomarski image. (C) Average percentage of TUNEL-positive cells (three or four mice were evaluated per condition and ∼2000–2500 cells counted per sample). The evaluation was performed by an investigator blinded to the genotypes of the mice from which the tissues were derived.
After tamoxifen administration, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were also performed on pancreatic tissues harvested from Sec23b-/fl CreErT+ mice and WT controls (Figure 6B, a–d). Three days after tamoxifen administration, the percentage of TUNEL-positive cells in Sec23b−/fl CreErT+ mice (6.08%) was higher than that in WT control mice (0.04%; Figure 6C). The same pattern was observed 7 d after tamoxifen administration (1.62 and 0.11%, respectively; Figure 6C). Consistent with these results, the expression of activated caspase 3 was increased in pancreata of Sec23b−/fl CreErT+ mice compared with WT control mice (Figure 6B, e and f).
DISCUSSION
Mice with germline deletion of Sec23b die perinatally, exhibiting massive pancreatic degeneration (Tao et al., 2012). Pancreas-specific Sec23b deletion in developing mouse embryos recapitulates the phenotype of mice with global SEC23B deficiency (Khoriaty et al., 2016), demonstrating that the defect in the latter mice is intrinsic to the pancreas. It is not clear whether this effect is mediated by the loss of the endocrine or exocrine pancreas or the development of intrauterine pancreatitis.
In this study, we evaluated the effect of SEC23B depletion in adult pancreatic acinar cells by generating mice with tamoxifen-inducible, acinar cell–specific SEC23B deficiency. Tissue was evaluated from 3 d to 2 wk after tamoxifen. Tamoxifen administration to these adult mice results in pancreatic cell loss, swollen ER with degeneration of exocrine cells, ER stress, and increased pancreatic acinar cell apoptosis but without an effect on viability of the mice. There were small numbers of white blood cells (primarily resident macrophages and lymphocytes) in pancreatic tissues of the latter mice; these infiltrates were minimal in comparison to the moderate inflammatory cell infiltration in the pancreatitis-positive control tissues. In addition, the pancreatitis control tissues exhibited a significant number of neutrophils, consistent with a primary inflammatory process in the pancreas. In contrast, the small numbers of macrophages observed in the pancreas of SEC23B-depleted mice is consistent with a clean-up process secondary to acinar degeneration rather than an active inflammatory process. In addition, amylase was not increased in the plasma of mice with acinar depletion of SEC23B, and carboxypeptidase cleavage was not observed, arguing against frank pancreatitis. Taken together, these data demonstrate that SEC23B is important for maintenance of function of adult murine pancreatic acinar cells. Specifically, the results are consistent with a role for SEC23B in forming the COPII coat, which promotes budding of transport vesicles off the ER, initiating passage through the secretory pathway and culminating in the production of ZG. Blockage of the early secretory pathway explains the reduction in ZG. Because some granules are still present, the blockage is not complete. Of interest, different digestive enzymes move through the pathway to the ZG at different rates. Chymotrypsinogen moves faster and shows a higher increase in concentration than amylase (Oprins et al., 2001). The slower movement of amylase may be related to the overall fall in tissue amylase compared with chymotrypsinogen, which does not fall in SEC23B deficiency.
In contrast to mice with germline SEC23B deficiency, Sec23b deletion in pancreatic acinar cells of adult mice is not lethal (a cohort of mice was followed for ∼2–3 wk after tamoxifen administration). One possible explanation is that these mice were observed for too short a time after tamoxifen administration to assess for mortality. Alternatively, selection over time against acinar cells with complete Sec23b excision could result in enrichment for normal acinal cells with intact Sec23b. Another possible explanation is that the small percentage of acinar cells without Sec23b excision might be sufficient for survival of these mice. It is also possible that Sec23b exerts a unique function during murine embryonic development, which is responsible for the damage in the setting of germline deletion.
Acinar cell death after Sec23b deletion could result from accumulation of exocrine pancreatic enzymes in the ER, resulting in ER stress and apoptosis. Consistent with this hypothesis, mutations in PRSS1, the gene encoding trypsinogen, that result in an increase of its activity have been associated with pancreatitis (Whitcomb et al., 1996a,b; Ferec et al., 1999). Similarly, mutation in SPINK1, which encodes a serine protease with antitrypsin activity, is associated with hereditary pancreatitis (Witt et al., 2000; Audrezet et al., 2002). However, these genes are involved in enhancing or inhibiting the activity of trypsin while still in the acinar cell. Deletion of SEC23B inhibits exit from the ER but is not expected to affect trypsin activation and for this reason may not promote pancreatitis in the adult mouse. Variants in procarboxypeptidase A1 in humans were shown to be associated with pancreatitis associated with ER stress but without trypsin activation (Witt et al., 2013). Further work on the type of ER stress induced by Sec23b deletion in murine acinar cells is warranted.
MATERIALS AND METHODS
Mice and PCR genotyping
A Sec23b floxed allele (Sec23bfl) and a Sec23b null allele (Sec23b−) were generated as previously described (Figure 1A; Khoriaty et al., 2014). Mice carrying a pancreatic acinar cell–specific, tamoxifen-inducible Cre-recombinase (CreErT) transgene (a generous gift from C. Logsdon, M.D. Anderson Cancer Center, Houston, TX; Ji et al., 2008) were crossed to Sec23b+/− mice to generate Sec23b+/− CreErT+ mice. CreErT mice express Cre recombinase under the control of the full-length elastase gene promoter, resulting in expression of Cre recombinase in nearly 100% of the acinar cells but not in duct or islet cells (Ji et al., 2008; Gurda et al., 2010). Genotyping for the Sec23bfl, Sec23b−, and CreErT alleles was performed as previously described (Ji et al., 2008; Khoriaty et al., 2014). All of the mice used in this study were either generated on the C57BL/6J background or crossed to the latter genetic background for more than six generations.
A cohort of WT mice were given cerulein (50 μg/kg) every hour for 7 h to induce pancreatitis as previously described (Sans et al., 2003). These mice, which exhibit increased plasma amylase and pancreatic cell damage, were used as positive control for pancreatitis.
Tamoxifen administration
Tamoxifen (purchased from Sigma-Aldrich) was dissolved in corn oil at 10 mg/ml. Mice at 5–6 wk of age were administered tamoxifen (3 mg tamoxifen per 40 g of mouse weight) via daily gavage for 4 d.
Blood glucose and amylase measurement
Blood glucose was measured using the OneTouch Ultra glucometer (LifeScan) or the Contour glucometer (Bayer), and plasma amylase was measured using Phadebas reagents (Magle Life Sciences) according to manufacturer’s instructions.
DNA, RNA, and protein isolation and measurement
Pancreas tissue samples were weighed and homogenized at 100 mg/1.5 ml solution of 5 mM MgCl2 plus 0.1% Triton X-100 and then sonicated. DNA and RNA were measured using the Qubit RNA HS Assay Kit (Q32852; Life Technologies) and Qubit dsDNA HS Assay Kit (Q32851; Life Technologies), respectively, according to manufacturer’s instructions. Protein was measured spectrophotometrically by using Bio-Rad protein assay reagent. Total pancreatic protein, DNA, and RNA were then calculated (Tashiro et al., 2004; Crozier et al., 2010).
qRT-PCR
RNA was isolated from pancreas tissues using the RNeasy Kit (Qiagen). cDNA synthesis was carried out using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen) with random primers. Power SYBR Green PCR Master Mix (Applied Biosystems) was used for qRT-PCR using the Applied Biosystems 7900HT Fast Real-Time PCR System. Each sample was analyzed in triplicate, and Gapdh (glyceraldehyde-3-phosphate dehydrogenase) or Atcb (β-actin) was used as internal control. Relative gene expression was determined using the 2−ΔΔCT method. Supplemental Table S1 lists primers used.
Western blot and antibodies
Total cell lysates were prepared from pancreas tissues, and Western blots (film visualization with chemiluminescence detection) were performed as previously described (Khoriaty et al., 2014). Mouse anti-actin and anti-GAPDH antibodies were obtained from Santa Cruz Biotechnology and Millipore, respectively. Rabbit anti-SEC23B and anti-SEC23A antibodies were generated as previously described (Khoriaty et al., 2014). Carboxypeptidase A1, amylase, chymotrypsin, and trypsin antibodies were obtained from R&D Systems (AF2765), Sigma-Aldrich (A8273), Fitzgerald (20C-CR1095R), and Santa Cruz Biotechnology (sc-67388), respectively. Quantitative Western blots (infrared fluorescence detection) were performed and analyzed as previously described (Khoriaty et al., 2016).
Hematoxylin and eosin staining and electron microscopy
Pancreas tissues were collected and fixed in 4% paraformaldehyde for histologic analysis. Tissues were processed, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Electron microscopy was performed on pancreas tissues as previously described (Crozier et al., 2009; Hou et al., 2015). In brief, minced pancreas was fixed in a mixture of 2% formaldehyde and 2% glutaraldehyde in phosphate-buffered saline, postfixed in 1% osmium tetroxide, and then dehydrated and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and evaluated with a Phillips CM-100 electron microscope.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Khoriaty et al., 2016) with anti-CD45 and anti-F4/80 antibodies purchased from Novus Biologicals (NB100-77417) and Abcam (ab6640), respectively.
TUNEL assay and caspase 3 immunostaining
The ApopTag Red In Situ Apoptosis Detection Kit (Chemicon, Millipore) was used to localize red fluorescent TUNEL-positive nuclei. Briefly, fresh-frozen sections of pancreas were fixed for 10 min with a 2:1 mixture of methanol and acetone at −20°C. Sections were treated with the kit reagents as specified by the manufacturer, coverslipped with mounting medium containing fluorescent nuclear stain 4′,6-diamidino-2-phenylindole (DAPI; Prolong Gold, Invitrogen), and viewed with a 40× objective of an Olympus BX-51 fluorescence microscope. Five randomly chosen DAPI-stained fields in sections from each animal were photographed, each followed by changing filters to photograph TUNEL fluorescence. DAPI-stained nuclei were counted using the Nuclei Counting program in MetaMorph software (Molecular Devices, Sunnyvale, CA), and TUNEL-positive nuclei were counted manually. The percentage of TUNEL-positive nuclei was calculated for each field (five determinations per mouse), and these values were then averaged for each mouse. This experiment was performed by an investigator blinded to the genotypes of the mice from which the pancreas tissues were harvested.
Immunofluorescence localization of caspase-3 (8G10 rabbit monoclonal antibody, Cell Signaling Technology, Danvers, MA; Alexa anti-rabbit 594, Thermo Fisher Scientific, Grand Island, NY) was performed on cryostat sections of pancreas tissue that had been fixed in 4% paraformaldehyde. Immunostaining for caspase-3 was merged with its corresponding Nomarski image to show acinar cell distribution of caspase-3.
Statistical analysis
The chi-square test was used to determine the statistical significance of the deviation from Mendelian ratios for mouse crosses. The Student’s t test was used to calculate the statistical significance of the differences between various parameters among different genotype groups. p < 0.05 is considered statistically significant.
Supplementary Material
Acknowledgments
We acknowledge Elizabeth Hughes, Keith Childs, and Thomas Saunders for preparation of gene-targeted mice and the Transgenic Animal Model Core of the University of Michigan’s Biomedical Research Core Facilities. This work was supported by National Institutes of Health Grants R01 HL039693 and P01-HL057346 (D.G.), K08 HL128794 (R.K.), and R37-DK041122 (J.A.W.). R.K. is the recipient of an American Society of Hematology Scholar Award. D.G. is a Howard Hughes Medical Institute investigator. Core support was provided by the University of Michigan Cancer Center (P30 CA046592) and Michigan Gastrointestinal Peptide Center (P30 DK34933).
Abbreviations used:
- CDAII
congenital dyserythropoietic anemia type II
- COPII
coat protein complex II
- CreErT
tamoxifen-inducible Cre recombinase transgene
- ER
endoplasmic reticulum
- RER
rough endoplasmic reticulum
- UPR
unfolded protein response
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-01-0001) on May 24, 2017.
REFERENCES
- Audrezet MP, Chen JM, Le Marechal C, Ruszniewski P, Robaszkiewicz M, Raguenes O, Quere I, Scotet V, Ferec C. Determination of the relative contribution of three genes-the cystic fibrosis transmembrane conductance regulator gene, the cationic trypsinogen gene, and the pancreatic secretory trypsin inhibitor gene-to the etiology of idiopathic chronic pancreatitis. Eur J Hum Genet. 2002;10:100–106. doi: 10.1038/sj.ejhg.5200786. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Helenius A. Cargo capture and bulk flow in the early secretory pathway. Annu Rev Cell Dev Biol. 2016;32:197–222. doi: 10.1146/annurev-cellbio-111315-125016. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Fermo E, Vercellati C, Boschetti C, Barcellini W, Iurlo A, Marcello AP, Righetti PG, Zanella A. Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum Mutat. 2009;30:1292–1298. doi: 10.1002/humu.21077. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Kim SD, Hata A, Haldeman-Englert C, Zackai EH, Naydenov C, Hamamoto S, Schekman RW, Kim J. Cranio-lenticulo-sutural dysplasia associated with defects in collagen secretion. Clin Genet. 2010;80:169–176. doi: 10.1111/j.1399-0004.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier SJ, D’Alecy LG, Ernst SA, Ginsburg LE, Williams JA. Molecular mechanisms of pancreatic dysfunction induced by protein malnutrition. Gastroenterology. 2009;137:1093–1101. doi: 10.1053/j.gastro.2009.04.058. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier SJ, Sans MD, Wang JY, Lentz SI, Ernst SA, Williams JA. CCK-independent mTORC1 activation during dietary protein-induced exocrine pancreas growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1154–G1163. doi: 10.1152/ajpgi.00445.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferec C, Raguenes O, Salomon R, Roche C, Bernard JP, Guillot M, Quere I, Faure C, Mercier B, Audrezet MP, et al. Mutations in the cationic trypsinogen gene and evidence for genetic heterogeneity in hereditary pancreatitis. J Med Genet. 1999;36:228–232. [PMC free article] [PubMed] [Google Scholar]
- Gorelick F, Jamieson J. Structure-function relationship in the pancreatic acinar cell. In: Johnson L, editor. Physiology of the Gastrointestinal Tract. San Diego, CA: Academic Press; 2012. pp. 1341–1360. [Google Scholar]
- Gurda GT, Crozier SJ, Ji B, Ernst SA, Logsdon CD, Rothermel BA, Williams JA. Regulator of calcineurin 1 controls growth plasticity of adult pancreas. Gastroenterology. 2010;139:609–619. doi: 10.1053/j.gastro.2010.04.050. 619.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Ernst SA, Stuenkel EL, Lentz SI, Williams JA. Rab27A is present in mouse pancreatic acinar cells and is required for digestive enzyme secretion. PLoS One. 2015;10:e0125596. doi: 10.1371/journal.pone.0125596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Song J, Tsou L, Bi Y, Gaiser S, Mortensen R, Logsdon C. Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis. 2008;46:390–395. doi: 10.1002/dvg.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoriaty R, Everett L, Chase J, Zhu G, Hoenerhoff M, McKnight B, Vasievich MP, Zhang B, Tomberg K, Williams J, et al. Pancreatic SEC23B deficiency is sufficient to explain the perinatal lethality of germline SEC23B deficiency in mice. Sci Rep. 2016;6:27802. doi: 10.1038/srep27802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoriaty R, Vasievich MP, Ginsburg D. The COPII pathway and hematologic disease. Blood. 2012;120:31–38. doi: 10.1182/blood-2012-01-292086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoriaty R, Vasievich MP, Jones M, Everett L, Chase J, Tao J, Siemieniak D, Zhang B, Maillard I, Ginsburg D. Absence of a red blood cell phenotype in mice with hematopoietic deficiency of SEC23B. Mol Cell Biol. 2014;34:3721–3734. doi: 10.1128/MCB.00287-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J. Transport between ER and Golgi. Curr Opin Cell Biol. 2000;12:445–449. doi: 10.1016/s0955-0674(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Lang MR, Lapierre LA, Frotscher M, Goldenring JR, Knapik EW. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat Genet. 2006;38:1198–1203. doi: 10.1038/ng1880. [DOI] [PubMed] [Google Scholar]
- Leach SD, Modlin IM, Scheele GA, Gorelick FS. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991;87:362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Menarguez JA, Geuze HJ, Slot JW, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- Oprins A, Rabouille C, Posthuma G, Klumperman J, Geuze HJ, Slot JW. The ER to Golgi interface is the major concentration site of secretory proteins in the exocrine pancreatic cell. Traffic. 2001;2:831–838. doi: 10.1034/j.1600-0854.2001.21112.x. [DOI] [PubMed] [Google Scholar]
- Sans MD, DiMagno MJ, D’Alecy LG, Williams JA. Caerulein-induced acute pancreatitis inhibits protein synthesis through effects on eIF2B and eIF4F. Am J Physiol Gastrointest Liver Physiol. 2003;285:G517–G528. doi: 10.1152/ajpgi.00540.2002. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Iolascon A, Verissimo F, Trede NS, Horsley W, Chen W, Paw BH, Hopfner KP, Holzmann K, Russo R, et al. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet. 2009;41:936–940. doi: 10.1038/ng.405. [DOI] [PubMed] [Google Scholar]
- Tao J, Zhu M, Wang H, Afelik S, Vasievich MP, Chen XW, Zhu G, Jensen J, Ginsburg D, Zhang B. SEC23B is required for the maintenance of murine professional secretory tissues. Proc Natl Acad Sci USA. 2012;109:E2001–E2009. doi: 10.1073/pnas.1209207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G784–G790. doi: 10.1152/ajpgi.00446.2003. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Jr, Amann ST, Toskes PP, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996a;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK, Jr, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996b;110:1975–1980. doi: 10.1053/gast.1996.v110.pm8964426. [DOI] [PubMed] [Google Scholar]
- Witt H, Beer S, Rosendahl J, Chen JM, Chandak GR, Masamune A, Bence M, Szmola R, Oracz G, Macek M, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet. 2013;45:1216–1220. doi: 10.1038/ng.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- Zhu M, Tao J, Vasievich MP, Wei W, Zhu G, Khoriaty RN, Zhang B. Neural tube opening and abnormal extraembryonic membrane development in SEC23A deficient mice. Sci Rep. 2015;5:15471. doi: 10.1038/srep15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.