Abstract
IMPORTANCE
Financial incentives to physicians or patients are increasingly used, but their effectiveness is not well established.
OBJECTIVE
To determine whether physician financial incentives, patient incentives, or shared physician and patient incentives are more effective than control in reducing levels of low-density lipoprotein cholesterol (LDL-C) among patients with high cardiovascular risk.
DESIGN, SETTING, AND PARTICIPANTS
Four-group, multicenter, cluster randomized clinical trial with a 12-month intervention conducted from 2011 to 2014 in 3 primary care practices in the northeastern United States. Three hundred forty eligible primary care physicians (PCPs) were enrolled from a pool of 421. Of 25 627 potentially eligible patients of those PCPs, 1503 enrolled. Patients aged 18 to 80 years were eligible if they had a 10-year Framingham Risk Score (FRS) of 20% or greater, had coronary artery disease equivalents with LDL-C levels of 120 mg/dL or greater, or had an FRS of 10% to 20% with LDL-C levels of 140 mg/dL or greater. Investigators were blinded to study group, but participants were not.
INTERVENTIONS
Primary care physicians were randomly assigned to control, physician incentives, patient incentives, or shared physician-patient incentives. Physicians in the physician incentives group were eligible to receive up to $1024 per enrolled patient meeting LDL-C goals. Patients in the patient incentives group were eligible for the same amount, distributed through daily lotteries tied to medication adherence. Physicians and patients in the shared incentives group shared these incentives. Physicians and patients in the control group received no incentives tied to outcomes, but all patient participants received up to $355 each for trial participation.
MAIN OUTCOMES AND MEASURES
Change in LDL-C level at 12 months.
RESULTS
Only patients in the shared physician-patient incentives group achieved reductions in LDL-C levels statistically different from those in the control group (8.5 mg/dL; 95% CI, 3.8–13.3; P = .002). For comparison of all 4 groups, P < .001.
| Low-Density Lipoprotein Cholesterol Level |
Incentives Group
|
|||
|---|---|---|---|---|
| Shared | Physician | Patient | Control | |
| Mean reduction (95% CI), mg/dL | 33.6 (30.1–37.1) | 27.9 (24.9–31.0) | 25.1 (21.6–28.5) | 25.1 (21.7–28.5) |
| Baseline, mg/dL | 160.1 | 159.9 | 160.6 | 161.5 |
| 12 Months, mg/dL | 126.4 | 132 | 135.5 | 136.4 |
CONCLUSIONS AND RELEVANCE
In primary care practices, shared financial incentives for physicians and patients, but not incentives to physicians or patients alone, resulted in a statistically significant difference in reduction of LDL-C levels at 12 months. This reduction was modest, however, and further information is needed to understand whether this approach represents good value.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01346189
Cardiovascular disease (CVD) is the leading cause of death in the United States, and clinical trials indicate that taking statins (HMG-CoA reductase inhibitors) to lower cholesterol reduces the risk of myocardial infarction by about 30%.1 Despite proven benefits, the relatively low cost, once-a-day dosing, and few adverse effects, the population effectiveness of statins is limited for 2 reasons. First, physicians underprescribe statins or fail to intensify treatment when indicated.2, 3 Second, approximately half of patients prescribed statins discontinue usage within a year, even among those surviving acute coronary syndromes.4–6 Poor adherence is associated with worse outcomes, higher hospitalization and mortality rates, and increased health care costs among patients with CVD.4, 5, 7–9
Three developments over the past decade offer promise in improving medication adherence and other health behaviors. First, recent insights from behavioral economics have improved understanding of human motivation. Second, wireless technologies, such as “smart” pill bottles, provide a way to connect with patients at relevant times. Third, new payment approaches put physician income at risk for achieving population health outcomes.
One approach to increase attention to patient lipid management is to tie physician financial incentives to clinical goals. Pay for performance is widespread in health care, although to date such programs have had limited effect on physician behavior in the United States.10–12 In the United Kingdom, much larger incentives to hospitals resulted in short-term but not long-term reductions in mortality.13, 14 A second approach is to provide financial incentives to patients for clinical goals. Both of these approaches might be enhanced by applying insights from behavioral economics.15 In this study, we compared incentives to physicians, incentives to patients, and incentives shared between physicians and patients—all incorporating design elements from behavioral economics and all aimed at reducing levels of low-density lipoprotein cholesterol (LDL-C) among primary care patients with hyperlipidemia and elevated CVD risk.
Methods
This multicenter, cluster randomized clinical trial compared 4 approaches to reduce LDL-C levels among patients with high CVD risk. Participating primary care physicians (PCPs) from the University of Pennsylvania (Penn Medicine, Philadelphia), Geisinger Clinic (Danville, Pennsylvania), and Harvard Vanguard Medical Associates (Boston, Massachusetts) were randomly assigned to 1 of 4 study groups: control, physician incentives, patient incentives, and shared physician-patient incentives. Eligible and participating patients were allocated to the same group as their participating PCP. The interventions continued for 12 months, and patients were followed up for an additional 3 months. The primary outcome was change in LDL-C level at 12 months. The study protocol was approved by the institutional review boards of the University of Pennsylvania and Geisinger Health System and the Partners human research committee at Harvard Vanguard Medical Associates. The study ran from 2011 to 2014, when all recruited patients had completed 15 months of follow-up (see Trial Protocol in the Supplement).
Study Populations
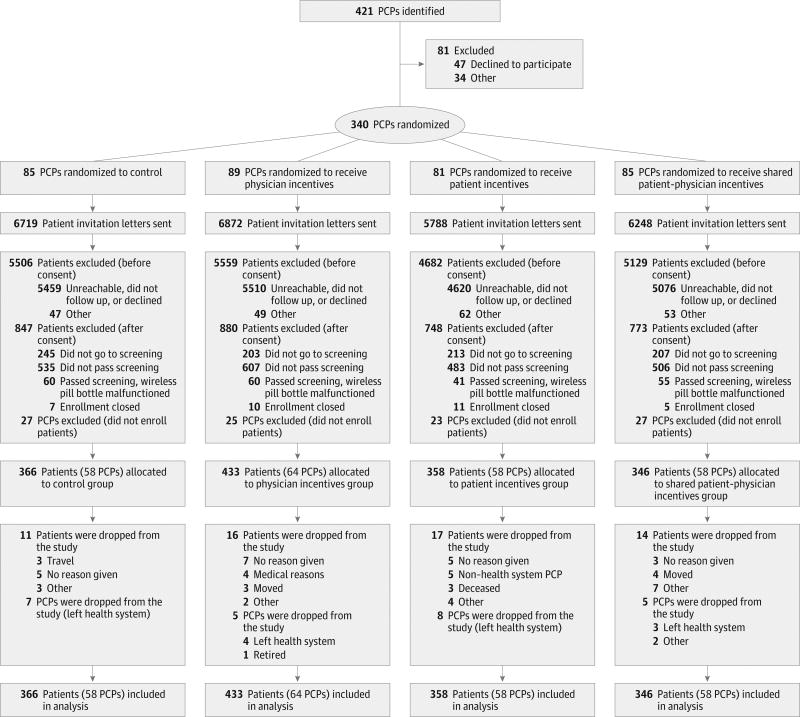
Primary care physicians practicing at each site were eligible if they had at least 5 patients likely to meet study inclusion criteria based on recent electronic health record data (Figure 1). Study coordinators conducted in-person sessions with eligible and interested physicians to describe study procedures, review patient lists, and conduct a baseline survey. A waiver of written informed consent for physicians was granted by the review boards at each site. If they agreed to participate, PCPs were randomized, informed of their assigned group, and provided with a handout detailing study procedures.
Figure 1. Flow Diagram of Physician and Patient Progress Through the Trial.
PCPs indicates primary care physicians.
Patients were eligible to participate if they were aged 18 to 80 years; had a designated consenting PCP; and had 1 of the following: a 10-year Framingham Risk Score (FRS)16 of 20% or greater, coronary artery disease (CAD) equivalents (diabetes, peripheral artery disease, ischemic CVD, arteriosclerotic CVD, stroke or transient ischemic attack, coronary artery bypass graft, coronary stenting, or coronary bypass anastomosis) with an LDL-C level of 120 mg/dL or greater (high-risk participants), or an FRS of 10% to 20% with an LDL-C level of 140 mg/dL (medium-risk participants). (To convert LDL-C to mmol/L, multiply by 0.0259.) Patients were excluded if they had previously experienced adverse effects to statins, had terminal illness making aggressive lipid management unsuitable, had alanine aminotransferase values of 80 U/L or greater (to convert to µkat/L, multiply by 0.0167), had active or progressive liver disease, or were unwilling or unable to provide informed consent.
Letters to potentially eligible patients described the study and enrollment using the University of Pennsylvania web plat-form Way to Health (described next) or by calling study staff. Patients not responding within 10 days were contacted by telephone by research staff unaware of the PCP group assignment. Patients could consent to participate online or through a paper consent form. Consenting patients completing baseline laboratory measures were enrolled if their LDL-C level met or exceeded the FRS-specific recruitment threshold. The assigned incentive design was then explained.
The study was executed on a customizable web-based plat-form that supports recruitment, consent, randomization, and data collection for clinical trials. The platform automates connections among peripheral devices (eg, electronic pill bottles), feedback to patients or physicians (via text, email, voice, or summary reports), self-administered surveys, and transfers of financial incentives to participants.17
Interventions
All patients were sent an electronic pill bottle (Vitality GlowCap) for statins. When opened, electronic pill bottles wirelessly transmit a signal to the web platform. Electronic pill bottles were set so they did not provide audible or visual reminders. Each patient in the 3 intervention groups was assigned a quarterly goal to reduce LDL-C levels by 10 mg/dL or more from the previous quarter’s target or to achieve or maintain an LDL-C level less than 100 mg/dL for high-risk participants or 130 mg/dL for medium-risk participants. Achievement of the quarterly goal made patients, physicians, or both eligible for incentives, which is likely to be achieved if patients are fully adherent to statins.18 Physicians in the 3 intervention groups received monthly reports of their participating patients’ LDL-C levels, and adherence and progress could be tracked by patients on the web platform if they chose.
Physicians in the physician incentives group accrued quarterly payments of $256 ($1024 maximum annual payment)for each enrolled patient meeting the quarterly goal, paid semi-annually. The design of this group overcomes some common limitations of existing pay-for-performance programs by providing more frequent information on relevant behaviors (monthly reports on adherence and LDL-C level), larger incentive payments, and separation of incentives from other larger streams of money to increase saliency.19 Patients in the physician incentives group received no goal-based payments.
Physicians in the patient incentives group received no payments; instead, their enrolled patients participated in an automatic daily lottery. Each patient was assigned a 2-digit number for the trial. Each day, a 2-digit number was randomly generated by the web platform and compared with each patient’s number. If both digits matched (1 in 100 chance), the patient could win $100. If 1 digit matched (18 in 100 chance), the patient could win $10, but patients were eligible to win only if they had taken their statin the day before. A patient fully adherent in statin use could therefore expect to earn a mean of $2.80 per day, or $1022 per year, equivalent to the physician payment for a patient meeting each quarterly goal.
This design incorporates several behavioral economic features. First, little additional effort was required from patients. Information was uploaded automatically by the electronic pill bottles, and messaging was pushed to patients. Second, patients were engaged daily, coinciding with typical daily medication use for lipid management. Third, “regret” lotteries were used: patients whose number came up in the lottery but who had not been adherent the prior day received a message indicating that they would have won if only they had taken their medication—a message designed to create a highly motivating sense of regret.20–23 Fourth, the relatively high probability of a small reward and the lower probability of a larger reward appealed to those focused on either the frequency of winning or the magnitude of the reward, and frequent smaller rewards increased the opportunity for regret. Fifth, although patients’ lottery winnings accrued to a personal account visible on the web platform, these earnings were received quarterly and only if patients met their quarterly LDL-C target. This approach leverages loss aversion—the tendency to be more motivated to avoid losses than achieve similarly sized gains24, 25—and offered the additional advantage that only verified reductions in LDL-C level were incentivized.
Physicians in the shared physician-patient incentives group received direct payments half the size of those in the physician incentives group, for a maximum total payment of $512 per enrolled patient. Patients in the shared physician-patient incentives group were also eligible for the daily lottery, but with payments of half the size of those in the patient incentives group. Thus, the total possible payouts were the same for all incentive groups.
Physicians and patients in the control group received no goal-based incentives. In all 4 groups, both physicians and patients received participation payments. Physicians were compensated via relative value unit credit to their practices for the initial and 12-month follow-up visits. Physicians who also participated in poststudy qualitative interviews received additional relative value unit credit. Patients were paid $75 for the baseline visit ($50 for blood draw and $25 for questionnaire completion); $40 each for blood draws at 3, 6, and 9 months; and $80 each for blood draws at 12 and 15 months (a total of up to $355).
Randomization Procedures
Within each site, physicians were randomized evenly to the 4 groups using variable permuted blocks. Study participants and operational staff were not blinded to group assignment, because knowledge of the incentives is essential to their mechanism, but study investigators and data analysts remained blinded until all follow-up data were obtained and primary analytic strategies were finalized.
End Points and Assessments
The primary end point was change in LDL-C level from enrollment to 12 months, measured using direct LDL-C26 from patient blood samples. Secondary end points included daily adherence measured by electronic pill bottles and change in LDL-C level from baseline to 15 months. Achievement of LDL-C goal and medication intensification were also assessed post hoc; the latter was defined as the initiation of a statin in a patient previously not taking statins or an increase in dose or drug potency in a patient currently taking a statin, using electronic physician prescribing records.
Baseline Variables
Physician information included hire date, demographics, training, and certification. Patient information included demographics, socioeconomic status, current medication use, and electronic health record data. Information on race/ethnicity was obtained through patient- or physician-completed surveys, with options defined by the investigators, to allow for assessment of any differences in intervention effectiveness by race/ethnicity.
Statistical Analysis
Although randomization occurred at the physician level, the patient was the unit of analysis for all primary and secondary outcomes. The primary analysis used mixed-effects models27 of direct LDL-C measured at baseline and at 3, 6, 9, 12, and 15 months, with random effects to adjust for clustering within physicians and correlation in repeated LDL-C assessments over time and fixed-effect indicators for study site, group, and time. Pairwise comparisons of the primary outcome for each active group against the usual care (control) group used Bonferroni adjustment of tests for interaction to maintain the type I error rate; corrected P values are reported and all P values are 2-sided. Secondary outcomes were tested using the nominal .05 type I error rate without correction. Secondary analyses included longitudinal binary mixed-effect regression modeling of quarterly LDL-C goal achievement; quarterly adherence, dichotomized at 80%7; and intensification. Adherence was calculated as the percentage of days with an electronic pill bottle opening; groups were compared via mixed-effects models similar to those for LDL-C level. Estimated rates of LDL-C goal achievement, adherence, and intensification by group were derived from these adjusted models, with site effects weighted by the relative proportion of participants.
Approximately 7% of patients were missing follow-up LDL-C values. Multiple imputation with 5 imputations was used, achieving 97% to 99% relative efficiency and ensuring in-range values. All analyses were conducted on each imputed data set; results were combined using the standard rules from Rubin.28 Primary results using imputed data were compared with those obtained from complete and all available data.
Power calculations were derived assuming comparison of each incentive group with control using a Bonferroni-corrected29 type I error of .017, followed by comparison of any incentive groups that showed significant differences from control using a sequential Holm-Bonferroni30 approach. The study was designed to have at least 80% power to detect differences in change in LDL-C level between any incentive group and control of 15 mg/dL and between any 2 incentive groups of 10 mg/dL. A conservative assumption of intraclass correlation coefficient of 0.04 within physicians was used. Simulation studies incorporating these parameters indicated the need for 1400 participants (350 per group). No interim analyses were planned or conducted. A data and safety monitoring board met prior to study launch and then approximately every 6 months to review study progress and any adverse events.
Analyses were conducted using SAS version 9.3 (SAS Institute) and Stata version 13.9 (StataCorp).
Results
Three hundred forty physicians were randomized; 25 627 of their patients were invited to participate, and 1503 were allocated to the groups of their primary care physicians (Figure 1). Most potentially eligible patients who did not enroll were unreachable, did not follow up after initial contact, or declined to participate. A small number of physicians and patients were dropped from the study, generally because they left the practices, but all randomized physicians and allocated patients were analyzed; 102 physicians had no allocated patients and so contributed no information to the trial.
Demographic characteristics of enrolled physicians (Table 1) and patients (Table 2) were similar across treatment groups. Mean baseline FRS among patients was 20%; approximately 35% of patients had CAD or equivalent. About 50% of patients were not taking statins at enrollment. Ninety-one percent of patients had LDL-C measured at the 12-month assessment.
Table 1.
Physician Characteristics by Groupa
| Total (N = 238) |
Control (n = 58) |
Patient Incentives (n = 58) |
Physician Incentives (n = 64) |
Shared Patient-Physician Incentives (n = 58) |
|
|---|---|---|---|---|---|
| No. of enrolled patients, median (IQR) | 5.0 (2.0–9.0) | 4.5 (3.0–9.0) | 4.0 (2.0–10.0) | 5.0 (2.5–9.0) | 5.0 (3.0–9.0) |
| Years in practice, median (IQR) | 18.0 (10.0–26.0) | 21.0 (9.00–28.0) | 16.0 (9.00–26.0) | 19.0 (10.0–27.0) | 16.0 (12.0–23.0) |
| Female, No. (%) | 89 (37.4) | 24 (41.4) | 20 (34.5) | 21 (32.8) | 24 (41.4) |
| Race/ethnicity, No. (%) | |||||
| White, non-Hispanic | 195 (82.98) | 46 (79.31) | 51 (87.93) | 52 (83.87) | 46 (80.70) |
| African American, non-Hispanic | 5 (2.13) | 1 (1.72) | 3 (5.17) | 1 (1.61) | 0 |
| Other non-Hispanic | 32 (13.62) | 10 (17.24) | 4 (6.90) | 9 (14.52) | 9 (15.79) |
| Hispanic | 3 (1.28) | 1 (1.72) | 0 | 0 | 2 (3.51) |
| Missing | 3 (1.26) | 0 | 0 | 2 (3.13) | 1 (1.72) |
Abbreviation: IQR, interquartile range.
No differences across columns were statistically significant.
Table 2.
Patient Characteristics by Groupa
| Total (N = 1503) |
Control (n = 366) |
Patient Incentives (n = 358) |
Physician Incentives (n = 433) |
Patient-Physician Incentives (n = 346) |
|
|---|---|---|---|---|---|
| Age, mean (SD), y | 61.99 (8.70) | 61.70 (8.89) | 62.41 (8.63) | 61.86 (8.67) | 62.03 (8.64) |
| Female sex | 639 (42.63) | 161 (44.11) | 164 (45.94) | 179 (41.53) | 135 (39.02) |
| Missing | 4 (0.26) | 0 | 1 (0.28) | 2 (0.46) | 1 (0.29) |
| Race/ethnicity, No. (%) | |||||
| White, non-Hispanic | 1199 (80.36) | 289 (79.40) | 265 (74.23) | 360 (83.53) | 285 (83.82) |
| African American, non-Hispanic | 232 (15.55) | 61 (16.76) | 72 (20.17) | 57 (13.23) | 42 (12.35) |
| Other non-Hispanic | 31 (2.08) | 7 (1.92) | 10 (2.80) | 8 (1.86) | 6 (1.76) |
| Hispanic | 30 (2.01) | 7 (1.92) | 10 (2.80) | 6 (1.39) | 7 (2.06) |
| Missing | 11 (0.73) | 2 (0.55) | 1 (0.28) | 2 (0.46) | 6 (1.73) |
| Annual household income, No. (%), $ | |||||
| <50 000 | 635 (43.64) | 163 (45.92) | 145 (41.43) | 193 (45.73) | 134 (40.85) |
| 50 000 to 100 000 | 523 (35.95) | 124 (34.93) | 129 (36.86) | 144 (34.12) | 126 (38.41) |
| >100 000 | 297 (20.41) | 68 (19.15) | 76 (21.71) | 85 (20.14) | 68 (20.73) |
| Education, No. (%) | |||||
| <College | 486 (32.49) | 129 (35.34) | 107 (29.97) | 148 (34.34) | 102 (29.74) |
| Some college | 433 (28.94) | 105 (28.77) | 102 (28.57) | 118 (27.38) | 108 (31.49) |
| College and postcollege graduate | 577 (38.57) | 131 (35.89) | 148 (41.46) | 165 (38.28) | 133 (38.78) |
| Marital status, No. (%) | |||||
| Single | 225 (15.05) | 59 (16.16) | 59 (16.53) | 64 (14.85) | 43 (12.57) |
| Married | 992 (66.35) | 231 (63.29) | 236 (66.11) | 286 (66.36) | 239 (69.88) |
| Other | 278 (18.60) | 75 (20.55) | 62 (17.37) | 81 (18.79) | 60 (17.54) |
| FRS, mean (SD), %b | 19.8 (8.7) | 20.1 (8.7) | 19.7 (8.6) | 20.1 (9.0) | 19.1 (8.7) |
| Pre-existing CAD, No. (%) | 516 (34.49) | 148 (40.44) | 110 (30.81) | 143 (33.49) | 115 (33.24) |
| Taking cholesterol-reducing medications at baseline, No. (%) | 712 (47.40) | 166 (45.46) | 180 (52.02) | 166 (46.37) | 200 (46.19) |
Abbreviations: CAD, coronary artery disease; FRS, Framingham Risk Score.
No differences across columns were statistically significant.
Range: 10%-20% represents medium risk of developing cardiovascular disease within 10 years; ≥20%, high risk.
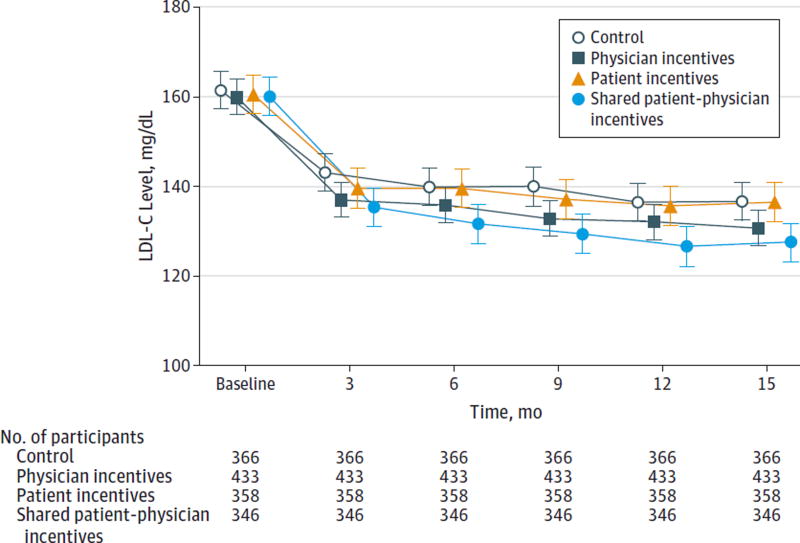
Patients in the control group had a mean reduction in LDL-C level of 25.1 mg/dL (95% CI, 21.7–28.5) from baseline (161.5 mg/dL) to 12 months (136.4 mg/dL) (Table 3). Patients in the shared physician-patient incentives group achieved a mean reduction in LDL-C level of 33.6 mg/dL (95% CI, 30.1–37.1) from baseline (160.1 mg/dL) to 12 months (126.4 mg/dL); patients in the physician incentives group achieved a mean reduction of 27.9 mg/dL (95% CI, 24.9–31.0) from baseline (159.9 mg/dL) to 12 months (132.0 mg/dL); and patients in the patient incentives group achieved a mean reduction of 25.1 mg/dL (95% CI, 21.6–28.5) from baseline (160.6 mg/dL) to 12 months (135.5 mg/dL) (P < .001 for comparison of all 4 groups). The observed relative rankings of these groups persisted at each assessment, including 3 months after the cessation of the intervention (Figure 2). Only patients in the shared physician-patient incentives group achieved a reduction in LDL-C level statistically different from those in the control group (8.5 mg/dL; 95% CI, 3.8–13.3, P = .002) (Table 3).
Table 3.
LDL-C Change, Adherence, and Medication Intensification by Intervention Groupa
| Control | Patient Incentives | Physician Incentives | Shared Patient-Physician Incentives |
|
|---|---|---|---|---|
| 12-mo Reduction in LDL-C level (95% CI), mg/dL [N = 1503] | 25.1 (21.7 to 28.5) | 25.1 (21.6 to 28.5) | 27.9 (24.9 to 31.0) | 33.6 (30.1 to 37.1) |
| Difference relative to control | 0.1 (−4.9 to 4.8) | 2.8 (−1.7 to 7.4) | 8.5 (3.8 to 13.3) | |
| P value | >.99 | .66 | .002 | |
| Not taking medication at baseline (n = 791) | 17.7 (13.5 to 21.8) | 16.2 (11.9 to 20.4) | 21.2 (17.4 to 25.1) | 24.8 (20.3 to 29.3) |
| P value | .62 | .21 | .02 | |
| Taking medication at baseline (n = 712) | 34.0 (28.7 to 39.2) | 35.5 (30.1 to 40.8) | 35.7 (31.1 to 40.5) | 41.9 (36.8 to 47.1) |
| P value | .70 | .62 | .03 | |
| Achievement of LDL-C goal, % (95% CI)b | 36 (31 to 42) | 40 (34 to 46) | 40 (35 to 46) | 49 (43 to 55) |
| P value | .37 | .35 | .003 | |
| Mean medication adherence, % (95% CI)c | 27 (23 to 31) | 34 (31 to 38) | 31 (27 to 38) | 39 (32 to 44) |
| P value | .01 | .22 | <.001 | |
| Not taking medication at baseline | 13 (9 to 18) | 17 (13 to 21) | 18 (13 to 23) | 21 (17 to 25) |
| P value | .23 | .19 | .01 | |
| Taking medication at baseline | 43 (38 to 47) | 54 (49 to 59) | 46 (42 to 50) | 56 (51 to 61) |
| P value | .001 | .25 | <.001 | |
| Patients with medication intensification, % (95% CI)d | 27 (22 to 32) | 25 (20 to 31) | 33 (28 to 38) | 38 (32 to 44) |
| P value | .67 | .09 | .004 | |
| Not taking medication at baseline | 28 (21 to 35) | 23 (17 to 31) | 33 (26 to 40) | 44 (36 to 52) |
| P value | .41 | .29 | .003 | |
| Taking medication at baseline | 26 (20 to 33) | 26 (19 to 34) | 33 (27 to 40) | 34 (26 to 42) |
| P value | .96 | .15 | .14 |
Abbreviations: CAD, coronary artery disease; FRS, Framingham Risk Score; LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: To convert LDL-C to mmol/L, multiply by 0.0259.
P values reflect comparison with control; for pairwise comparisons of the primary outcome of reduction in 12-month LDL-C levels, Bonferroni adjusted P values are reported. Multiple imputation was used for the approximately 9% of participants missing ≥1 follow-up LDL-C measurements. Achievement of LDL-C goal and medication intensification were analyzed post hoc, with percentages generated from mixed models that adjusted for study site and treatment group as fixed effects and physician as a random effect.
Set at the higher of 40 mg/dL below the participant’s baseline LDL-C value, 100 mg/dL for high-risk participants (10-year FRS ≥20% or CAD equivalents with enrollment LDL-C ≥120 mg/dL), or 130 mg/dL for medium-risk participants (10-year FRS 10%-20% with enrollment LDL-C ≥140 mg/dL).
Calculated as a proportion (total number of pill bottle opening days during the 12-month study period divided by 365).
Defined as the initiation of a statin for a participant previously not taking statins or an increase in dose or drug potency for a participant currently taking a statin, using electronic physician prescribing records.
Figure 2. Mean LDL-C Levels by Quarter in Intervention and Control Groups.
To convert low-density lipoprotein cholesterol (LDL-C) to mmol/L, multiply by 0.0259. Error bars indicate 95% CIs.
Patients in the shared physician-patient incentives group also achieved LDL-C reductions significantly different from those of the patient incentives group (8.6 mg/dL, 95% CI, 3.7–13.5, P = .003) and the physician incentives group (5.7 mg/dL, 95% CI, 1.1–10.3, P = .03). In post hoc analyses at 12 months, 49% of patients in the shared physician-patient incentives group had achieved their LDL-C goal compared with 40% in physician incentives, 40% in patient incentives, and 36% in control (P = .03 for comparison of all 4 groups). At 15 months, 3 months after stopping all incentives, LDL-C values remained stable. The improvement in LDL-C was qualitatively larger in the one-half of participants taking statins at study entry (control: 34.0 mg/dL, patient incentives: 35.5 mg/dL, physician incentives: 35.7 mg/dL, shared physician-patient incentives: 41.9 mg/dL) than in the one-half of participants not taking statins at entry (control: 17.7 mg/dL, patient incentives: 16.2 mg/dL, physician incentives: 21.2 mg/dL, shared physician-patient incentives: 24.8 mg/dL).
By 12 months, 38% of patients in the shared physician-patient incentives group had their medication intensified compared with 33% in physician incentives, 25% in patient incentives, and 27% in control; only the difference between shared physician-patient incentives and control was statistically significant (P = .004) (Table 3).
Over the 12-month study period, incentive payments totaled a mean of $3246 per enrolled physician in physician incentives, $172 per enrolled patient in patient incentives, and $1597 per enrolled physician and $118 per enrolled patient in shared physician-patient incentives. Physician incentives reflect quarterly payments of $256 or $128 for each participating patient meeting that quarter’s LDL-C goal in the physician incentive or shared incentive group, respectively. Patient incentives reflect lottery accumulations over adherent days during each quarter—an expected value of $2.80 per day or $1.40 per day in the patient incentive and shared incentive groups, respectively, and were distributed only if the patient also met that quarter’s LDL-C goal. Posttrial review of payment logs revealed that occasionally physicians were inadvertently paid despite their patients not meeting goal. These payments amounted to approximately 5.4% of the total payout.
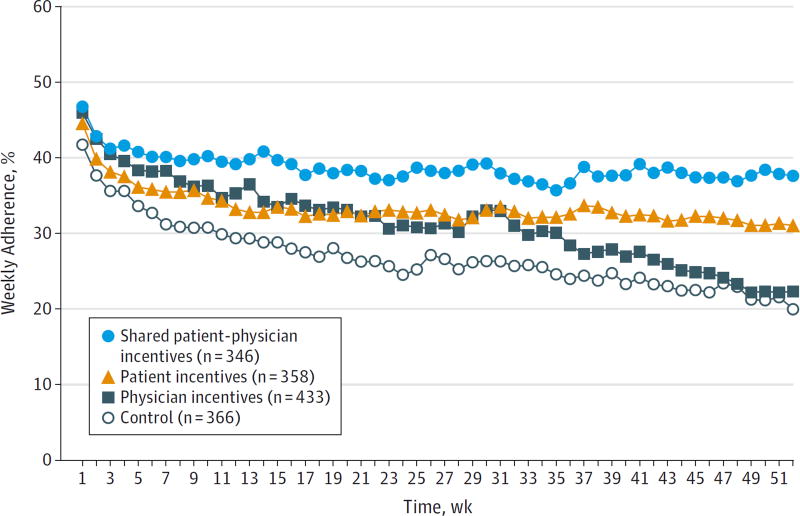
Weekly adherence rates in all groups declined over the study period (Figure 3), but adherence remained highest among patients in the shared physician-patient incentives group. Mean total adherence over the 12 months was 39% in shared physician-patient incentives, 31% in physician incentives, 34% in patient incentives, and 27% in control (P < .001) (Table 3). Patients in the shared physician-patient incentives group were more likely to exhibit sustained adherence (defined as ≥80%) than patients in control (P = .002) and patients in physician incentives (P = .002); there were no significant differences in adherence between patients in the shared physician-patient incentives group and patient incentives group. Patients who were taking statins at study entry showed substantially increased medication adherence compared with control if they were assigned to patient incentives or shared physician-patient incentives (control: 43%, patient incentives: 54%, physician incentives: 46%, shared physician-patient incentives: 56%).
Figure 3. Mean Weekly Medication Adherence by Intervention Group.
Adherence was calculated by dividing the number of pill bottle openings per week by 7. Standard deviation for the shared patient-physician incentives group was 1.8%; patient incentives, 2.4%; physician incentives, 5.5%; and control, 4.4%.
Discussion
This trial, which to our knowledge is the first to test physician, patient, and shared incentives with equivalent value, has 4 findings. First, an approach using shared financial incentives for patients and physicians reduced LDL-C level more than control and more than physician-only or patient-only incentives. This outcome is supported by the finding that 49% of the patients in the shared patient and physician incentive group achieved the LDL-C goal in comparison with 36% to 40% in the other 3 groups. The superiority of a shared approach makes sense because success at LDL-C reduction is likely to be driven by both provision of medication by physicians and patient adherence to that medication. Consistent with this hypothesis, patients in the shared group were more likely to receive medication intensification and to adhere to medication use than patients in other groups. A second finding, however, was that neither physician nor patient incentives on their own lowered LDL-C level significantly more than the control.
The absence of an effect from physician incentives alone is important. Physician financial incentives have been deployed for decades to motivate improved processes or outcomes of care, and it seems intuitive and self-evident that paying physicians more for better quality should improve performance. The lack of improvement in LDL-C level, despite potential physician incentives of up to $1024 per patient, offers the first controlled evidence that adding these incentives to a fee-for-service payment model may not improve medication-related intermediate outcomes.
Third, although previous studies have shown effectiveness of daily lotteries for medication adherence,23, 31 we observed a modest effect on LDL-C level. When results were stratified by statin use at study start, however, patients taking statins at baseline showed large increases in adherence if they received incentives. This suggests that the patient incentive was ineffective at getting patients started with statins but effective at increasing adherence among those patients already taking statins. Because more than half of patients were not taking statins at baseline, the overall effect of the incentives on LDL-C level may therefore have been dampened.
Fourth, although medication adherence was higher in the shared incentive and patient incentive groups, it was low in all groups. Many previous studies have documented poor medication adherence among patients with chronic disease,4, 32, 33 and hyperlipidemia is without the immediate symptoms that might motivate adherence. In addition, by targeting patients at higher-than-average cardiovascular risk who are not at LDL-C goals, we selected for patients who were likely nonadherent.
Most previous evaluations of pay-for-performance programs have involved observational studies, some of which lacked control groups or had other significant methodological limitations.12 These studies typically have shown modest effects but were limited by either small incentive amounts per patient (for example, $1 for each percentage point increase in the rate of patients referred for mammography)34 or “all-or-nothing” threshold goals. In addition, pay-for-performance programs typically have used standard economic incentives in which participants were simply offered fixed amounts for achieving a particular outcome or behavior, rather than leveraging behavioral economic principles.15, 35 The most relevant randomized clinical trial to date testing the effect of physician incentives in improving outcomes used 4 groups aimed at improving adherence to hypertension guidelines within the Veterans Affairs system: incentives provided to individual physicians, practice-level incentives split equally between physicians and nonphysician providers, a combined incentive program in which physicians received both an individual physician performance incentive and a share of the practice incentive, and control. Only the physician incentive group was significantly more effective than control, and effects on blood pressure were modest (about 9% more patients met the recommended blood pressure targets) and did not persist beyond 12 months.36 The maximum size of incentives for primary care physicians was approximately 1.6% of annual income, with nurse team members receiving more than $500 in incentives in the team and combined groups; while incentives were relatively small, this reflected the real-world constraints of the program and, as this study illustrates, increasing the size of a quality incentive per patient may not be sufficient to change physician behavior in the context of a fee-for-service payment system that still predominantly rewards physicians for volume.
This study has limitations. Patients in the control group received electronic pill bottles and may have been more adherent than is typical for similar nonenrolled patients because they were under observation. The mean LDL-C reduction of 26.6 mg/dL among control patients could be explained by patients knowing their actions were being witnessed or by regression to the mean. Although either the physician or patient incentive might be more effective when compared with a true “usual-care” control without electronic pill bottles, electronic pill bottles were included in the control group to ensure that measurement of adherence was the same across all groups. This bias is conservative in its main effect, although it should temper conclusions about the ineffectiveness of the physician and patient incentives if either were used alone. In addition, all participants received up to $355 in payments for completing basic study milestones; these participation incentives may have led to a significantly more motivated group of participants overall than would occur with a true usual-care comparison group.
Second, more than one-half of patients were not taking statins at baseline, so the intervention relied on improving statin initiation and not just adherence. Third, a mean of only about 6 patients per physician enrolled, limiting the total size of the potential rewards to physicians from this initiative and thereby reducing the likelihood they would exert themselves to win those rewards. Fourth, adherence information came from pill bottle opening. Patients receiving incentives (patient incentives or shared incentives) had more motivation to use their bottles assiduously and report malfunctions; medication adherence may be relatively understated in the control and physician incentives groups. Nevertheless, the primary outcome was not adherence, but reduction in LDL-C level.
Fifth, this study emphasized achieving LDL-C targets, consistent with existing guidelines for lipid management.37 As this study was concluding, new guidelines were issued for lipid management that deemphasized specific LDL-C goals,38 which could have altered the management of patients in this trial; however, those guidelines were introduced toward the end of the trial and were unlikely to affect process or outcomes differentially across groups. Our analysis of LDL-C goal achievement was conducted post hoc. Sixth, the study enrolled only about 6% of the patients eligible, and although all participants were high risk, those who participated likely were not a representative sample of all such patients. Finally, given the relatively modest effect size, the intervention may not be cost-effective; a follow-up cost-effectiveness analysis will be conducted.
Conclusions
In primary care practices, shared financial incentives for physicians and patients, but not incentives to physicians or patients alone, resulted in a statistically significant difference in reduction of LDL-C levels at 12 months. This reduction was modest, however, and further information is needed to understand whether this approach represents good value.
Supplementary Material
Acknowledgments
Funding/Support: This study received support from the National Institute on Aging (RC4 AG039114 to Drs Asch and Volpp).
Role of the Funder/Sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Asch and Volpp had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Asch, Troxel, Stewart, Jones, Rosenthal, Volpp.
Acquisition, analysis, or interpretation of data: Asch, Troxel, Stewart, Sequist, Jones, Hirsch, Hoffer, Zhu, Wang, Hodlofski, Frasch, Weiner, Finnerty, Gangemi, Volpp.
Drafting of the manuscript: Asch, Troxel.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Troxel, Zhu, Wang.
Obtained funding: Asch, Volpp.
Administrative, technical, or material support: Asch, Sequist, Jones, Hirsch, Hoffer, Hodlofski, Weiner, Finnerty, Gangemi, Volpp.
Study supervision: Asch, Stewart, Sequist, Jones, Hoffer, Frasch, Volpp.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Asch and Volpp reported being principals and owners of VAL Health. Dr Troxel reported serving on the scientific advisory board of VAL Health. Dr Jones reported having received grants from Geisinger Health System, Merck, AstraZeneca, Genentech, and the National Association of Chain Drug Stores, and other support from the Academy of Managed Care Pharmacy. Dr Volpp reported having served as a consultant for CVS Caremark and having received grants from CVS Caremark, Humana, Merck, Weight Watchers, Discovery (South Africa). No other disclosures were reported.
Additional Contributions: We thank Ronald Barg, MD, and Charles F. Orellana, MD (University of Pennsylvania), Thomas R. Graff, MD (Chartis Group), and Thomas Isaacs, MD (Atrius Health), for help fielding this study. We also thank Constantine Gatsonis, PhD (Brown University), Donald Lloyd-Jones, MD, ScM (Northwestern University), and Eugene Z. Oddone, MD, MHS (Duke University), for their service on the study’s data and safety monitoring board. All but Dr Orellana received compensation for this assistance.
References
- 1.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Austin PC, Mamdani MM, Juurlink DN, Alter DA, Tu JV. Missed opportunities in the secondary prevention of myocardial infarction: an assessment of the effects of statin underprescribing on mortality. Am Heart J. 2006;151(5):969–975. doi: 10.1016/j.ahj.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Albert NM, Birtcher KK, Cannon CP, et al. Factors associated with discharge lipid-lowering drug prescription in patients hospitalized for coronary artery disease (from the Get With the Guidelines database) Am J Cardiol. 2008;101(9):1242–1246. doi: 10.1016/j.amjcard.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 5.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166(17):1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 6.Hudson M, Richard H, Pilote L. Parabolas of medication use and discontinuation after myocardial infarction: are we closing the treatment gap? Pharmacoepidemiol Drug Saf. 2007;16(7):773–785. doi: 10.1002/pds.1414. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn DF, Dobson RT, Blackburn JL, Wilson TW, Stang MR, Semchuk WM. Adherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors following a first cardiovascular event: a retrospective cohort study. Can J Cardiol. 2005;21(6):485–488. [PubMed] [Google Scholar]
- 9.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002;88(3):229–233. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal MB, Frank RG, Li Z, Epstein AM. Early experience with pay-for-performance: from concept to practice. JAMA. 2005;294(14):1788–1793. doi: 10.1001/jama.294.14.1788. [DOI] [PubMed] [Google Scholar]
- 11.Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care? Ann Intern Med. 2006;145(4):265–272. doi: 10.7326/0003-4819-145-4-200608150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Houle SK, McAlister FA, Jackevicius CA, Chuck AW, Tsuyuki RT. Does performance-based remuneration for individual health care practitioners affect patient care? a systematic review. Ann Intern Med. 2012;157(12):889–899. doi: 10.7326/0003-4819-157-12-201212180-00009. [DOI] [PubMed] [Google Scholar]
- 13.Sutton M, Nikolova S, Boaden R, Lester H, McDonald R, Roland M. Reduced mortality with hospital pay for performance in England. N Engl J Med. 2012;367(19):1821–1828. doi: 10.1056/NEJMsa1114951. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen SR, Meacock R, Turner AJ, et al. Long-term effect of hospital pay for performance on mortality in England. N Engl J Med. 2014;371(6):540–548. doi: 10.1056/NEJMoa1400962. [DOI] [PubMed] [Google Scholar]
- 15.Loewenstein G, Volpp KG, Asch DA. Incentives in health: different prescriptions for physicians and patients. JAMA. 2012;307(13):1375–1376. doi: 10.1001/jama.2012.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosworth HB, Almirall D, Weiner BJ, et al. The implementation of a translational study involving a primary care based behavioral program to improve blood pressure control: the HTN-IMPROVE study protocol (01295) Implement Sci. 2010;5:54. doi: 10.1186/1748-5908-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asch DA, Volpp KG. On the way to health. LDI Issue Brief. 2012;17(9):1–4. [PubMed] [Google Scholar]
- 18.Rosenson RS. Statins: actions, side effects, and administration. [Accessed September 4, 2015];UpToDate. http://www.uptodate.com/contents/statins-actions-side-effects-and-administration?source=machineLearning&search=statin+efficacy&selectedTitle=1∼150 §ionRank=1&anchor=H3#H3.
- 19.Read D, Loewenstein G, Rabin M. Choice bracketing. J Risk Uncertain. 1999;19(1):171–197. [Google Scholar]
- 20.Connolly T, Butler DU. Regret in economic and psychological theories of choice. J Behav Decis Making. 2006;19(2):139–154. [Google Scholar]
- 21.Chapman GB, Coups EJ. Emotions and preventive health behavior: worry, regret, and influenza vaccination. Health Psychol. 2006;25(1):82–90. doi: 10.1037/0278-6133.25.1.82. [DOI] [PubMed] [Google Scholar]
- 22.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmel SE, Troxel AB, Loewenstein G, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J. 2012;164(2):268–274. doi: 10.1016/j.ahj.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47(2):263–292. [Google Scholar]
- 25.Thaler RH, Tversky A, Kahneman DR, Schwartz A. The effect of myopia and loss aversion on risk taking: an experimental test. Q J Econ. 1997;112(2):647–661. [Google Scholar]
- 26.Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem. 2002;48(2):236–254. [PubMed] [Google Scholar]
- 27.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 28.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: J. Wiley & Sons; 1987. [Google Scholar]
- 29.Luczak K, Piestrzeniewicz K, Maciejewski M, et al. Long-lasting durability of Carpentier-Edwards pericardial bovine bioprosthesis in mitral position: a case report of unfavourable post redo valve surgery course. Kardiol Pol. 2010;68(7):806–808. [PubMed] [Google Scholar]
- 30.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 31.Volpp KG, Loewenstein G, Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 33.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Grady KE, Lemkau JP, Lee NR, Caddell C. Enhancing mammography referral in primary care. Prev Med. 1997;26(6):791–800. doi: 10.1006/pmed.1997.0219. [DOI] [PubMed] [Google Scholar]
- 35.Loewenstein G, Asch DA, Volpp KG. Behavioral economics holds potential to deliver better results for patients, insurers, and employers. Health Aff (Millwood) 2013;32(7):1244–1250. doi: 10.1377/hlthaff.2012.1163. [DOI] [PubMed] [Google Scholar]
- 36.Petersen LA, Simpson K, Pietz K, et al. Effects of individual physician-level and practice-level financial incentives on hypertension care: a randomized trial. JAMA. 2013;310(10):1042–1050. doi: 10.1001/jama.2013.276303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 38.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.