Abstract
Background
Sexual dimorphisms are well recognized in various cardiac diseases such as ischemic cardiomyopathy (ICM), hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM). Thorough understanding of the underlying genetic programs is crucial to optimize treatment strategies specified for each gender. By performing meta-analysis and microarray analysis, we sought to comprehensively characterize the sexual dimorphisms in the healthy and diseased heart at the level of both mRNA and miRNA transcriptome.
Results
Existing mRNA microarray data of both mouse and human heart were integrated, identifying dozens/ hundreds of sexually dimorphic genes in healthy heart, ICM, HCM, and DCM. These sexually dimorphic genes overrepresented gene ontologies (GOs) important for cardiac homeostasis. Further, microarray of miRNA, isolated from mouse sham left ventricle (LV) (n = 6 & n = 5 for male & female) and chronic MI LV (n = 19 & n = 19) and from human normal LV (n = 6 & n = 6) and ICM LV (n = 4 & n = 5), was conducted. This revealed that 13 mouse miRNAs are sexually dimorphic in MI and 6 in normal heart. In human, 3 miRNAs were sexually dimorphic in ICM and 15 in normal heart. These data revealed miRNA-mRNA networks that operate in a sexually-biased fashion.
Conclusions
mRNA and miRNA transcriptome of normal and disease heart show significant sex differences, which might impact the cardiac homeostasis. Together this study provides the first comprehensive picture of the genome-wide program underlying the heart sexual dimorphisms, laying the foundation for gender specific treatment strategies.
Background
Sexual dimorphisms are well recognized in various cardiac diseases [1] [2]. Ischemic cardiomyopathy (ICM) including myocardial infarction (MI) develops later in women, but once established, it contributes more persistent symptoms and higher mortality than in men [3–13]. Hypertrophic cardiomyopathy (HCM) reportedly shows similar trends [14–18]. On the contrary, more prevalent in men is dilated cardiomyopathy (DCM), both familial and myocarditis-induced [19–28]. Importantly, similar observations have been reported in rodent models of ICM [29,30], HCM [31–35], and DCM[36–39], offering powerful models to elucidate the underlying molecular mechanisms. However, study results focusing on the effect of sex hormones so far have been conflicting [31–33,36,38,40–43]. Therefore, the whole picture of sexual dimorphism in the heart remains unclear.
Several research teams thus investigated the mRNA transcriptome in mouse MI [44], HCM [31,32,45], DCM[39], and human DCM [46,47], and found sexually dimorphic genes. However, whether or not such sex differences also exist in human ICM and HCM is still unknown. In addition, no study has investigated the sex difference of cardiac disease at the level of miRNAs, important players in cardiac functions and diseases [48]. Comprehensive understanding of the mRNA- and miRNA-level genetic programs underlying the heart sexual dimorphisms will expectedly improve clinical outcome by facilitating the development of gender specific treatment strategies.
Here, by conducting meta-analysis of mRNA transcriptome and performing miRNA microarray analysis of mouse/ human disease samples, we set out to characterize the heart sexual dimorphisms at the level of both mRNA and miRNA transcriptome. mRNA meta-analysis identified dozens/ hundreds of sexually dimorphic genes in ICM, HCM, DCM, and normal heart. These genes over-represented GOs important for cardiac homeostasis, suggesting the functional significance of their sex difference. Next we investigated the miRNA in ICM and normal heart and found significant sex difference. Computational analyses suggest that these sexually dimorphic mRNAs and miRNAs form sex-specific miRNA-mRNA networks. Together these data provide the first comprehensive picture of the genome-wide program underlying the heart sexual dimorphisms, laying the foundation for the gender specific treatment strategies.
Methods
An expanded Methods section is available in S1 Methods. All microarray data have been submitted to the National Center for Biotechnology Information gene expression and hybridization array data repository (GSE76604).
Myocardial infarction modeling
The left anterior descending (LAD) coronary artery of mice aged 10 weeks was surgically ligated to create extensive MI. The ventricular septum of the areas at risk of ischemia was sampled on post-operative day 28.
Patient selection and tissue collection
Human tissue samples, acquired during post-mortem examination and frozen in liquid nitrogen, were provided by the Department of Pathology, Tokyo Metropolitan Geriatric Hospital. Autopsy and medical research were performed with written consent by the families under the Act of Postmortem Examination. This work was approved by the ethical committee of the Tokyo Metropolitan Geriatric Hospital (no.240208). Age- and sex-matched cohorts were selected to compare healthy hearts to those with post-MI LV remodeling. Border zone for myocardial infarction was sampled for microarray analysis (S1 Methods).
RNA library preparation, microarray, and data processing
Total RNA was extracted from samples using Sepasol (Sepasol-RNA I super G, nakalai tesque, Japan), and microarray analysis was performed using Affymetrix GeneChip® miRNA 3.0 Arrays.
Public microarray data integration
Relevant studies were searched in the NCBI Gene Expression Omnibus (GEO)[49] and their raw data were downloaded and processed in R[50] according to each array platform. We used 4 mouse studies (GSE23294[44], GSE18224[31,32], GSE6970[45], GSE35182[39]) and 6 human studies (GSE57338[51], GSE29819[52], GSE22253[53], GSE26887[54], GSE52601[55], GSE36961 (Hebl VB, Bos JM, Oberg AL, Sun Z, Herman DS, Teekakirikul P, Seidman JG, Seidman CE, dos Remedios CG, Schaff HV, Dearani JA, Ommen SR, Brozovich FV, Ackerman MJ, unpublished data, [2012])) reporting the mRNA transcriptome of both genders belonging to either normal/ ICM/ HCM/ DCM (Table 1). The cross-platform normalization was performed using COMBAT method[56].
Table 1. Characteristics of the microarray data used in this study.
| GSE | Disease | Age (s.d.) * | Sample Size (M_F) | Platform name | d.a.o† | Procedure |
|---|---|---|---|---|---|---|
| Mouse | ||||||
| GSE23294 | Normal | 12~15 | 10 | Illumina MouseWG-6 v2.0 expression beadchip | 3 | Sham |
| (5_5) | ||||||
| GSE23294 | AMI | 12~15 | 10 | Illumina MouseWG-6 v2.0 expression beadchip | 3 | LAD ligation |
| (5_5) | ||||||
| GSE18224 | Normal | 10 | 8 | Affymetrix Mouse Genome 430 2.0 Array | 63 | Sham |
| (4_4) | ||||||
| GSE18224 | HCM | 10 | 8 | Affymetrix Mouse Genome 430 2.0 Array | 63 | TAC |
| (4_4) | ||||||
| GSE6970 | Normal | 12~14 | 8 | Affymetrix Mouse Expression 430A Array | 16 | Sham |
| (4_4) | ||||||
| GSE6970 | HCM | 12~14 | 8 | Affymetrix Mouse Expression 430A Array | 16 | TAC |
| (4_4) | ||||||
| GSE35182 | Normal | 6~8 | 6 | Affymetrix Mouse Gene 1.0 ST Array | 90 | Sham |
| (3_3) | ||||||
| GSE35182 | DCM | 6~8 | 6 | Affymetrix Mouse Gene 1.0 ST Array | 90 | CVB3-induced myocarditis |
| (3_3) | ||||||
| Human | ||||||
| GSE57338 | Normal | 49.4 (15.0) | 136 | Affymetrix Human Exon ST1.1 arrays | ||
| (73_63) | ||||||
| GSE57338 | ICM | 59.1 (7.4) | 95 | Affymetrix Human Exon ST1.1 arrays | ||
| (81_14) | ||||||
| GSE57338 | DCM | 51.2 (14.0) | 82 | Affymetrix Human Exon ST1.1 arrays | ||
| (63_19) | ||||||
| GSE29819 | Normal | 45.2 (18.8) | 5 | Affymetrix Human Genome U133 Plus 2.0 Array | ||
| (3_2) | ||||||
| GSE29819 | DCM | 57.6 (6.1) | 7 | Affymetrix Human Genome U133 Plus 2.0 Array | ||
| (3_4) | ||||||
| GSE22253 | Normal | 47.9 (12.6) | 107 | Affymetrix Human Gene 1.0 ST Array | ||
| (55_52) | ||||||
| GSE26887 | Normal | 48.4 (2.6) | 5 | Affymetrix Human Gene 1.0 ST Array | ||
| (2_3) | ||||||
| GSE26887 | ICM | 62.2 (12.1) | 19 | Affymetrix Human Gene 1.0 ST Array | ||
| (18_1) | ||||||
| GSE52601 | Normal | 52 (10.6) | 3 | Illumina HumanHT-12 V4.0 expression beadchip | ||
| (3_0) | ||||||
| GSE52601 | ICM | 67 (2.6) | 4 | Illumina HumanHT-12 V4.0 expression beadchip | ||
| (3_1) | ||||||
| GSE52601 | DCM | 56 (9.4) | 4 | Illumina HumanHT-12 V4.0 expression beadchip | ||
| (3_1) | ||||||
| GSE36961 | Normal | 37.2 (15.2) | 39 | Illumina HumanHT-12 V3.0 expression beadchip | ||
| (19_20) | ||||||
| GSE36961 | HCM | 46.6 (18.8) | 106 | Illumina HumanHT-12 V3.0 expression beadchip | ||
| (54_52) | ||||||
* Unit is week for mouse and year for human
†Days after onset
Differential expression analysis
The normalized data were analyzed using R and the Bioconductor limma package[57]. All p-values were adjusted for false discovery rate correction (FDR < 0.05). Since cardiac sex difference presumably reflects the mild biases in transcriptome-wide gene expression, 1.2 fold change was considered biologically meaningful. Hence our detection criteria were: FDR < 0.05 and fold change > 1.2.
Data analysis
Statistical analyses were conducted in R unless specified otherwise. p < 0.05 was considered significant.
Results
Study design
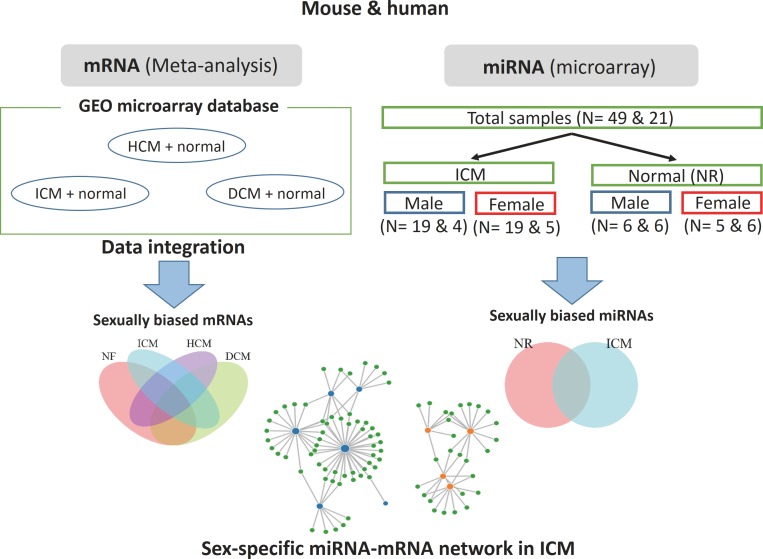
In this study, we sought to characterize the sex difference in mRNA and miRNA transcriptome (Fig 1). Since GEO database offered several mRNA microarray data of both genders of disease heart, we conducted meta-analysis to assess the mRNA sex difference. For miRNA data, for which such data was not available, we performed microarray analysis.
Fig 1. Workflow of this study.
For mRNA, public microarray data were integrated with cross-platform normalization. The integrated mRNA data included normal (NR) and ICM samples as well as HCM and DCM. For miRNA data, miRNA microarray analysis was conducted using 49 mouse samples and 21 human samples. These samples included normal and ICM samples. Sample sizes are indicated in the bracket (mouse & human). In both mRNA and miRNA microarray analyses, male and female expression levels were compared within each disease group. Finally, mRNA and miRNA data from ICM samples were used to identify sexually biased miRNA-mRNA networks.
Integrating public microarray data reveals significant sex difference of mRNA expression in normal/ disease heart
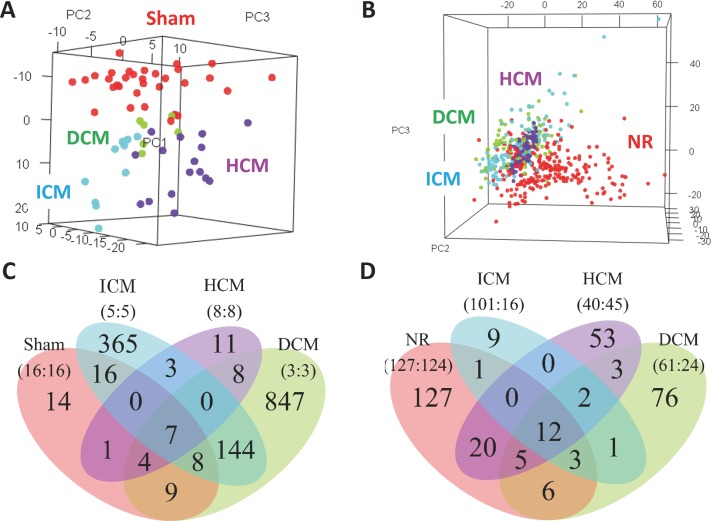
First, we asked if disease heart shows sex difference in mRNA expression. From GEO database, we found 4 mouse studies and 6 human studies that reported the transcriptome of the normal/ ICM/ HCM/ DCM (Table 1). The sample size of mouse and human data totaled 64 and 616, respectively. These data were each pre-processed according to their array platform and then integrated with COMBAT cross-platform normalization method[56]. Principal component analysis (PCA) confirmed that the cross-platform normalization effectively removed the batch effect–the noise arising from the fact that each data is generated by different labs and platforms (Fig 2, S1 Fig).
Fig 2. mRNA array data integration.
(A, B) PCA of the integrated mouse (A) and human (B) data. Samples of the same health condition are grouped together, indicating that our cross-platform normalization procedure effectively removed the batch effect.(C, D), Venn’s diagram of the results of male-female comparison in mouse (C) and human (D). Dozens/ hundreds of genes were found to be sexually biased in each health condition Sample sizes are shown in bracket (male: female).
To assess whether sex difference exists in our combined meta-data, we checked if sex can be discriminated on the basis of the transcriptomic principal components (PCs). PCs are the products of PCA, a popular dimensionality reduction technique. In this analysis, the expression values of all genes are linearly combined to construct a small set of new variables so that they best retain the transcriptome information. When we performed machine-learning discrimination using PCs, male and female were discriminated almost perfectly (S2–S4 Figs), indicating the existence of sex difference. Likewise, health conditions were also effectively discriminated, confirming that different diseases acquire different transcriptome (S5 Fig).
Consistently, we identified a number of genes sexually biased in all the health conditions (Fig 2C and 2D, S1 and S2 Tables). As expected, these genes were enriched on sex chromosomes among several other chromosomes, possibly accounting for their sex difference (S6 Fig). The overrepresented GOs included angiogenesis, cardiac muscle growth, regulation of heart contraction, and response to wounding (S3 Table), suggesting the functional importance of these sexually dimorphic genes in heart disease. These data are consistent with previous reports [32,58–60].
miRNA array sample characteristics
Next we asked if the similar sex difference also exists in miRNA expression. To this end we conducted miRNA microarray analysis using ICM and normal heart samples of both mouse and human. Murine ICM model samples were prepared by surgically ligating the LAD coronary artery and were sacrificed 1 month later. The hearts were macroscopically validated to exhibit the LV free wall thinning and dilatation (S7 Fig). Human RNA samples were obtained during post-mortem examination. Patient characteristics are summarized in Table 2.
Table 2. Clinical characteristics of the study subjects.
| Normal | ICM | |
|---|---|---|
| Sample number | 12 | 9 |
| Age, mean (s.d.) | 79.83 (6.76) | 79.22 (4.81) |
| Male, ratio | 50 | 44.44 |
| BMI, kg/m2 mean (s.d.) | 17.04 (7.03) | 19.5 (6.93) |
| Medical history, ratio | ||
| AS | 0 | 0.11 |
| LVH | 0 | 0.56 |
| Hypertension | 0.42 | 0.89 |
| DM | 0 | 0.67 |
| Smoking | 0.55 | 0.43 |
ICM: Ischemic cardiomyopathy, BMI: body mass index, AS: aortic stenosis, LVC: left ventricular hypertrophy, DM: Diabetes mellitus
Sex difference of miRNA expression in normal heart and ICM
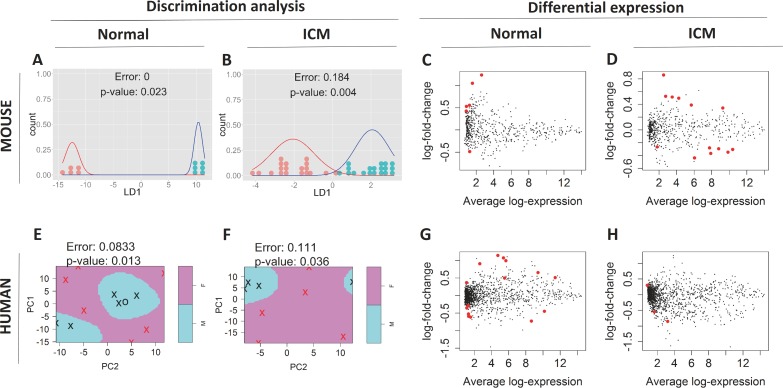
We profiled expression of 1088 mouse mature miRNAs using a high-throughput Affymetrix platform. 592 miRNAs were expressed above detection threshold in at least one condition and were used for subsequent analyses. We confirmed the reliability of our expression profiling by qPCR (S8 Fig). Machine-learning discrimination on the basis of PCs discriminated male and female almost perfectly, indicating the existence of sex difference (Fig 3A and 3B, S9 and S10 Figs). Likewise, health conditions were discriminated effectively, confirming the change in transcriptome post MI (S11 Fig). Differential expression analysis identified 6 miRNAs as sexually biased in normal heart, and 13 in MI (Fig 3C and 3D, Table 3). As expected, substantially larger number of miRNAs were differentially expressed between normal and MI compared with sexually biased miRNAs (S4 Table). No miRNA showed sex difference both in normal and MI heart, implying that the sex difference in miRNA changes when the heart suffers MI.
Fig 3. miRNA microarray comparison result.
(A, B) Sex discrimination of mouse miRNA array data of sham (A) and ICM (B) by LDA based on PCs. Each dot indicates one mouse sample. Shown curves are fitted normal distributions. Red: female, blue: male. Male and female samples are grouped together on the number line of a linear discriminant (LD1), and thus are well discriminated with p-value < 0.05 (C, D) MA plot of sex comparison in sham (C) and ICM (D) mouse data. Each dot indicates one miRNA. Sexually dimorphic miRNAs are colored red. (E, F) Sex discrimination of human miRNA array data of normal (E) and ICM (F) by SVM based on PCs. Crosses indicate data used as support vectors and circles the other. Red crosses/ circles indicate female samples and black crosses/ circles indicate male. Pink-/ blue- shaded regions indicates female/ male regions determined by SVM, respectively. Male and female samples are well discriminated with p-value < 0.05. (G, H) MA plot of sex comparison in normal (G) and ICM (H) patient data. The LOOCV error rates and their associated p-values are shown in (A), (B), (E), (F).
Table 3. Sexually dimorphic miRNAs.
| miRNA | logFC (M/F) | FDR |
|---|---|---|
| Mouse Sham | ||
| mmu-miR-190a-3p | 1.23154 | 2.15E-02 |
| mmu-miR-509-5p | 1.04725 | 2.15E-02 |
| mmu-miR-743b-3p | 0.52788 | 2.15E-02 |
| mmu-miR-669k-3p | 0.55455 | 4.36E-02 |
| mmu-miR-1b-3p | 0.42497 | 4.72E-02 |
| mmu-miR-218-5p | -0.4849 | 4.88E-02 |
| Mouse MI | ||
| mmu-miR-505-5p | 0.85863 | 4.14E-04 |
| mmu-miR-744-5p | 0.38993 | 1.13E-02 |
| mmu-miR-210-3p | -0.3669 | 1.13E-02 |
| mmu-miR-30e-5p | -0.3482 | 1.19E-02 |
| mmu-miR-30b-5p | -0.306 | 1.56E-02 |
| mmu-miR-29b-3p | -0.4383 | 2.06E-02 |
| mmu-miR-19b-3p | -0.2945 | 2.06E-02 |
| mmu-miR-193a-5p | 0.49665 | 2.06E-02 |
| mmu-miR-23a-5p | 0.52715 | 0.02063 |
| mmu-miR-142a-5p | -0.2667 | 0.02589 |
| mmu-miR-664-5p | 0.51445 | 0.03407 |
| mmu-miR-133a-5p | -0.282 | 0.03755 |
| mmu-miR-214-3p | 0.34282 | 0.04969 |
| Human normal | ||
| hsa-miR-558 | -0.5248 | 4.41E-03 |
| hsa-miR-3187-5p | 0.90705 | 5.25E-03 |
| hsa-miR-365a-3p | -0.6169 | 5.25E-03 |
| hsa-miR-4669 | 1.07475 | 5.25E-03 |
| hsa-miR-1261 | 0.36894 | 5.25E-03 |
| hsa-miR-193b-3p | -0.4488 | 5.98E-03 |
| hsa-miR-4735-3p | -0.2836 | 5.98E-03 |
| hsa-miR-148a-5p | -0.3558 | 5.98E-03 |
| hsa-miR-181c-5p | 0.9944 | 0.00598 |
| hsa-miR-4284 | -0.7285 | 0.007 |
| hsa-miR-150-3p | 1.14222 | 0.00708 |
| hsa-miR-4263 | -0.5913 | 0.00708 |
| hsa-miR-4745-5p | 0.65563 | 0.01521 |
| hsa-miR-4634 | 0.49747 | 0.04076 |
| hsa-miR-4516 | 0.51108 | 0.04189 |
| Human ICM | ||
| hsa-miR-3615 | -0.8528 | 2.97E-02 |
| hsa-miR-4423-5p | 0.29816 | 2.97E-02 |
| hsa-miR-4709-3p | -0.5457 | 4.66E-02 |
Next we profiled expression of 1725 human mature miRNAs using the same Affymetrix platform. 1559 miRNAs were expressed above detection threshold in at least one condition. In accordance with mouse, male and female were discriminated almost perfectly on the basis of PCs, indicating that the existence of sex difference is conserved in human heart as well (Fig 3E and 3F, S9 and S10 Figs). 15 and 3 miRNAs were detected as sexually biased in normal heart and ICM, respectively (Fig 3G and 3H, Table 3). Again, no miRNA showed sex difference both in normal and ICM heart.
Interestingly, in mouse nor human, the sexually biased miRNAs were not enriched on sex chromosomes (S12 Fig), implying a dedicated genetic program that creates the sexual biases of these miRNAs.
Sexually biased miRNAs in ICM are predicted to target cardiomyopathy pathways and are rich in known regulators of cardiac diseases
To gain insight into the sexually dimorphic miRNAs’ functionality in ICM, we performed the target GO/ pathway enrichment analysis on the basis of the target prediction analysis. DIANA microT-CDS software[61,62] and DAVID GO/ pathway enrichment analysis software[63,64] were used for this analysis. DAVID GO/ pathway enrichment analysis was performed with default parameters to rule out the authors’ arbitrariness. The result revealed that the sexually dimorphic miRNAs of ICM and normal heart preferentially target the cardiomyopathy pathways and GOs such as cardiac muscle growth, angiogenesis, apoptosis, and cation transport, important GOs for heart homeostasis (S5 Table). This raises the possibility that these miRNAs account for the sex difference in susceptibility and prognosis of ICM. Consistently, as many as 8 out of 13 sexually biased miRNAs in mouse MI were known to regulate cardiac diseases[65–80] (S6 Table). Likewise, 2 out of 6 in mouse normal heart[81–87], and 2 out of 15 in human normal heart[88–91] were known regulators of cardiac diseases.
miRNAs and mRNAs form sexually biased networks in ICM
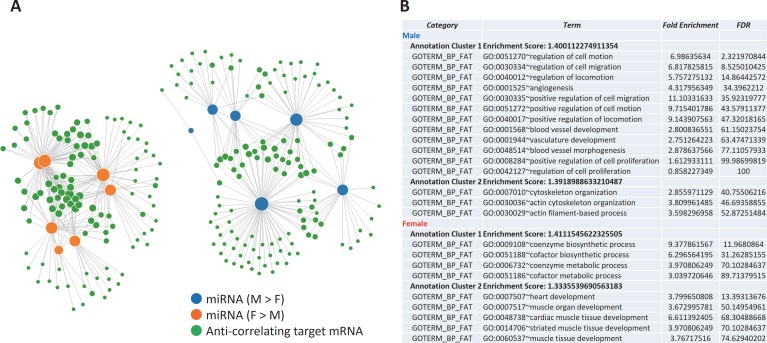
Since we successfully detected sexually dimorphic mRNAs and miRNAs in ICM, we next searched for sexually dimorphic miRNA-target mRNA relationships from the lists of sexually dimorphic mRNAs, miRNAs, and their predicted targets (S7 Table) in mouse MI. We found that these mRNAs are targeted by multiple miRNAs, forming sexually biased miRNA—mRNA networks (Fig 4A, S7 Table). The genes involved in the male-biased network (hence downregulated in male) over-represented GOs such as angiogenesis, and female-biased network over-represented GOs such as heart development (Fig 4B).
Fig 4. Sexually biased miRNA-mRNA networks in mouse MI.
(A) sexually dimorphic miRNAs and mRNAs form sexually biased networks. Blue and orange nodes represent miRNAs expressed higher in male and female, respectively. Green nodes represent target mRNAs. Each link represents one miRNA targeting one mRNA. Node size reflects the number of links. The link length reflects the confidence of the miRNA-mRNA target prediction (the closer, the more confident). A number of genes are targeted by multiple miRNAs, forming sexually biased miRNA-mRNA networks. (B,C) Over-represented GOs of the network component genes specifically down-regulated in male/ female ICM (B). An enrichment score of < 1.3 corresponds to a p-value of < 0.05 (detaled explanation is available as supporting information). See S5 Table for complete list of miRNAs and mRNAs involved.
Discussion
In this work, by integrating the existing mRNA microarray data of mouse and human, we found a number of sexually dimorphic genes in normal heart, ICM, HCM, and DCM, confirming the previous literature and further revealing new genes. These genes over-represented GOs important for heart homeostasis. We further asked if similar sex difference also exists in miRNA transcriptome, and by conducting miRNA microarray analysis of murine MI models and human ICM patients, we found that the miRNA transcriptome shows significant sex difference. Many of these miRNAs were known regulators of cardiac diseases. Computational analysis revealed that these sexually dimorphic miRNAs likely form sexually biased miRNA-mRNA networks in ICM, which potentially impact the prognosis. This offers the first comprehensive picture of the sex difference in mammalian cardiac diseases (ICM, HCM, DCM) at the level of mRNA transcriptome, and for the first time reports the sex difference of diseased heart at the level of miRNA.
Basal/ disease heart shows sex difference at the level of mRNA transcriptome
Thus far mRNA-level sex difference in mouse MI[44], HCM[31,32,45], DCM[39], and human DCM[46,47] have been reported separately. However, such data of human ICM and HCM are currently lacking. Importantly, no report has provided the picture of transcriptome-level sex differences in heart disease in a comprehensive fashion. Thus, in this study we set out to comprehensively characterize the genetic program underlying the heart sexual dimorphisms.
Meta-analysis is becoming increasingly powerful as the registered expression microarray data accumulate in the GEO database. Here we adopted this technique to investigate the transcriptome-wide sex difference of the heart. The result showed that each cardiac disease has a number of sexually dimorphic genes. The numbers of sexually dimorphic genes in diseased murine heart detected in this meta-analysis were roughly comparable to those reported in at least 2 of the 4 original studies, although the other 2 studies could not be compared to our results because they reported the sex difference without discriminating the healthy and disease samples. By analyzing the raw data of each study separately, we confirmed that an unexpectedly small number of sexually biased genes in normal murine heart (< 100) is attributable to the little overlap of the results of the original studies, and that our result reflects the overlapped genes (data not shown). These observations support the validity of our meta-analysis results.
Interestingly, our results suggest that some of the sexually dimorphic genes are common to some or all of the 4 diseases (Fig 2C and 2D). Since women have less occurrence but have higher risk after establishment of ICM[3–13] and HCM[14–18], sexually dimorphic genes common to ICM and HCM are potentially good targets for gender-specific treatments. Likewise, sexually dimorphic genes in DCM might be good candidates of drug targets since men are protected from DCM[19–25].
An important limitation of the human part of our study is the intergroup differences. Analysis of human disease transcriptome is often complicated due to the confounding biological noises such as age, body habitus, race, comorbidities, and medications. Although we could control for intergroup age differences (S13 Fig), the nature of our analysis—the integration of transcriptome data conducted in different labs with different aims—made it hard to control for the other variables. It is possible that these intergroup differences are underlying relatively small number of sexually biased genes detected in human, especially for disease samples where the sample sizes of women were relatively limited (Fig 2). Still, the set of sexually dimorphic genes presented in this study offers the first valuable clue to understanding the genetic program underlying the sexual dimorphism in heart disease. As for mouse, our analysis did not suffer from this problem because each of these data was generated to identify sexually dimorphic genes. Still, it is important to note that one of these data (Sham + DCM) was generated from a genetic background (BALB6/cJ) different from the rest (C57BL/6). This calls for a caution in directly comparing our data of DCM with the other health conditions. Concerning the validity of the sex difference of each health condition, on the other hand, this DCM data of BALB6/cJ was the only data used to compute the sex difference in DCM. This means that, in exploring the sex difference of heart diseases, samples of different genetic backgrounds are handled practically separately. Hence, the heterogeneity in the genetic background is unlikely to confound our result in this respect.
Normal/ ICM heart shows sex difference at the level of miRNA transcriptome
mRNAs have been thought to be the primary players in genetic programs. Accordingly, researchers studying the sex difference of mouse/ human cardiac diseases have focused on the mRNA transcriptome[31,32,39,44–47]. However, evidence is accumulating that many aspects of cardiac diseases are critically influenced by miRNAs[48]. Indeed, sexually biased miRNAs have been implicated in sexually dimorphic diseases such as neurodegenerative disorders[92] and metabolic syndrome[93]. It is therefore natural to hypothesize their role in the sex difference of cardiac disease. Consistently, we discovered several miRNAs in disease heart differentially expressed between sexes (Fig 3, Table 3).
The list of sexually dimorphic miRNAs presented in this study, however, is likely only a tip of the iceberg—the fact that male and female can be accurately discriminated on the basis of their transcriptomic PCs indicates that a larger number of genes are differentially expressed between sexes (Fig 3A, 3B, 3E and 3F). As for our mouse data, one factor that might have limited the detection power is the effect of estrous cycle. We did not match the estrus cycle of females in hope of capturing the “average” sexual dimorphism. This likely resulted in the hyper variability of the female samples. In support of this, correlation between female samples were significantly weaker than males in MI (S14 Fig), although in sham, the sample sizes were too small to assess the correlation difference. Consistently, larger number of genes showed > 1.2 fold sex differences (99 and 23 genes in sham and MI, respectively) than genes deemed statistically significant (6 and 13 genes in sham and MI, respectively). We predict that a population of miRNAs larger than detected in this study are differentially expressed between sexes. Additional studies on larger number of samples will be of considerable interest.
Sexually dimorphic miRNAs might be regulated by sex hormones
The expression of sex-biased miRNAs could stem from both sex chromosome and sex hormone effects[94]. X-chromosome is highly enriched in miRNAs, and approximately 15% of genes encoded by the inactive X-chromosome in humans escape inactivation[95], although in mouse this extent appears less[96]. We thus checked the chromosomal enrichment of the sexually dimorphic miRNAs in disease heart but found no such enrichment (S12 Fig). Sex steroid hormones–estradiol, progesterone and testosterone–also have been suggested to regulate miRNA expression in the context of cancer and brain[97,98]. Also supporting the role of sex hormones are studies reporting evidence that sex hormones induce the sex difference of genes in heart, albeit conflicting[31–33,36,38,40–43]. It would be interesting to assess this hypothesis, for example by measuring the binding of sex hormone receptors to the promoters of sexually dimorphic miRNAs. Measuring this by ChIP analysis and comparing it between sexes would provide a good insight into this hypothesis. Also, counterintuitive as it may seem that we observed miRNA sex differences in patients of > 70 years of age, literature do exist that report differences in autosomal gene expression in men vs postmenopausal women[99,100]. Although neither of these reports discusses the possible mechanisms of autosomal sex differences, they do support the existence of post-menopausal sex difference in autosomal gene expression.
Sexually biased miRNA-mRNA networks operate in ICM
We found that in murine ICM model, sexually dimorphic miRNAs seem to target the sexually dimorphic genes, forming sexually biased miRNA-mRNA networks. The genes repressed in male and female over-represented GOs such as angiogenesis and heart development, respectively (Fig 4B). This supports the following scenario: after developing ICM, male heart represses genes involved in angiogenesis, leading to the worse prognosis. In female heart, genes involved in heart development are repressed, preventing the harmful reactivation of the fetal cardiac gene program[101]. Although this network analysis was not applicable to human ICM due to the limited number of sexually dimorphic miRNAs detected, considering the shared phenotypic sex differences in cardiac diseases, the sexually biased networks revealed in mouse MI likely operate in human as well.
Note an important limitation that the miRNAs and mRNA data were not obtained from the same samples nor from samples of the same timing post MI (miRNA from chronic MI versus mRNA from acute MI). This means that the miRNA and mRNA data presented in this work are, strictly speaking, not directly comparable. Unfortunately the strong batch effect observed in our samples, which was effectively removed in our microarray data on the basis of transcriptome, did not allow us to directly confirm the sexual dimorphism of these genes in our samples by qPCR. Confirming the sexual dimorphism of these genes using batch-free chronic MI samples would be an important next step.
If the presented networks indeed operate and cause symptomatic sex differences in ICM, simultaneously targeting the components of these networks might enable highly effective and specific treatment strategies. In addition, considering the shared phenotypic sexual dimorphisms of the other cardiac diseases such as HCM and DCM, it is conceivable that sexually biased networks similarly operate in these cardiac diseases. Future miRNA microarray analyses are awaited to clarity this tempting possibility.
In summary, this study comprehensively characterized the sex difference of cardiac diseases at the level of miRNA and mRNA transcriptome, laying the foundation for the gender specific treatment strategies.
Conclusions
The existing mRNA microarray data of both mouse and human heart were integrated, identifying sexually dimorphic genes in cardiac diseases (ICM, HCM, and DCM). These genes over-represented GOs essential for heart homeostasis. Furthermore, microarray of miRNA isolated from mouse/ human ICM and normal heart samples was conducted, identifying sexually dimorphic miRNAs. Computational analysis revealed miRNA-mRNA networks that operate in a sexually biased fashion. Together this study provides the first comprehensive picture of the genome-wide program underlying the heart sexual dimorphisms, laying the foundation for the gender specific treatment strategies.
Ethics approval and consent to participate
Human tissue samples, acquired during post-mortem examination and frozen in liquid nitrogen, were provided by the department of pathology, Tokyo Metropolitan Geriatric Hospital after the approval from the ethical committee.
Our experimental procedures and protocols of animals were approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine, and performed in accordance with the US Animal Welfare Act.
Supporting information
(A-D) PCA of (A, B) mouse and (C, D) human metadata before batch effect correction. (E-F) PCA of (E, F) mouse and (G, H) human metadata after batch effect correction. Each dot represents each sample, colored by (A, E, C, G) batch or (B, F, D, H) disease. Samples of the same health condition are clustered together after normaliztion, indicating that normalization effectively removed the batch effect.
(TIF)
Sex was clearly discriminated in each disease. (A-D) mouse, (E-H), human. (A,E) Normal, (B,F) ICM, (C,G) HCM, and (D,H) DCM. The LOOCV error rate and its p-value are shown in each graph. Shown curves are the fitted normal distributions of each sex.
(TIF)
(A-D) mouse, (E-H), human. (A,E) Normal, (B,F) ICM, (C,G) HCM, and (D,H) DCM.
(TIF)
The weight distribution of PC1 used for the discriminant analysis in S2 Fig (A-D) mouse, (E-H), human. (A,E) Normal, (B,F) ICM, (C,G) HCM, and (D,H) DCM. PC weights are not dominated by small number of genes, but rather it appears many genes contribute to PC1. The other PCs showed similar weight distributions (data not shown).
(TIF)
(A-C) mouse, (D-F) human. (A,D)LDA results. The LOOCV error rate and its p-value are shown above each plot. (B,E) the cumulative variance explained by PCs used for LDA. (C,F) The weight distribution of PC1.
(TIF)
Chromosome enrichment of sexually dimorphic mRNAs of (A,B,E,F) mouse, (C,D,G,H) human. (A,C) male-biased genes in normal heart, (B,D) female-biased genes in normal heart. (E,G) male-biased genes in ICM, (F,H) female-biased genes in ICM. Light blue: the ratio of chromosome of the biased genes detected in this study. Dark blue: the ratio of chromosome of all the genes considered in this study. Sexually biased genes are enriched on several chromosomes when compared with all the genes considered in this study. *p < 0.05.
(TIF)
Shown are the ventricle side. M: male, F: female, sh: sham, MI: myocardial infarction (e.g. F_MI: female MI). The hearts exhibit post-myocardial infarction left ventricle (LV) remodeling with LV free wall thinning and dilatation. *if the fibrotic change was clearer from dorsal view, dorsal side is shown (labeled *).
(TIF)
X axes show the Cq values of each sample subtracted from that of a reference gene (mmu-miR-23a-3p). Y axes show the signal intensities of the microarray. Both axes are in log2 scale. Overall the miRNA microarray results seem to be consistent with that of qPCR. miRNAs that showed high variance between samples in the microarray show strong consistency between microarray and qPCR (mmu-miR-208b-3p, mmu-miR-3473a, mmu-miR-709). 5 out of 7 randomly chosen miRNAs with significant sex difference post MI also showed significant consistency.
(TIF)
PC weights of the miRNAs of (A, B) mouse and (C, D) human miRNA array data used for discriminant analysis in Fig 3. (A, C) normal (B, D) ICM.
(TIF)
(A,B) correspond to Fig 3A, 3B, 3C and 3D to Fig 3E and 3F.
(TIF)
Normal and ICM samples are well discriminated. (A) mouse (B) human. Red indicates ICM and blue indicates normal heart. The LOOCV error rate is shown in each graph. Shown curves are the fitted normal distributions of each health condition. The LOOCV error rate is shown in each graph.
(TIF)
Chromosome enrichment of sexually dimorphic miRNAs of (A,B,E,F) mouse and (C,D,G,H) human. (A,C) male-biased genes in normal heart, (B,D) female-biased genes in normal heart. (E,G) male-biased genes in ICM, (F,H) female-biased genes in ICM. Light blue: the ratio of chromosome of the biased genes detected in this study. Dark blue: the ratio of chromosome of all the genes considered in this study. Sexually biased genes are enriched on several chromosomes when compared with all the genes considered in this study. *p < 0.05.
(TIF)
Within each disease, age was effectively controlled between genders. NR: normal. * p < 0.05, n.s. not significant.
(TIF)
Correlations between all possible pairs of samples of the same group (e.g. female ICM) are plotted. (A) sham (B) ICM. In ICM, female samples have lower correlations than male. Welch’s two-sample t-test was used. *p < 0.05.
(TIF)
(XLSX)
(XLSX)
In mouse NF and human ICM, the number of sexually dimorphic genes detected was not sufficient for clustering analysis and thus the corresponding columns are left blank. Clusters are shown in a decreasing order according to enrichment score (indicated in bracket). Enrichment score > 1.0 (equivalent to p < 0.10) was deemed significant.
(XLSX)
(XLSX)
All clusters presented show enrichment score > 1.3 (equivalent to p-value < 0.05), although individual categories within clusters often show p-values > 0.05.
(XLSX)
(XLSX)
In miRupdown’ and ‘mRNAupdown’, 1 means stronger expression in male and -1 in female. miTGscore indicates the confidence of miRNA-mRNA targeting prediction according to the DIANA microT CDS algorhithm.
(XLSX)
(RTF)
Acknowledgments
We thank Dr. Katsuhiko Shirahige and Keiko Nakagawa (IMCB, the University of Tokyo) for help in microarray analysis. Dr. Shirahige generously provided Affymetrix Fluidics Station 450 and Affymetrix GeneChip Scanner 3000 7G. Keiko Nakagawa provided detailed instructions as to the microarray procedure. We also thank Dr. Tomoya Kitani (Kyoto Prefectural School of Medicine) and Dr. Hatsune Makino (IMCB, the University of Tokyo) for help in managing samples. Dr. Kitani managed the mice used for MI model making. Dr. Makino managed the human samples.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Japan Agency for Medical Research and Development: 16bk0104012h0004 (JT); Japan Society for the Promotion of Science: 25291049 (JT); Takeda Medical Research Foundation: 2013 (JT); and Banyu Life Science Foundation International: 2012 (JT). This study was supported by Nanken-Kyoten, TMDU.
References
- 1.Meyer S, Van Der Meer P, Van Tintelen JP, Van Den Berg MP. Sex differences in cardiomyopathies. Eur J Heart Fail. 2014;16: 238–247. doi: 10.1002/ejhf.15 [DOI] [PubMed] [Google Scholar]
- 2.Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5: 425–38. doi: 10.1038/nrd2032 [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Parsons L, Every NR, Barron H V, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341: 217–225. doi: 10.1056/NEJM199907223410401 [DOI] [PubMed] [Google Scholar]
- 4.Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: Results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27: 1408–1415. doi: 10.1093/eurheartj/ehl040 [DOI] [PubMed] [Google Scholar]
- 5.Olson MB, Kelsey SF, Matthews K, Shaw LJ, Sharaf BL, Pohost GM, et al. Symptoms, myocardial ischaemia and quality of life in women: Results from the NHLBI-sponsored WISE study. Eur Heart J. 2003;24: 1506–1514. doi: 10.1016/S0195-668X(03)00279-3 [DOI] [PubMed] [Google Scholar]
- 6.Vaccarino V, Krumholz HM, Berkman LF, Horwitz RI. Sex Differences in Mortality After Myocardial Infarction: Is There Evidence for an Increased Risk for Women? Circulation. 1995;91: 1861–1871. doi: 10.1161/01.CIR.91.6.1861 [DOI] [PubMed] [Google Scholar]
- 7.Bueno H, Vidan MT, Almazan A, Lopez-Sendon JL, Delcan JL. Influence of Sex on the Short-term Outcome of Elderly Patients With a First Acute Myocardial Infarction. Circulation. 1995;92: 1133–1140. doi: 10.1161/01.CIR.92.5.1133 [DOI] [PubMed] [Google Scholar]
- 8.With C. In-Hospital and 1-Year Mortality in 1, 524 Women After Myocardial Infarction. 1991; 484–491. [DOI] [PubMed] [Google Scholar]
- 9.Shapira I, Isakov a, Burke M, Almog C. Cardiac rupture in patients with acute myocardial infarction. Chest. 1987;92: 219–223. [DOI] [PubMed] [Google Scholar]
- 10.Kostis JB, Wilson a C, O’Dowd K, Gregory P, Chelton S, Cosgrove NM, et al. Sex differences in the management and long-term outcome of acute myocardial infarction. A statewide study. MIDAS Study Group. Myocardial Infarction Data Acquisition System. Circulation. 1994;90: 1715–1730. doi: 10.1161/01.CIR.90.4.1715 [DOI] [PubMed] [Google Scholar]
- 11.Vaccarino V, Abramson JL, Veledar E, Weintraub WS. Sex differences in hospital mortality after coronary artery bypass surgery: Evidence for a higher mortality in younger women. Circulation. 2002;105: 1176–1181. doi: 10.1161/hc1002.105133 [DOI] [PubMed] [Google Scholar]
- 12.Hammett CJK, Oxenham HC, Baldi JC, Doughty RN, Ameratunga R, French JK, et al. Effect of six months’ exercise training on C-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol. 2004;44: 2411–2413. doi: 10.1016/j.jacc.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 13.Antoniucci D, Valenti R, Moschi G, Migliorini a, Trapani M, Santoro GM, et al. Sex-based differences in clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am J Cardiol. 2001;87: 289–293. [DOI] [PubMed] [Google Scholar]
- 14.Carroll JD, Carroll EP, Feldman T, Ward DM, Lang RM, McGaughey D, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86: 1099–1107. doi: 10.1161/01.CIR.86.4.1336 [DOI] [PubMed] [Google Scholar]
- 15.Olivotto I, Maron MS, Adabag a. S, Casey S a., Vargiu D, Link MS, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46: 480–487. doi: 10.1016/j.jacc.2005.04.043 [DOI] [PubMed] [Google Scholar]
- 16.Kubo T, Kitaoka H, Okawa M, Hirota T, Hayato K, Yamasaki N, et al. Gender-specific differences in the clinical features of hypertrophic cardiomyopathy in a community-based Japanese population: Results from Kochi RYOMA study. J Cardiol. Japanese College of Cardiology; 2010;56: 314–319. doi: 10.1016/j.jjcc.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Lin CL, Chiang CW, Shaw CK, Chu PH, Chang CJ, Ko YL. Gender differences in the presentation of adult obstructive hypertrophic cardiomyopathy with resting gradient: a study of 122 patients. Jpn Circ J. 1999;63: 859–864. doi: 10.1253/jcj.63.859 [DOI] [PubMed] [Google Scholar]
- 18.Liao Y, Cooper RS, Mensah GA, McGee DL. Left Ventricular Hypertrophy Has a Greater Impact on Survival in Women Than in Men. Circulation. 1995;92: 805–810. doi: 10.1161/01.CIR.92.4.805 [DOI] [PubMed] [Google Scholar]
- 19.Magnani JW, Suk Danik HJ, Dec GW, DiSalvo TG. Survival in biopsy-proven myocarditis: A long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151: 463–470. doi: 10.1016/j.ahj.2005.03.037 [DOI] [PubMed] [Google Scholar]
- 20.Caforio ALP, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28: 1326–1333. doi: 10.1093/eurheartj/ehm076 [DOI] [PubMed] [Google Scholar]
- 21.Mason JW, O’Connell JB, Herskowitz a, Rose NR, McManus BM, Billingham ME, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333: 269–275. doi: 10.1056/NEJM199508033330501 [DOI] [PubMed] [Google Scholar]
- 22.van Rijsingen I a W, Nannenberg E a., Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, et al. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail. 2013;15: 376–384. doi: 10.1093/eurjhf/hfs191 [DOI] [PubMed] [Google Scholar]
- 23.Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of Titin Causing Dilated Cardiomyopathy. N Engl J Med. 2012;366: 619–628. doi: 10.1056/NEJMoa1110186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Codd MB, Sugrue DD, Gersh BJ, Melton LJ. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80: 564–572. doi: 10.1161/01.CIR.80.3.564 [DOI] [PubMed] [Google Scholar]
- 25.Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart. 2009;95: 1925–1930. doi: 10.1136/hrt.2008.164061 [DOI] [PubMed] [Google Scholar]
- 26.Sainani GS, Krompotic E, Slodki SJ. Adult heart disease due to the Coxsackie virus B infection. Medicine (Baltimore). 1968;47: 133–147. [DOI] [PubMed] [Google Scholar]
- 27.Fulminant THE, Of F, Hepatitis E. the American Journal. 1946;XXII. [Google Scholar]
- 28.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101: 425–484. [PMC free article] [PubMed] [Google Scholar]
- 29.Shioura KM, Geenen DL, Goldspink PH. Sex-related changes in cardiac function following myocardial infarction in mice. Am J Physiol Regul Integr Comp Physiol. 2008;295: R528–R534. doi: 10.1152/ajpregu.90342.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavasin M a, Tao Z, Menon S, Yang X-P. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75: 2181–2192. doi: 10.1016/j.lfs.2004.04.024 [DOI] [PubMed] [Google Scholar]
- 31.Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, et al. Female sex and estrogen receptor-  attenuate cardiac remodeling and apoptosis in pressure overload. 2010; 1597–1606. doi: 10.1152/ajpregu.00825.2009 [DOI] [PubMed] [Google Scholar]
- 32.Kararigas G, Fliegner D, Gustafsson J-Å, Regitz-Zagrosek V. Role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol Genomics. 2011;43: 438–446. doi: 10.1152/physiolgenomics.00199.2010 [DOI] [PubMed] [Google Scholar]
- 33.Xin H-B, Senbonmatsu T, Cheng D-S, Wang Y-X, Copello J a, Ji G-J, et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416: 334–338. doi: 10.1038/416334a [DOI] [PubMed] [Google Scholar]
- 34.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96: 7059–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelhardt S, Hein L, Keller U, Klämbt K, Lohse MJ. Inhibition of Na+-H+ exchange prevents hypertrophy, fibrosis, and heart failure in β1-adrenergic receptor transgenic mice. Circ Res. 2002;90: 814–819. doi: 10.1161/01.RES.0000014966.97486.C0 [DOI] [PubMed] [Google Scholar]
- 36.Arimura T, Onoue K, Takahashi-Tanaka Y, Ishikawa T, Kuwahara M, Setou M, et al. Nuclear accumulation of androgen receptor in gender difference of dilated cardiomyopathy due to lamin A/C mutations. Cardiovasc Res. 2013;99: 382–94. doi: 10.1093/cvr/cvt106 [DOI] [PubMed] [Google Scholar]
- 37.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett M a, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178: 6710–6714. doi: 10.4049/jimmunol.178.11.6710 [DOI] [PubMed] [Google Scholar]
- 38.Lyden DC, Olszewski J, Feran M, Job LP, Huber S a. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126: 432–438. [PMC free article] [PubMed] [Google Scholar]
- 39.Coronado MJ, Brandt JE, Kim E, Bucek A, Bedja D, Abston ED, et al. Testosterone and interleukin-1 increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. AJP Hear Circ Physiol. 2012;302: H1726–H1736. doi: 10.1152/ajpheart.00783.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavasin M a Tao Z-Y, Yu A-L Yang X-P. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol. 2006;290: H2043–H2050. doi: 10.1152/ajpheart.01121.2005 [DOI] [PubMed] [Google Scholar]
- 41.Nikolic I, Liu D, Bell J a., Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol. 2007;42: 769–780. doi: 10.1016/j.yjmcc.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 42.Babiker F a., De Windt LJ, Van Eickels M, Grohe C, Meyer R, Doevendans P a. Estrogenic hormone action in the heart: Regulatory network and function. Cardiovasc Res. 2002;53: 709–719. doi: 10.1016/S0008-6363(01)00526-0 [DOI] [PubMed] [Google Scholar]
- 43.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular Disease Outcomes During 6.8 Years of Hormone Therapy. Jama. 2002;288: 49 doi: 10.1001/jama.288.1.49 [DOI] [PubMed] [Google Scholar]
- 44.Chen Q, Williams R, Healy CL, Wright CD, Wu SC, O’Connell TD. An association between gene expression and better survival in female mice following myocardial infarction. J Mol Cell Cardiol. Elsevier Ltd; 2010;49: 801–811. doi: 10.1016/j.yjmcc.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witt H, Schubert C, Jaekel J, Fliegner D, Penkalla A, Tiemann K, et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med. 2008;86: 1013–1024. doi: 10.1007/s00109-008-0385-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haddad GE, Saunders LJ, Crosby SD, Carles M, del Monte F, King K, et al. Human cardiac-specific cDNA array for idiopathic dilated cardiomyopathy: sex-related differences. Physiol Genomics. 2008;33: 267–277. doi: 10.1152/physiolgenomics.00265.2007 [DOI] [PubMed] [Google Scholar]
- 47.Molina-Navarro MM, Roselló-Lletí E, Ortega A, Tarazón E, Otero M, Martínez-Dolz L, et al. Differential gene expression of cardiac ion channels in human dilated cardiomyopathy. PLoS One. 2013;8: 1–10. doi: 10.1371/journal.pone.0079792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joladarashi D, Thandavarayan RA, Babu SS, Krishnamurthy P. Small engine, big power: microRNAs as regulators of cardiac diseases and regeneration. Int J Mol Sci. 2014;15: 15891–911. doi: 10.3390/ijms150915891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edgar R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30: 207–210. doi: 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R: a language and environment for statistical computing [Internet]. 2015 [cited 10 Nov 2015]. Available: http://www.gbif.org/resource/81287
- 51.Liu Y, Morley M, Brandimarto J, Hannenhalli S, Hu Y, Ashley E a., et al. RNA-Seq identifies novel myocardial gene expression signatures of heart failure. Genomics. 2015;105: 83–89. doi: 10.1016/j.ygeno.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaertner a., Schwientek P, Ellinghaus P, Summer H, Golz S, Kassner a., et al. Myocardial transcriptome analysis of human arrhythmogenic right ventricular cardiomyopathy. Physiol Genomics. 2012;44: 99–109. doi: 10.1152/physiolgenomics.00094.2011 [DOI] [PubMed] [Google Scholar]
- 53.Pilbrow AP, Folkersen L, Pearson JF, Brown CM, McNoe L, Wang NM, et al. The chromosome 9p21.3 coronary heart disease risk allele is associated with altered gene expression in normal heart and vascular tissues. PLoS One. 2012;7: 1–11. doi: 10.1371/journal.pone.0039574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greco S, Fasanaro P, Castelvecchio S, D'Alessandra Y, Arcelli D, Di Donato M, et al. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes. 2012;61: 1633–1641. doi: 10.2337/db11-0952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akat KM, Moore-McGriff D, Morozov P, Brown M, Gogakos T, Correa Da Rosa J, et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc Natl Acad Sci U S A. 2014;111: 11151–6. doi: 10.1073/pnas.1401724111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8: 118–127. doi: 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- 57.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. 2015;43 doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Champion HC, Breton E, et al. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur Heart J. 2010;31: 1188–1196. doi: 10.1093/eurheartj/ehp549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boheler KR, Volkova M, Morrell C, Garg R, Zhu Y, Margulies K, et al. Sex- and age-dependent human transcriptome variability: Implications for chronic heart failure. 2003; doi: 10.1073/pnas.0436564100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isensee J, Witt H, Pregla R, Hetzer R, Regitz-Zagrosek V, Noppinger PR. Sexually dimorphic gene expression in the heart of mice and men. J Mol Med (Berl). 2008;86: 61–74. doi: 10.1007/s00109-007-0240-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paraskevopoulou MD, Georgakilas G, Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, et al. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41: 169–173. doi: 10.1093/nar/gkt393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reczko M, Maragkakis M, Alexiou P, Grosse I, Hatzigeorgiou a G. Functional microRNA targets in protein coding sequences. Bioinformatics. 2012;28: 771–776. doi: 10.1093/bioinformatics/bts043 [DOI] [PubMed] [Google Scholar]
- 63.Huang DW, Sherman BT, Lempicki R a. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4: 44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 64.Huang DW, Sherman BT, Lempicki R a. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37: 1–13. doi: 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. American Society for Clinical Investigation; 2012;122: 1222–32. doi: 10.1172/JCI59327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. Nature Publishing Group; 2007;13: 613–8. doi: 10.1038/nm1582 [DOI] [PubMed] [Google Scholar]
- 67.Chen M, Ma G, Yue Y, Wei Y, Li Q, Tong Z, et al. Downregulation of the miR-30 family microRNAs contributes to endoplasmic reticulum stress in cardiac muscle and vascular smooth muscle cells. Int J Cardiol. 2014;173: 65–73. doi: 10.1016/j.ijcard.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 68.Chen S, Puthanveetil P, Feng B, Matkovich SJ, Dorn GW, Chakrabarti S. Cardiac miR-133a overexpression prevents early cardiac fibrosis in diabetes. J Cell Mol Med. 2014;18: 415–21. doi: 10.1111/jcmm.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danielson LS, Park DS, Rotllan N, Chamorro-Jorganes A, Guijarro M V, Fernandez-Hernando C, et al. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013;27: 1460–7. doi: 10.1096/fj.12-221994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding W, Li J, Singh J, Alif R, Vazquez-Padron RI, Gomes SA, et al. miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE-/- mice. Cardiovasc Res. 2015;106: 131–42. doi: 10.1093/cvr/cvv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duan Q, Yang L, Gong W, Chaugai S, Wang F, Chen C, et al. MicroRNA-214 Is Upregulated in Heart Failure Patients and Suppresses XBP1-Mediated Endothelial Cells Angiogenesis. J Cell Physiol. 2015;230: 1964–73. doi: 10.1002/jcp.24942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.el Azzouzi H, Leptidis S, Dirkx E, Hoeks J, van Bree B, Brand K, et al. The hypoxia-inducible microRNA cluster miR-199a∼214 targets myocardial PPARδ and impairs mitochondrial fatty acid oxidation. Cell Metab. Elsevier; 2013;18: 341–54. doi: 10.1016/j.cmet.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 73.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122: S124–31. doi: 10.1161/CIRCULATIONAHA.109.928424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Izarra A, Moscoso I, Levent E, Cañón S, Cerrada I, Díez-Juan A, et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem cell reports. Elsevier; 2014;3: 1029–42. doi: 10.1016/j.stemcr.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M, Hu X, Zhu J, Zhu C, Zhu S, Liu X, et al. Overexpression of miR-19b impairs cardiac development in zebrafish by targeting ctnnb1. Cell Physiol Biochem. Karger Publishers; 2014;33: 1988–2002. doi: 10.1159/000362975 [DOI] [PubMed] [Google Scholar]
- 76.Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li P-F. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106: 12103–8. doi: 10.1073/pnas.0811371106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lv G, Shao S, Dong H, Bian X, Yang X, Dong S. MicroRNA-214 Protects Cardiac Myocytes Against H 2 O 2 -Induced Injury. J Cell Biochem. 2014;115: 93–101. doi: 10.1002/jcb.24636 [DOI] [PubMed] [Google Scholar]
- 78.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106: 166–75. doi: 10.1161/CIRCRESAHA.109.202176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105: 13027–32. doi: 10.1073/pnas.0805038105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang K, Lin Z-Q, Long B, Li J-H, Zhou J, Li P-F. Cardiac hypertrophy is positively regulated by MicroRNA miR-23a. J Biol Chem. 2012;287: 589–99. doi: 10.1074/jbc.M111.266940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karakikes I, Chaanine AH, Kang S, Mukete BN, Jeong D, Zhang S, et al. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc. 2013;2: e000078 doi: 10.1161/JAHA.113.000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan Z, Sun X, Ren J, Li X, Gao X, Lu C, et al. miR-1 Exacerbates Cardiac Ischemia-Reperfusion Injury in Mouse Models. Salloum FN, editor. PLoS One. Public Library of Science; 2012;7: e50515 doi: 10.1371/journal.pone.0050515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. Nature Publishing Group; 2007;13: 486–91. doi: 10.1038/nm1569 [DOI] [PubMed] [Google Scholar]
- 84.Cao Q, Dong P, Wang Y, Zhang J, Shi X, Wang Y. miR-218 suppresses cardiac myxoma proliferation by targeting myocyte enhancer factor 2D. Oncol Rep. Spandidos Publications; 2015;33: 2606–2612. Available: http://www.spandidos-publications.com/or/33/5/2606/abstract doi: 10.3892/or.2015.3861 [DOI] [PubMed] [Google Scholar]
- 85.Chiavacci E, Dolfi L, Verduci L, Meghini F, Gestri G, Evangelista AMM, et al. MicroRNA 218 mediates the effects of Tbx5a over-expression on zebrafish heart development. PLoS One. Public Library of Science; 2012;7: e50536 doi: 10.1371/journal.pone.0050536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fish JE, Wythe JD, Xiao T, Bruneau BG, Stainier DYR, Srivastava D, et al. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development. 2011;138: 1409–19. doi: 10.1242/dev.060046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ Res. 2010;107: 1336–44. doi: 10.1161/CIRCRESAHA.110.227926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang Y, Wang Y, Park K-M, Hu Q, Teoh J-P, Broskova Z, et al. MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc Res. 2015;106: 387–97. doi: 10.1093/cvr/cvv121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, Kong M, Jiang D, Qian J, Duan Q, Dong A. MicroRNA-150 aggravates H2O2-induced cardiac myocyte injury by down-regulating c-myb gene. Acta Biochim Biophys Sin (Shanghai). 2013;45: 734–41. doi: 10.1093/abbs/gmt067 [DOI] [PubMed] [Google Scholar]
- 90.Liu Z, Ye P, Wang S, Wu J, Sun Y, Zhang A, et al. MicroRNA-150 protects the heart from injury by inhibiting monocyte accumulation in a mouse model of acute myocardial infarction. Circ Cardiovasc Genet. 2015;8: 11–20. doi: 10.1161/CIRCGENETICS.114.000598 [DOI] [PubMed] [Google Scholar]
- 91.MiR-155 and miR-148a reduce cardiac injury by inhibiting NF-κB pathway during acute viral myocarditis [Internet]. [cited 9 Oct 2015]. Available: http://www.europeanreview.org/article/7707 [PubMed] [Google Scholar]
- 92.Mellios N, Galdzicka M, Ginns E, Baker SP, Rogaev E, Xu J, et al. Gender-specific reduction of estrogen-sensitive small RNA, miR-30b, in subjects with schizophrenia. Schizophr Bull. 2012;38: 433–43. doi: 10.1093/schbul/sbq091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y-T, Tsai P-C, Liao Y-C, Hsu C-Y, Juo S-HH. Circulating microRNAs have a sex-specific association with metabolic syndrome. J Biomed Sci. Journal of Biomedical Science; 2013;20: 72 doi: 10.1186/1423-0127-20-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morgan CP, Bale TL. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol Sex Differ. Biology of Sex Differences; 2012;3: 22 doi: 10.1186/2042-6410-3-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434: 400–404. doi: 10.1038/nature03479 [DOI] [PubMed] [Google Scholar]
- 96.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20: 614–22. doi: 10.1101/gr.103200.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klinge CM. MiRNAs and estrogen action. Trends Endocrinol Metab. Elsevier Ltd; 2012;23: 223–233. doi: 10.1016/j.tem.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morgan CP, Bale TL. Early Prenatal Stress Epigenetically Programs Dysmasculinization in Second-Generation Offspring via the Paternal Lineage. J Neurosci. 2011;31: 11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jansen R, Batista S, Brooks AI, Tischfield JA, Willemsen G, van Grootheest G, et al. Sex differences in the human peripheral blood transcriptome. BMC Genomics. BioMed Central; 2014;15: 33 doi: 10.1186/1471-2164-15-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trabzuni D, Ramasamy A, Imran S, Walker R, Smith C, Weale ME, et al. Widespread sex differences in gene expression and splicing in the adult human brain. Nat Commun. Nature Publishing Group; 2013;4: 2771 doi: 10.1038/ncomms3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin Heavy Chain Isoform Expression in the Failing and Nonfailing Human Heart. Circ Res. 2000;86: 386–390. doi: 10.1161/01.RES.86.4.386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A-D) PCA of (A, B) mouse and (C, D) human metadata before batch effect correction. (E-F) PCA of (E, F) mouse and (G, H) human metadata after batch effect correction. Each dot represents each sample, colored by (A, E, C, G) batch or (B, F, D, H) disease. Samples of the same health condition are clustered together after normaliztion, indicating that normalization effectively removed the batch effect.
(TIF)
Sex was clearly discriminated in each disease. (A-D) mouse, (E-H), human. (A,E) Normal, (B,F) ICM, (C,G) HCM, and (D,H) DCM. The LOOCV error rate and its p-value are shown in each graph. Shown curves are the fitted normal distributions of each sex.
(TIF)
(A-D) mouse, (E-H), human. (A,E) Normal, (B,F) ICM, (C,G) HCM, and (D,H) DCM.
(TIF)
The weight distribution of PC1 used for the discriminant analysis in S2 Fig (A-D) mouse, (E-H), human. (A,E) Normal, (B,F) ICM, (C,G) HCM, and (D,H) DCM. PC weights are not dominated by small number of genes, but rather it appears many genes contribute to PC1. The other PCs showed similar weight distributions (data not shown).
(TIF)
(A-C) mouse, (D-F) human. (A,D)LDA results. The LOOCV error rate and its p-value are shown above each plot. (B,E) the cumulative variance explained by PCs used for LDA. (C,F) The weight distribution of PC1.
(TIF)
Chromosome enrichment of sexually dimorphic mRNAs of (A,B,E,F) mouse, (C,D,G,H) human. (A,C) male-biased genes in normal heart, (B,D) female-biased genes in normal heart. (E,G) male-biased genes in ICM, (F,H) female-biased genes in ICM. Light blue: the ratio of chromosome of the biased genes detected in this study. Dark blue: the ratio of chromosome of all the genes considered in this study. Sexually biased genes are enriched on several chromosomes when compared with all the genes considered in this study. *p < 0.05.
(TIF)
Shown are the ventricle side. M: male, F: female, sh: sham, MI: myocardial infarction (e.g. F_MI: female MI). The hearts exhibit post-myocardial infarction left ventricle (LV) remodeling with LV free wall thinning and dilatation. *if the fibrotic change was clearer from dorsal view, dorsal side is shown (labeled *).
(TIF)
X axes show the Cq values of each sample subtracted from that of a reference gene (mmu-miR-23a-3p). Y axes show the signal intensities of the microarray. Both axes are in log2 scale. Overall the miRNA microarray results seem to be consistent with that of qPCR. miRNAs that showed high variance between samples in the microarray show strong consistency between microarray and qPCR (mmu-miR-208b-3p, mmu-miR-3473a, mmu-miR-709). 5 out of 7 randomly chosen miRNAs with significant sex difference post MI also showed significant consistency.
(TIF)
PC weights of the miRNAs of (A, B) mouse and (C, D) human miRNA array data used for discriminant analysis in Fig 3. (A, C) normal (B, D) ICM.
(TIF)
(A,B) correspond to Fig 3A, 3B, 3C and 3D to Fig 3E and 3F.
(TIF)
Normal and ICM samples are well discriminated. (A) mouse (B) human. Red indicates ICM and blue indicates normal heart. The LOOCV error rate is shown in each graph. Shown curves are the fitted normal distributions of each health condition. The LOOCV error rate is shown in each graph.
(TIF)
Chromosome enrichment of sexually dimorphic miRNAs of (A,B,E,F) mouse and (C,D,G,H) human. (A,C) male-biased genes in normal heart, (B,D) female-biased genes in normal heart. (E,G) male-biased genes in ICM, (F,H) female-biased genes in ICM. Light blue: the ratio of chromosome of the biased genes detected in this study. Dark blue: the ratio of chromosome of all the genes considered in this study. Sexually biased genes are enriched on several chromosomes when compared with all the genes considered in this study. *p < 0.05.
(TIF)
Within each disease, age was effectively controlled between genders. NR: normal. * p < 0.05, n.s. not significant.
(TIF)
Correlations between all possible pairs of samples of the same group (e.g. female ICM) are plotted. (A) sham (B) ICM. In ICM, female samples have lower correlations than male. Welch’s two-sample t-test was used. *p < 0.05.
(TIF)
(XLSX)
(XLSX)
In mouse NF and human ICM, the number of sexually dimorphic genes detected was not sufficient for clustering analysis and thus the corresponding columns are left blank. Clusters are shown in a decreasing order according to enrichment score (indicated in bracket). Enrichment score > 1.0 (equivalent to p < 0.10) was deemed significant.
(XLSX)
(XLSX)
All clusters presented show enrichment score > 1.3 (equivalent to p-value < 0.05), although individual categories within clusters often show p-values > 0.05.
(XLSX)
(XLSX)
In miRupdown’ and ‘mRNAupdown’, 1 means stronger expression in male and -1 in female. miTGscore indicates the confidence of miRNA-mRNA targeting prediction according to the DIANA microT CDS algorhithm.
(XLSX)
(RTF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.