Abstract
Novel, tumor-selective therapies are needed to increase the survival rate of pancreatic cancer patients. K-Ras-mutant-driven NAD(P)H:quinone oxidoreductase 1 (NQO1) is over-expressed in pancreatic tumor vs associated normal tissue, while catalase expression is lowered compared to levels in associated normal pancreas tissue. ARQ761 undergoes a robust, futile redox cycle in NQO1+ cancer cells, producing massive hydrogen peroxide (H2O2) levels; normal tissues are spared by low NQO1 and high catalase expression. DNA damage created by ARQ761 in pancreatic cancer cells ‘hyperactivates’ PARP1, causing metabolic catastrophe and NAD+-keresis cell death. NQO1: catalase levels (high in tumor, low in normal tissue) are an attractive therapeutic window to treat pancreatic cancer. Based on a growing body of literature, we are leading a clinical trial to evaluate the combination of ARQ761 and chemotherapy in patients with pancreatic cancer.
Keywords: β-lapachone, pancreatic cancer, experimental therapeutics, cancer metabolism, hyperpolarization
Introduction
Pancreatic cancer has risen in incidence and is a historically recalcitrant disease that is expected to be the second leading cause of cancer-related deaths by 20201,2. Current therapies against pancreatic cancer lack rationale that exploit cancer-specific targets, are subject to inherent resistance mechanisms and are ineffective against non-cycling cancer cells. Effective treatment for pancreatic cancer will require use of agents that cause tumor-specific cell death, independent of oncogenic driver mutations such as Kras and p53, or apoptotic processes (e.g., caspase-independent). A majority of cancers lack functional p53, have activated or mutant tumor driver mutations, and/or have defects in apoptotic pathways that confer growth advantages and drug resistance3,4. Agents that kill pancreatic cancer cells irrespective of growth state (i.e., effective against stationary and log-phase cells) and target primary and metastatic tumor cells are needed, since resistant, growth-arrested cancer (stem-like) cells can repopulate a tumor or metastasize.
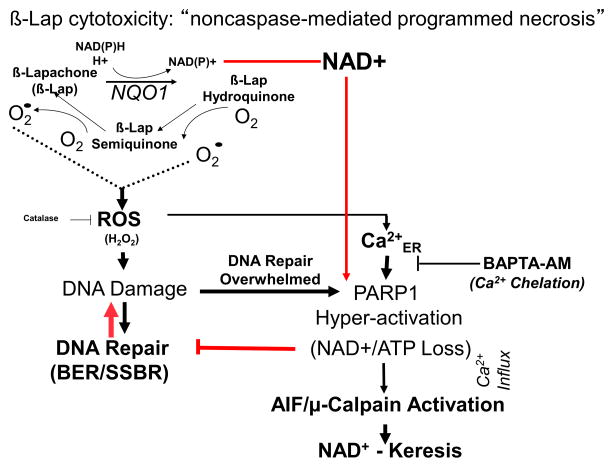
NAD(P)H:quinone oxidoreductase 1 (NQO1) expression is an exploitable, tumor-specific target for pancreatic cancer therapies. Elevated NQO1 expression is noted in many early forms of cancers such as in pancreatic intraepithelial neoplasia (PanINs)5, prostatic intraepithelial neoplasia6,7 and breast ductal carcinoma in situ8,9 and further increase in this enzyme occurs as these cancers progress.10 NQO1 bioactivatable drugs are a unique class of rare quinones that include β-lapachone (β-lap, ARQ761 in clinical form) and deoxynyboquinones.11 NQO1 catalyzes the two-electron oxidoreduction of β-lap to generate an unstable hydroquinone that spontaneously reacts with two oxygen molecules in a two-step back-reaction to regenerate the original compound.12 NQO1-dependent futile redox cycling oxidizes ~60 moles of NAD(P)H to create ~120 moles of reactive oxygen species (ROS) in ~2 mins.11,13 High levels of superoxide dismutase (SOD) in cancers ultimately generates long-lived and cell membrane-permeable hydrogen peroxide (H2O2) that diffuses into the nucleus and causes massive oxidative base and single strand break DNA lesions. Rapid accumulation of DNA lesions overwhelms the cell’s DNA repair capacity and causes ‘hyperactivation’ of poly(ADP-ribose) polymerase-1 (PARP1). Rapid protein PARylation, including PAR-PARP1, severe NAD+/ATP depletion, massive DNA lesions and repair inhibition follows.11 ROS (H2O2) formation only occurs while pools of NAD(P)H are available for NQO1-driven futile redox cycling of the drug. A lethal dose of β-lap induces caspase-independent programmed necrosis (i.e. NAD+-keresis) (Figure 1). 6,7,11,10
Figure 1.
β-Lap causes unique NQO1-specific antitumor lethality irrespective of a wide range of driver or carrier mutations. A, NQO1-mediated cell death by β-lap. Note the combined futile cycle mechanism of NQO1-mediated redox cycling and H2O2-induced PARP1 hyperactivation reduce NAD(P)H equivalents that stimulates NAD+ elimination by GAPD-SNO-NAD conjugation (glycolysis inhibition) and PARP1 ADP-ribosylation.
Concomitant NQO1 overexpression and loss of catalase are a hallmark of many solid cancers. NQO1 is over-expressed in >85% of resected pancreatic cancer samples and is preferentially expressed in pancreatic cancer vs. non-cancer adjacent pancreas.14,15 A majority (>90%) of pancreatic tumors have over-expressed NQO1 levels, believed mainly due to mutant K-Ras driven transcription.5,16 We assessed NQO1 mRNA levels in 462 pancreatic adenocarcinoma tissues vs. associated normal tissue, where uniformly elevated NQO1 levels were noted, consistent with our prior data17. As also noted in non-small cell lung cancer, head and neck, prostate, and breast cancers6,8,12,18, we also demonstrated a reciprocal loss of expression of catalase in pancreatic adenocarcinoma tumors, with elevated catalase and low NQO1 levels in associated normal tissue.16 Thus, NQO1: catalase ratios in pancreatic adenocarcinoma tumor vs associated normal pancreas tissue of patients is predicted to be an important and highly exploitable therapeutic target. Similar NQO1: catalase ratios at the mRNA level have been reported in pancreatic cancer. 5,16,17,19,20 This distribution of redox cycle-related enzymes in tumor vs. normal tissue strongly suggest that NQO1 bioactivatable drugs (i.e., β-lapachone (β-lap) are ideally suited for treatment of pancreatic adenocarcinoma that have a high rate of NQO1 expression. Thus, NQO1: catalase ratios represent an exploitable therapeutic window for treatment of pancreatic adenocarcinomas.11,16,21
β-Lapachone provides synergistic antitumor activity against preclinical human pancreatic cancer xenografts in vivo as a single agent, as well as when combined with standard of care chemotherapy. β-Lapachone is highly efficacious against human MiaPaCa2 xenografts in athymic nude mice due to increased drug PK (and tumor-selective PD) effects in the pancreas.22 Efficacy was demonstrated by a high number of cures in mice seen at 20–30 mg/kg HPβCD-β-lap against MiaPaCa2 xenografts. When administered in combination with DNA damaging agents (e.g., ionizing radiation), doses of HPβCD-β-lap as low as 5 mg/kg are highly efficacious where 100% cures were seen at 300 days. Additionally, efficacy was verified by ex vivo tumor luciferase activities 14–60 days post-therapy.22,23 Significant inhibition of 18FDG uptake is seen when post-treatment efficacy is evaluated 7 days later, suggesting that glucose metabolism was strongly suppressed during tumor regression in preclinical models.23,24 Beta-lapachone synergized with multiple DNA damaging agents12,18,25,26, including gemcitabine24,23 and paclitaxel.27,28 Mechanistically, combination therapies are predicted to enhance DNA damage to a threshold that triggers PARP1 hyperactivation and cell death at sublethal doses of both the DNA damaging agent(s) and β-lapachone.6,11,16,29
We are leading the clinical development of a novel NQO1 bioactivatable drug, β-lapachone (used as HPβCD-β-lap, ARQ761). The major toxicities of ARQ761 monotherapy have been methemoglobinemia and hemolytic anemia. Other toxicities normally associated with conventional cytotoxic regimens such as nausea, neutropenia and thrombocytopenia were not observed. Single-agent clinical activity has been seen starting at the first dose level studied (195 mg/m2).30
NQO1 expression is being developed as a companion diagnostic with ARQ761 and is used as an integral biomarker on clinical trials. Archival formalin-fixed, paraffin embedded tissue was tested for NQO1 expression using an immunohistochemistry (IHC) assay. In the first in human phase 1 clinical trial of monotherapy ARQ761, NQO1 expression was found to be a principal determinant of disease response. After evaluation of 19 patients with advanced solid tumors, there was a positive trend (P=0.06) between clinical benefit and tumor NQO1 expression. NQO1 expression by IHC was subsequently used as an enrichment/enrollment biomarker and only patients with NQO1-positive tumors were enrolled. A histo-score (H-score) was assigned based on intensity of NQO1 expression and prevalence of positive tumor cells. An H-Score of ≥200 (range 0–300) has been determined to be the cut off in defining NQO1 expression as positive or negative.30 Future studies will validate the NQO1:catalase ratio in patient tumors as a biomarker of response, and determine patterns of expression across multiple tumor types (including pancreas, non-small lung, triple negative breast and bladder cancers), changes in expression with treatment and the relationship of NQO1 expression with molecular biomarkers (e.g., KRAS).
Rationale for Phase 1b clinical trial of ARQ761 with gemcitabine and nab-paclitaxel in advanced pancreatic cancer
The combination of gemcitabine and nab-paclitaxel has demonstrated improved overall survival compared to single agent gemcitabine alone and has emerged as the new standard reference chemotherapy for patients with advanced pancreatic cancer and good performance status31. Based on the data above, we demonstrated synergistic anti-tumor effects with beta-lapachone (ARQ761) plus gemcitabine in pre-clinical models. We propose building on gemcitabine plus nab-paclitaxel chemotherapy backbone by combining this doublet with ARQ761. We anticipate this combination will be safe and tolerable and result in substantial synergy and antitumor efficacy in patients with advanced pancreatic cancer.
As drug levels are cleared from circulation rapidly, biweekly dosing is feasible. Such a rapid rise and clearance of drug levels is ideally suited for the ‘kiss of death’ responses of NQO1 bioactivatable drugs, such as β-lapachone, where such treatments take advantage of the therapeutic window of NQO1-mediated burst of H2O2 and subsequent PARP1 hyperactivation in tumor and spare these effects on normal tissue. This affords an irreversible cell death mechanism as long as 4–6 μM doses are achieved for 1–2 h.
Clinical trial: phase 1b clinical trial of ARQ761 treatment with gemcitabine/nab-paclitaxel chemotherapy in pancreatic cancer
This is a single arm, open label, multi-institution phase 1b clinical trial for patients with advanced pancreatic cancer (NCT02514031). The primary objective is to determine the maximum tolerated dose (MTD) of ARQ761 when administered in combination with gemcitabine and nab-paclitaxel. Secondary objectives include determining 1) the safety and tolerability of the combination, 2) clinical activity as defined by overall response rate (ORR), progression free survival (PFS) and time to progression (TTP) and 3) pharmacokinetic profile of ARQ761 when administered in combination with chemotherapy. To further enhance our understanding of this treatment strategy, additional exploratory objectives will be performed. This includes correlation of NQO1 expression with clinical outcomes and determination of change in PAR-PARP1, gamma-H2AX formation, and NAD+/ATP losses in patient biopsies.
Inclusion criteria include patients with histologically or cytologically confirmed pancreatic adenocarcinoma that is metastatic, unresectable, or recurrent. Patients may have received, at most, one line of prior non-gemcitabine chemotherapy for metastatic/unresectable disease. Patients must have measurable disease is required per RECIST criteria 1.132 and good performance status as defined by Eastern Cooperative Group performance status of 0 or 1. Patients with untreated brain metastases are excluded. Other exclusion criteria include pregnant women, laboratory abnormalities, uncontrolled intercurrent illness or social situations that would limit compliance with or tolerability of study requirements or conditions that may confound the ability to interpret data from the study. Although all tumors will be retrospectively analyzed for NQO1 status using the IHC assay, NQO1 status is not used as an enrichment biomarker in this clinical trial. Based on published reports, we expect the rate of NQO1 expression to be greater than 85% in pancreatic adenocarcinoma.14,15 Also, by enrolling patients regardless of NQO1 status, the investigators will learn about the predictive effect of NQO1 in the pancreatic cancer population which can help inform future ARQ761 clinical trial designs.
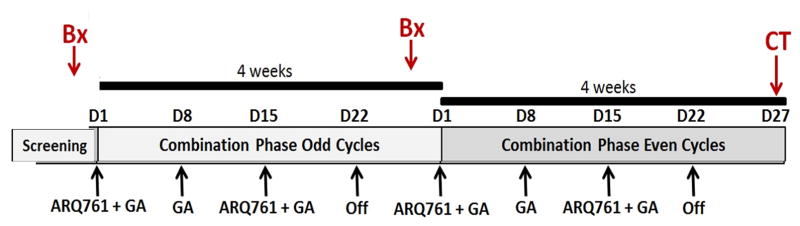
Treatment schema
Treatment will include gemcitabine, nab-paclitaxel, and ARQ761. Patient treatment will consist of gemcitabine and nab-paclitaxel administered intravenously on days 1, 8, 15 -every 28 days, as per the MPACT clinical trial (figure 2). 31
Figure 2.
Phase 1b clinical trial of ARQ761 combined with gemcitabine and nab-paclitaxel in patients with advanced pancreatic cancer
ARQ761 will be delivered on days 1 and 15 as an intravenous infusion to start 60 mins after completion of gemcitabine and nab-paclitaxel to allow for incorporation of gemcitabine and additional DNA damage created in combination with nab-paclitaxel. Mechanistically, ARQ761 (β-lap) will synergize best (requiring the least levels of both drug within the tumor) at a time when extensive DNA lesions created by gemcitabine and nab-paclitaxel are present, allowing PARP1 hyperactivaton.12,22,33–35
Starting dose of gemcitabine (1000 mg/m2) + nab-paclitaxel (125 mg/m2) follow the doses used in the MPACT clinical trial.31 The maximum tolerated dose (MTD) of ARQ761 will be determined by dose-titrating using a standard 3+3 method, as highlighted in Table 1.36 Three patients within a dose level must be observed for one cycle (28 days) before accrual to the next higher dose level may begin. If a subject is withdrawn from the study prior to completing 22 days of combination therapy without experiencing a Dose limiting toxicity (DLT) prior to withdrawal, an additional subject may be added to that dose level. If a subject misses 2 doses for reasons other than adverse events, an additional subject may be added to that dose level. For the dose expansion phase of the study, there will be no replacements of subjects made.
Table 1.
Dose escalation schema for the Phase 1b clinical trial of ARQ761 combined with gemcitabine and nab-paclitaxel in patients with advanced pancreatic cancer.
| Dose Level | ARQ761 IV D1, 15 |
Gemcitabine D1, 8, 15 |
Nab-paclitaxel D1, 8, 15 |
|---|---|---|---|
| −3 | 136 mg/m2 | 1000 mg/m2 | 125 mg/m2 |
| −2 | 156 mg/m2 | 1000 mg/m2 | 125 mg/m2 |
| −1 | 175 mg/m2 | 1000 mg/m2 | 125 mg/m2 |
| 1 | 195 mg/m2 | 1000 mg/m2 | 125 mg/m2 |
| 2 | 290 mg/m2 | 1000 mg/m2 | 125 mg/m2 |
| 3 | 390 mg/m2 | 1000 mg/m2 | 125 mg/m2 |
| Expansion | MTD | 1000 mg/m2 | 125 mg/m2 |
DLT will be defined by the occurrence of any of the following toxicities possibly or probably related to drug during cycle 1 as defined by the NCI Common Terminology Criteria for Adverse Events (CTCAE version 4.0)
-
Grade 3 or 4 hemolysis or hemolytic anemia, except
Transfusion for hemolysis or hemolytic anemia will be considered a DLT only if Hgb < 8.0 g/dL (Grade 3 anemia)
Any drug related grade 3 or grade 4 non-hematological toxicity, except alopecia, or asymptomatic elevation of hepatic transaminases thought to be secondary to gemcitabine.
Grade ≥ 3 diarrhea, vomiting, or nausea that persists for > 3 days despite optimal supportive care.
Grade 4 neutropenia lasting more than 5 days or febrile neutropenia.
Platelets <50,000/μl for longer than 5 days or any platelet count <25,000/μl.
Grade 3 or 4 hyperbilirubinemia that does not recover to Grade 1 within 7 days
Missing two consecutive weekly doses of gemcitabine and nab-paclitaxel because of unresolved toxicity.
Grade 3 hypoxia observed at the end of infusion of ARQ761 on the pulse oximetry readout without clinically significant symptoms will not be considered as a DLT. Arterial Blood Gas (ABG) will be obtained at the discretion of the investigator. Arterial O2 saturation levels will be used to evaluate hypoxia if required.
Any other toxicity possibly or probably related to treatment in the view of the investigator that represents a clinically significant hazard to the patient.
Anemia or lymphopenia is not considered a DLT unless as specified above.
Infusion related reaction Grade ≥ 4 or Grade 3 infusion-related reaction that persists for more than 24 hours or that precludes administration of the full dose of the investigational drug.
Tumor response and toxicity monitoring
Disease response and progression will be evaluated in this study using the international criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST) Committee.32 Any patient who receives treatment on this protocol will be evaluable for toxicity. Toxicity will be assessed according to the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE), version 4.0. The most common adverse events noted with the MPACT study that resulted in a dose reduction, delay or withholding of chemotherapy was neutropenia and thrombocytopenia.31 We will follow standard recommended dose modifications for gemcitabine and nab-paclitaxel per the MPACT trial.
Pharmacokinetic and pharmacodynamic sampling
Serial venous blood samples will be drawn from each subject during the first cycle on days 1 and 15 for determination of plasma levels of ARQ761. Serial venous blood samples will be drawn from each subject before and three hours after cycle 1 days 1 and 15 infusions of study drug for biomarker analyses.
Tumor biopsies will be performed at baseline (if not available) and after cycle 1. The goal of the planned laboratory correlative studies is discussed in the exploratory objectives indicated above. The purpose of the follow up biopsy is to inform the mechanism of action of ARQ761 and chemotherapy and will drive the translational aspect of the study. The planned analysis includes immunohistochemistry (IHC) analysis of NQO1, catalase, TUNEL, Ki-67 and γ-H2AX by IHC. Additional analysis by western blot for μ-calpain and PARP cleavage will be performed.
Conclusion
Rational hypothesis driven treatments for pancreatic cancer that exploit a well-defined mechanism of action are needed. Difficulty in obtaining pancreatic cancer tumor tissue has hampered the ability to develop markers of response and predictors of efficacy. This phase 1 clinical trial explores a novel bioactivatable compound to target pancreatic cancer therapy. Pharmacodynamic markers will validate NQO1: catalase expression as a predictive biomarker of response.
Synopsis.
Using a defined mechanism of action of a unique NAD(P)H:quinone oxidoreductase 1 (NQO1) bioactivatable compound, beta-lapachone (ARQ761), and multiple therapeutic agents we defined specific synergistic modalities for the treatment of pancreatic cancers, which express high levels of this enzyme. We are leading a clinical trial of beta-lapachone plus chemotherapy in patients with advanced pancreatic cancer.
Acknowledgments
Research Support/Funding: Pancreatic Cancer Action Network (PanCan)/Gateway for Cancer Research/Rising Tide Foundation Clinical Continuation Grant (14-65-25-BOOT), PanCan Translational Research Grant (15-65-25-Boot) to MSB, DAB and DL; and NIH/NCI grant CA102792 to DAB, and a National Cancer Institute Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01) (to D.E.G.). NCI Cancer Center Support Grant to UT Southwestern Medical Center (5P30CA142543-07) MSB, DEG, XJX, DAB, Cancer Prevention and Research Institute of Texas (CPRIT award RP170003) to RL, Arqule Pharmaceuticals
Footnotes
Conflicts of interest/Disclaimers: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Matrisian L, Aizenberg R, Rosenzweig A. Pancreatic Cancer Action Network Special Report: The alarming rise of pancreatic cancer deaths in the United States: Why we need to stem the tide today. 2012 http://wwwpancanorg/section_research/reports/pdf/incidence_report_2012pdf.
- 3.Gessner C, Liebers U, Kuhn H, et al. BAX and p16INK4A are independent positive prognostic markers for advanced tumour stage of nonsmall cell lung cancer. The European Respiratory Journal. 2002;19:134–40. doi: 10.1183/09031936.02.00219402. [DOI] [PubMed] [Google Scholar]
- 4.Gu CD, Osaki T, Oyama T, et al. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer: impact on recurrence and Survival. Annals of Surgery. 2002;235:133–9. doi: 10.1097/00000658-200201000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Bey EA, Li LS, et al. Prostate cancer radiosensitization through poly(ADP-Ribose) polymerase-1 hyperactivation. Cancer Res. 2010;70:8088–96. doi: 10.1158/0008-5472.CAN-10-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Chin SF, Blanco E, et al. Intratumoral delivery of beta-lapachone via polymer implants for prostate cancer therapy. Clin Cancer Res. 2009;15:131–9. doi: 10.1158/1078-0432.CCR-08-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin A, Lopez de Cerain A, Hamilton E, et al. DT-diaphorase and cytochrome B5 reductase in human lung and breast tumours. Br J Cancer. 1997;76:923–9. doi: 10.1038/bjc.1997.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menzel HJ, Sarmanova J, Soucek P, et al. Association of NQO1 polymorphism with spontaneous breast cancer in two independent populations. Br J Cancer. 2004;90:1989–94. doi: 10.1038/sj.bjc.6601779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madajewski B, Boatman MA, Chakrabarti G, Boothman DA, Bey EA. Depleting Tumor-NQO1 Potentiates Anoikis and Inhibits Growth of NSCLC. Mol Cancer Res. 2016;14:14–25. doi: 10.1158/1541-7786.MCR-15-0207-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Dong Y, Bey EA, et al. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res. 2012;72:3038–47. doi: 10.1158/0008-5472.CAN-11-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bey EA, Bentle MS, Reinicke KE, et al. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci U S A. 2007;104:11832–7. doi: 10.1073/pnas.0702176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. The Journal of biological chemistry. 2000;275:5416–24. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 14.Awadallah NS, Dehn D, Shah RJ, et al. NQO1 expression in pancreatic cancer and its potential use as a biomarker. Appl Immunohistochem Mol Morphol. 2008;16:24–31. doi: 10.1097/PAI.0b013e31802e91d0. [DOI] [PubMed] [Google Scholar]
- 15.Lyn-Cook BD, Yan-Sanders Y, Moore S, Taylor S, Word B, Hammons GJ. Increased levels of NAD(P)H: quinone oxidoreductase 1 (NQO1) in pancreatic tissues from smokers and pancreatic adenocarcinomas: A potential biomarker of early damage in the pancreas. Cell Biol Toxicol. 2006;22:73–80. doi: 10.1007/s10565-006-0156-3. [DOI] [PubMed] [Google Scholar]
- 16.Huang XME, Moore ZR, Yao J, Dong Y, Chakrabarti G, Kilgore J, SIlvers MA, Patidar PL, Cholka A, Fattah F, Cha Y, Anderson GG, Kusko R, Peyton M, Yan J, Xie XJ, Sarode V, Williams N, Minna JD, Beg MS, Gerber DE, Bey EA, Boothman DA. Leveraging An NQO1 Bioactivatable Drug For Tumor-Selective Use Of Poly(ADPribose) Polymerase (PARP) Inhibitors. Cancer Cell. 2016;30:940–52. doi: 10.1016/j.ccell.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ough M, Lewis A, Bey EA, et al. Efficacy of beta-lapachone in pancreatic cancer treatment: exploiting the novel, therapeutic target NQO1. Cancer Biol Ther. 2005;4:95–102. doi: 10.4161/cbt.4.1.1382. [DOI] [PubMed] [Google Scholar]
- 18.Bentle MS, Bey EA, Dong Y, Reinicke KE, Boothman DA. New tricks for old drugs: the anticarcinogenic potential of DNA repair inhibitors. J Mol Histol. 2006;37:203–18. doi: 10.1007/s10735-006-9043-8. [DOI] [PubMed] [Google Scholar]
- 19.Lewis AM, Ough M, Hinkhouse MM, Tsao MS, Oberley LW, Cullen JJ. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol Carcinog. 2005;43:215–24. doi: 10.1002/mc.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes & development. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bey EA, Bentle MS, Reinicke KE, et al. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11832–7. doi: 10.1073/pnas.0702176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li LS, Bey EA, Dong Y, et al. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of beta-lapachone for pancreatic cancer therapy. Clin Cancer Res. 2011;17:275–85. doi: 10.1158/1078-0432.CCR-10-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Rigell CJ, Zhai G, et al. Antagonistic effects of anti-EMMPRIN antibody when combined with chemotherapy against hypovascular pancreatic cancers. Mol Imaging Biol. 2014;16:85–94. doi: 10.1007/s11307-013-0665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H, Rigell CJ, Zhai G, et al. Antagonistic Effects of Anti-EMMPRIN Antibody When Combined with Chemotherapy Against Hypovascular Pancreatic Cancers. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging. 2013 doi: 10.1007/s11307-013-0665-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentle MS, Reinicke KE, Bey EA, Spitz DR, Boothman DA. Calcium-dependent modulation of poly(ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. J Biol Chem. 2006;281:33684–96. doi: 10.1074/jbc.M603678200. [DOI] [PubMed] [Google Scholar]
- 26.Bentle MS, Reinicke KE, Dong Y, Bey EA, Boothman DA. Nonhomologous end joining is essential for cellular resistance to the novel antitumor agent, beta-lapachone. Cancer Res. 2007;67:6936–45. doi: 10.1158/0008-5472.CAN-07-0935. [DOI] [PubMed] [Google Scholar]
- 27.D’Anneo A, Augello G, Santulli A, et al. Paclitaxel and beta-lapachone synergistically induce apoptosis in human retinoblastoma Y79 cells by downregulating the levels of phospho-Akt. J Cell Physiol. 222:433–43. doi: 10.1002/jcp.21983. [DOI] [PubMed] [Google Scholar]
- 28.Li CJ, Li YZ, Pinto AV, Pardee AB. Potent inhibition of tumor survival in vivo by beta-lapachone plus taxol: combining drugs imposes different artificial checkpoints. Proc Natl Acad Sci U S A. 1999;96:13369–74. doi: 10.1073/pnas.96.23.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EJ, Ji IM, Ahn KJ, et al. Synergistic effect of ionizing radiation and beta-Lapachone against RKO human colon adenocarcinoma cells. Cancer Res Treat. 2005;37:183–90. doi: 10.4143/crt.2005.37.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerber D, Arriaga Y, Beg MS, Dowell JE, Schiller JH, Frankel AE, Leff R, Meek C, Bolluyt J, Fatunde O, Martinez Phase 1 correlative study of ARQ761, a β-lapachone analogue that promotes NQ01-mediated programmed cancer cell necrosis. Eur J Cancer. 2014:84–5. [Google Scholar]
- 31.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Tagliarino C, Pink JJ, Dubyak GR, Nieminen AL, Boothman DA. Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J Biol Chem. 2001;276:19150–9. doi: 10.1074/jbc.M100730200. [DOI] [PubMed] [Google Scholar]
- 34.Tagliarino C, Pink JJ, Reinicke KE, Simmers SM, Wuerzberger-Davis SM, Boothman DA. Mu-calpain activation in beta-lapachone-mediated apoptosis. Cancer biology & therapy. 2003;2:141–52. doi: 10.4161/cbt.2.2.237. [DOI] [PubMed] [Google Scholar]
- 35.Bey EA, Reinicke KE, Srougi MC, et al. Catalase abrogates beta-lapachone-induced PARP1 hyperactivation-directed programmed necrosis in NQO1-positive breast cancers. Molecular cancer therapeutics. 2013;12:2110–20. doi: 10.1158/1535-7163.MCT-12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101:708–20. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]