Abstract
Malaria is a disease that has a major impact in many developing nations, especially on the African continent. There is a need to develop new therapeutics and prophylactic treatments against it. A trisubstituted pyrrole was recently found to inhibit infection of mammalian hepatocytes by Plasmodium sporozoites, but the target of this agent in not known. In this study trisubstituted pyrrole derivatives with different substituents on a piperidinyl nitrogen were prepared. We determined if modifications of the piperidinyl nitrogen would accommodate a drug–biotin linking strategy for affinity purification of the trisubstituted pyrrole’s target protein(s).
Keywords: Trisubstituted pyrrole, Malaria, Sporozoite, Hepatocyte
Malaria is caused by protozoan parasites of the genus, Plasmodium. It affects approximately 200 million people every year, resulting in about 650,000 deaths.1 The infection is initiated by a bite from an infected mosquito, which releases Plasmodium sporozoites into the host. Sporozoites infect hepatocytes where they replicate to form tens of thousands of liver stage parasites. The liver stage parasites are released into the blood stream where they infect erythrocytes to begin the symptomatic phase of a malaria infection. Since infection of hepatocytes by sporozoites is the first obligatory step in a malaria infection, this step represents an attractive goal for causal prophylaxis of malaria.
It was shown that the trisubstituted pyrrole (TSP) 4-[2-(4-fluorophenyl)- 5-(1-methylpiperidine-4-yl)-1H-pyrrol-3-yl]pyridine (1, Fig. 1) has activity against both erythrocytic2 and sporozoite3 stages of the parasite, in vitro and in vivo. TSP’s inhibition of the parasite’s erythrocytic stages results primarily from inhibition of a cGMP-dependent protein kinase (PKG) in the parasite.2,4 In contrast, sporozoites do not express PKG. Sporozoites that lack the PKG gene retain their ability to infect hepatocytes,5 and infection of hepatocytes by these sporozoites is still inhibited by TSP.3 These data demonstrate that sporozoites express an additional unidentified protein target(s) of the TSP that is essential for sporozoite invasion of the liver. These proteins represent potentially new targets for the development of novel therapeutics to prevent malarial infection. In the work presented here, TSP derivatives having different substituents on the piperidinyl nitrogen were prepared and evaluated to determine if modification of the piperidinyl nitrogen would accommodate a drug–biotin linking strategy to affinity purify and subsequently identify the target protein(s) of this new agent.
Figure 1.

TSP.
There have been no structure–activity-relationship studies to determine which structural features of TSP are important for inhibition of sporozoite infection. Thus a small set of control compounds was initially synthesized in order to determine if the piperidinyl nitrogen in TSP is amenable to structural changes that afford retention of activity. It was envisioned that if substitution of this secondary amine could be varied to afford analogs that maintained inhibition activity, then attachment of a linker–biotin moiety at this position would afford TSP derivatives for affinity purification of TSP’s protein target(s). Three control compounds (parent TSP 1), 9 and 10) were tested to confirm activity of TSP,12 and to determine if analogs with structural variation at the piperidinyl nitrogen retained activity (Schemes 1–3).
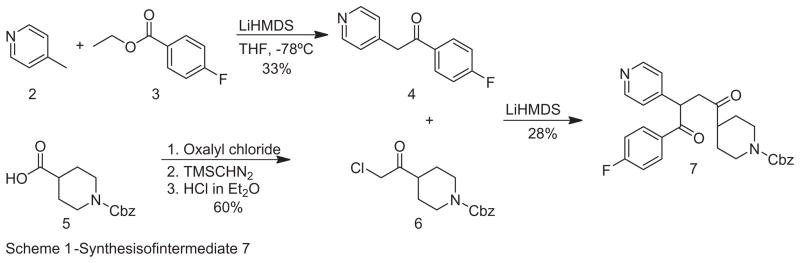
Scheme 1.
Synthesis of intermediate 7.
Scheme 3.
Synthesis of final products 13 and 14.
Synthesis of TSP (compound 1) and the piperidinyl-modified TSP derivatives was accomplished with modification of previously reported syntheses.6,7 Commercially available 4-picoline ( 2) was reacted with LiHMDS, followed by addition of ethyl-4-fluorobenzoate 3) to give ketone 4 (Scheme 1). N-CBZ piperidine-4-carboxylic acid 5) was converted to the acid chloride with oxalyl chloride, and then reacted with trimethylsilyldiazomethane, followed by the addition of hydrogen chloride in ether to yield α-chloro ketone, 6.8
The coupling of 4 with 6 to give diketone 7 was achieved in modest yield (Scheme 1). Diketone 7 was then reacted with ammonium acetate in acetic acid to yield pyrrole 8 (Scheme 2). Reduction of the N-CBZ protecting group of pyrrole 8 to the N-methyl group using LiAlH4 in refluxing THF afforded the parent TSP, 1 (Scheme 2).7 Alternatively, removal of the CBZ protecting group from 8 was achieved by dissolving 8 in acetic acid followed by bubbling HCl gas through the solvent9 to give target compound and key intermediate 9, which bears the unsubstituted piperidinyl amine that can undergo further chemical modification (Scheme 2). For the purposes of this study, a crosslinking moiety having a primary amine was desired at the piperidinyl nitrogen in order to determine if such a substituted derivative would retain activity, and if so to then couple this amine with linker–biotin groups. To this end, ethyl amine substituted target molecule 10 was synthesized by reacting intermediate 9 with 2-(tert-butoxycarbonylamino) ethyl bromide in the presence of triethylamine, followed by removal of the Boc protecting group with TFA (Scheme 2).
Scheme 2.
Synthesis of intermediate 6.
The three initial compounds, TSP synthesized in this study (compound 1), the desmethyl derivative (compound 9), and the ethylamine substituted derivative (compound 10) were tested and compared for their ability to inhibit invasion of hepatocytes by sporozoites using previously published procedures (Fig. 2).3 Briefly, P. berghei sporozoites were added to the human hepatoma cell line, HepG2, in the presence of different compounds. Sporozoite infectivity was determined by quantifying the number of intracellular liver stages that developed 40 h post-infection.
Figure 2.
Inhibition of sporozoite infection of hepatocytes by parent TSP (1) and derivatives 9 and 10.
As reported earlier, parent TSP inhibited the sporozoite infection of hepatocytes by over 90% at a concentration of 2 μM. Desmethyl derivative 9 and ethylamino-substituted derivative 10 also show near complete inhibition of sporozoite infectivity at a concentration of 10 μM (Fig. 2). This data indicates that structural variation at the secondary amine of the piperidine ring is possible and does not result in significant loss of activity. Moreover, this data suggests that future modification of the piperidine ring may yield more potent derivatives as well.
Having shown that changing substitution of the piperidinyl nitrogen from methyl to an ethylamine linker group did not result in a loss of activity, the synthesis of biotin-containing derivatives of TSP was undertaken. Compound 10 was coupled with two different biotin containing conjugates, NHS esters 11 and 12 (Scheme 3), to give the TSP–linker–biotin conjugates 13 and 14, respectively.
The depth of a putative binding pocket for TSP in the protein target(s) is unknown. Thus, the two linkers chosen to separate the biotin moiety from the TSP core are quite long. This approach was taken in order to avoid a situation where the linker was too short for the TSP core to interact with its target protein(s) while the biotin moiety simultaneously interacts with an affinity purification column. Furthermore, the LC–LC linker (11) was chosen because the hydrophobicity of the long alkyl chains are thought to increase membrane permeability.10 The PEG4 linker (12) was chosen to impart extra water solubility, as well as to decrease membrane permeability.11 It was hypothesized that the hydrophobic TSP–linker–biotin compound (13) would display greater activity than that of the more hydrophilic TSP–linker–biotin compound (14) if the drug target(s) are intracellular. Thus, these two linking strategies were chosen in an attempt to gather information about the cellular location of the protein target(s) and to ensure that the TSP–linker–biotin conjugates would be able to interact with both the target protein(s) and avidin/streptavidin column in chorus.
TSP–linker–biotin compounds 13 and 14 each showed essentially no inhibition of sporozoite infectivity of hepatocytes at either 2 or 10 μM concentrations (Fig. 3). It is possible this loss of activity is due to a loss of binding affinity for the protein target of TSP that is responsible for observed activity. The large biotin–linker moieties may impede the ability of compounds 13 and 14 to interact efficiently with the binding site of the target protein(s). However, it is also possible that the protein target(s) are intracellular, and regardless of linker used the large compounds simply lack sufficient cellular penetration.
Figure 3.
Inhibition of sporozoite infection of hepatocytes by biotin–linker–TSPs 13 and 14.
It was shown in previous work studying anticoccidial activity of TSP and TSP analogs that N-desmethyl TSP or TSP derivatives having a basic amine were potent inhibitors of parasite cGMP-dependent protein kinase in vitro, but greatly reduced activity was observed for these compounds with cells.6 Thus the reduced ability of compounds 9 and 10 to inhibit infection of hepatocytes as compared to parent TSP (Fig. 2) may also be due to decreased cellular penetration. These combined results are consistent with the target protein being intracellular. Decrease or loss of potency for the compounds in this study is likely due to their decreased permeability into the parasite. In any case, the two biotin-containing compounds were judged to have insufficient activity to move forward with affinity purification of the unknown protein target(s).
In summary, derivatives of TSP (1) were synthesized in order to assess whether structural variation at the piperidinyl nitrogen would give analogs that maintain the ability to inhibit sporozoite invasion, and thus are amenable to biotinylation and subsequent pull-down of TSP-binding sporozoite protein(s). It was found that structural variation at the piperidinyl nitrogen is tolerated. However, until the target protein can be identified and activity with the target determined directly, it is unclear if structural modifications to TSP such as with compounds 9 and 10 here result in reduced activity due to reduced target binding or due to reduced cellular penetration. Thus until the target of TSP can be identified and isolated, further structure–function studies to find more potent TSP-based inhibitors of sporozoite infectivity will be confounded by both target binding and cell penetration affecting activity. In addition, this work demonstrates that addition of large, biotin-containing substituents to the piperidine nitrogen is not likely a viable strategy to identify the cellular targets of TSP; biotin–linker–TSP conjugates 13 and 14 were inactive. Although it is possible the biotin conjugates might bind the protein target in cell lysate, where the barrier of cellular penetration is removed. Given the importance of identifying the target protein(s) of TSP in sporozoites, alternative methods involving the synthesis of bi-functional TSPs are being pursued. These TSPs will be designed to include a photoreactive aryl moiety to irreversibly bind the TSP to its target protein(s), and a small bioorthogonal reactive substituent on the piperidinyl ring, such as a propargyl group that is capable of coupling to an azide-linked biotin via click chemistry.
Acknowledgments
American Foundation for Pharmaceutical Education—2012 Predoctoral Fellowship to TRT.
American Chemical Society Division of Medicinal Chemistry Fellowship sponsored by Richard B. Silverman—2012 Predoctoral Fellowship to TRT.
Footnotes
Supplementary data
Supplementary data (detailed experimental methods and compound characterization) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2013.01.010.
References and notes
- 1.WHO. World Malaria Report 2011. World Health Organization; Geneva: 2011. [Last accessed 10/17/2012]. http://www.who.int/malaria/world_malaria_report_2011/en. [Google Scholar]
- 2.Diaz CA, Allocco J, Powles MA, Yeung L, Donald RGK, Anderson JW, Liberator PA. Mol Biochem Parasitol. 2006;146:78. doi: 10.1016/j.molbiopara.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Panchal D, Bhanot P. Antimicrob Agents Chemother. 2010;54:4269. doi: 10.1128/AAC.00420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor HM, McRobert L, Grainger M, Sicard A, Dluzewski AR, Hopp CS, Holder AA, Baker DA. Eukaryot Cell. 2010;9:37. doi: 10.1128/EC.00186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falae A, Combe A, Amaladoss A, Carvalho T, Menard R, Bhanot P. J Biol Chem. 2010;285:3282. doi: 10.1074/jbc.M109.070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biftu T, Feng D, Ponpipom M, Girotra N, Liang GB, Qian X, Bugianesi R, Simeone J, Chang L, Gurnett A, Liberator P, Dulski P, Leavitt PS, Crumley T, Misura A, Murphy T, Rattray S, Samaras S, Tamas T, Mathew J, Brown C, Thompson D, Schmatz D, Fisher M, Wyvratt M. Bioorg Med Chem Lett. 2005;15:3296. doi: 10.1016/j.bmcl.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 7.De Laszlo SE, Mantlo NB, Ponticello GS, Selnick HG, Liverton NJ. WO9705877 Worldwide Patent.
- 8.Kinzel O, Llauger-Bufi L, Pescatore G, Rowley M, Schultz-Fademrecht C, Monteagudo E, Fonsi M, Paz OG, Fiore F, Steinkuhler C, Jones P. J Med Chem. 2009;52:3453. doi: 10.1021/jm9004303. See also, Supplementary data for synthetic methods and compound characterization. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ishai D, Berger A. J Org Chem. 1952;17:1564. [Google Scholar]
- 10. [last accessed 10/17/2012];Piercenet product website. http://www.piercenet.com/browse.cfm?fldID=C78BA046-14C2-4533-8141-C096246FC78B.
- 11. [last accessed 10/17/2012];Piercenet product website. http://www.piercenet.com/browse.cfm?fldID=E2C9ED6C-AAC4-4755-AC33-BA209926523B.
- 12.Previous studies employed TSP from another source (see, Ref. 3), thus TSP was prepared and tested here to provide a direct comparison of activities with the TSP analogs as well as to confirm reported activity.