Abstract
This study was designed to assess the occurrence and concentrations of a broad range of contaminants of emerging concern (CECs) from three local estuaries within a large estuarine ecosystem. In addition to effluent from two wastewater treatment plants (WWTP), we sampled water and whole-body juvenile Chinook salmon (Oncorhynchus tshawytscha) and Pacific staghorn sculpin (Leptocottus armatus) in estuaries receiving effluent. We analyzed these matrices for 150 compounds, which included pharmaceuticals, personal care products (PPCPs), and several industrial compounds. Collectively, we detected 81 analytes in effluent, 25 analytes in estuary water, and 42 analytes in fish tissue. A number of compounds, including sertraline, triclosan, estrone, fluoxetine, metformin, and nonylphenol were detected in water and tissue at concentrations that may cause adverse effects in fish. Interestingly, 29 CEC analytes were detected in effluent and fish tissue, but not in estuarine waters, indicating a high potential for bioaccumulation for these compounds. Although concentrations of most detected analytes were present at relatively low concentrations, our analysis revealed that overall CEC inputs to each estuary amount to several kilograms of these compounds per day. This study is unique because we report on CEC concentrations in estuarine waters and whole-body fish, which are both uncommon in the literature. A noteworthy and unexpected finding was the preferential bioaccumulation of CECs in free-ranging juvenile Chinook salmon relative to staghorn sculpin, a benthic species with relatively high site fidelity.
Keywords: Estuary, fish, wastewater effluent, pharmaceuticals, personal care products
Graphical abstract
1. Introduction
Contaminants of emerging concern (CECs) constitute a wide range of chemicals for which there is limited data on occurrence, environmental fate, and toxicity. Represented in this class of environmental contaminants are pharmaceutical and personal care products (PPCPs) and a number of industrial compounds such as polybrominated diphenyl ethers (PBDEs), perfluorinated compounds (PFCs), alkylphenols, bisphenol A, phthalates, and current-use pesticides. Many of these compounds are present in our rivers, estuaries, and coastal areas from wastewater treatment plant (WWTP) effluent discharging via outfalls to these water bodies. Other sources of CECs to waterways include discharges from industrial sources and aquaculture operations, in addition to runoff from impervious surfaces, landfills, biosolids application, and agricultural and farming activities (Gaw et al., 2014).
Most of these CECs are potent human and animal medicines that are used for various purposes, many of which are then excreted as the parent compound or as metabolites that flow into WWTPs. Some of these compounds are eliminated or reduced in concentration by treatment practices that vary among facilities or are sorbed to biosolids and removed from the waste stream (Lubliner et al., 2010; Oulton et al., 2012). By contrast, some CECs are poorly removed by WWTP processing or are discharged to surface waters, including streams, estuaries, or open marine waters due to secondary bypass or combined sewer overflows, (Lubliner et al., 2010; Phillips et al., 2012).
There are several important factors to consider in assessing the environmental risk of CECs in estuarine waters, as well as other aquatic habitats. These include; the extent of product usage among local human populations, physical-chemical parameters (i.e. water solubility, hydrolysis, photodegradation, and adsorption to sediment and biosolids), rates of bioaccumulation, chemical potency, and potential toxicity to aquatic organisms and aquatic-dependent wildlife. Among these aforementioned factors, bioaccumulation and comparative toxicity to aquatic species constitutes the largest data gap in assessing ecological risk.
Over 4,000 approved drug products are currently available (U.S. Food and Drug Administration, 2015) under various formulations and approximately 1100 are unique prescription and over-the-counter compounds comprising a large number of chemical classes and mechanisms of action (MoA). A consensus value of 324 drug targets has been proposed by Overington et al. (2006) for all classes of therapeutic drugs. A recent study of 12 fish species from a variety of families concluded that 65 – 86% of human drug targets are conserved in diverse fish species (Brown et al., 2014); therefore it is reasonable to assume that many of these drugs will also affect fish. Of the hundreds of chemicals that are likely present in the Puget Sound ecosystem, only a small percentage are currently monitored or regulated and there is little or no toxicity information for the vast majority of these compounds. Many of these are common household chemicals that pass through wastewater treatment, have been approved for use and/or consumption by the general public, and are generally considered to be non-toxic. However, the higher-than-expected levels for some of these chemicals in aquatic organisms and possibly aquatic-dependent wildlife along with critical gaps in toxicological and risk assessment data underscores their importance for further investigation in the context of environmental and public health concerns (Roos et al., 2012; Arnold et al., 2014).
Relatively comprehensive analyses of CECs in the marine or estuarine ecosystem within the United States are uncommon. Notable exceptions for U.S. waters include the analysis of CECs in effluent and marine waters in southern California (Vidal-Dorsch et al., 2012) and Charleston Harbor (Hedgespeth et al., 2012), receiving waters in four estuaries along the Texas coast (Scott et al., 2015), San Francisco Bay (Klosterhaus et al., 2013), and Lubliner et al., (2010) who reported on effluent concentrations from WWTPs in Puget Sound, Washington. As far as we know, there are no studies that tested for a large suite of CECs in whole-body fish in marine waters.
Our approach in the present study involved a review of the literature that resulted in a prioritized list of 102 PPCPs, 17 hormones, and 31 industrial compounds to serve as a representative subset of CECs that we identified as a potential concern in the estuarine waters of Puget Sound, Washington, USA. Our primary goal was to determine the occurrence and concentrations of CECs in WWTP effluent, estuary water, and two fish species occupying different habitats with different life histories and compare among locations and matrices.
2. Methods
2.1. Selection of field sites
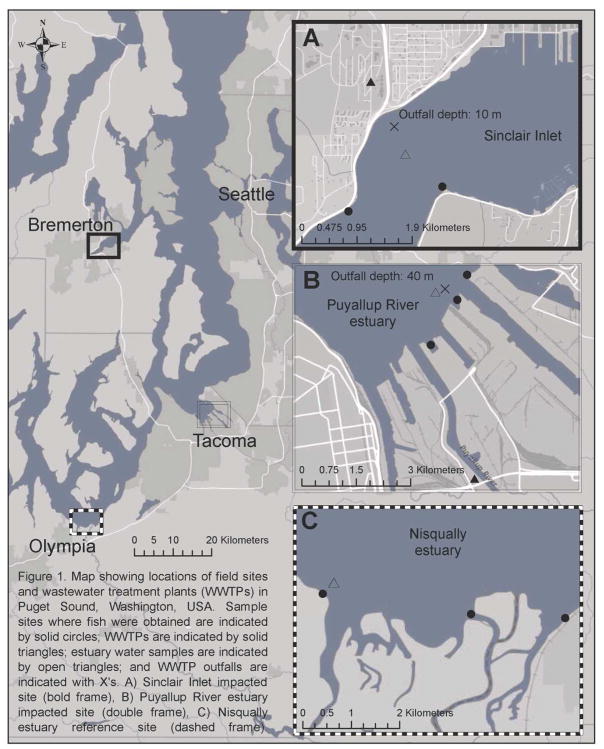
We selected three local estuaries as focal points for our study, including two estuaries that receive effluent from WWTPs and one as a reference site that is not known to have direct inputs from WWTP effluent. One contaminated site was Sinclair Inlet, which receives effluent from the Bremerton Westside WWTP (Figure 1). The effluent outfall is located approximately 170 meters from shore at a depth of 10 meters below mean lower low water (MLLW) in Sinclair Inlet. Sinclair Inlet has one other known source of effluent from the South Kitsap Water Reclamation Facility with a design flow of 16 million liters/d (MLD) (South Kitsap Water Reclamation Facility 2013). The other contaminated site selected was the Puyallup River estuary, which receives effluent from the Tacoma Central WWTP (Figure 1). The discharge outfall is at 40 meters MLLW and approximately 370 meters northwest from the mouth of the Blair Waterway in Commencement Bay. The Puyallup River basin contains 8 additional WWTPs with a combined permitted effluent volume of 63 MLD with flows generally running much lower (Pierce County 2010). The Nisqually estuary was selected as a minimally-contaminated reference site, and has been used in numerous studies as a reference site (Meador 2014). Table 1 contains additional details for each site. Two fish species that commonly occur in Puget Sound estuaries were selected for assessing bioaccumulation of CECs. Specifically, Pacific staghorn sculpin (Leptocottus armatus) was selected for biomonitoring because this species found widely in Puget Sound and U.S. west coast temperate waters, generally exhibits high site fidelity, and may reside in estuaries for extended periods (Tasto, 1976). Juvenile Chinook salmon (Oncorhynchus tshawytscha) were selected based on their residence time (up to several weeks) in local estuaries where contaminants are often concentrated (Healey 1991). Chinook salmon were selected over other salmonids that do not exhibit this life history trait (Meador 2014). We also collected hatchery-reared juvenile Chinook salmon from the Voight’s Creek hatchery on the Puyallup River for comparison to fish collected in the estuary. Fish were collected under a Washington State Scientific Collection Permit 13—046 and ESA Section 10(a)(1)(A) permit 17798. All methods for obtaining, transporting, and tissue sampling of fish were approved by the University of Washington Institutional Animal Care and Use Committee (protocol number 4096-01). Details of all sampling methods used in this study are reported in Yeh et al., (2013).
Figure 1.
Map of Puget Sound and estuaries sampled with locations of wastewater treatment plant outfalls and sampling sites.
Map showing locations of field sites and wastewater treatment plants (WWTPs) in Puget Sound, Washington, USA. Sample sites where fish were obtained are indicated by solid circles; WWTPs are indicated by solid triangles; estuary water samples are indicated by open triangles; and WWTP outfalls are indicated with X’s. A) Sinclair Inlet impacted site (bold frame), B) Puyallup River estuary impacted site (double frame), C) Nisqually estuary reference site (dashed frame).
Table 1.
Sampling locations, water and fish collection data, composition of chemistry composites, and estuary parameters.
| Puyallup estuary | Sinclair Inlet | Nisqually estuary | Voight’s Creek Hatchery | |
|---|---|---|---|---|
| Collection data | ||||
|
|
||||
| Coordinates | 47°16′35.4″N | 47°32′24.4″N | 47°05′56.4″N | 47°04′58.8″N |
| 122°24′58.0″W | 122°39′44.3″W | 122°42′01.8″W | 122°10′40.8″W | |
| Sample dates fish | 21 Aug 2013, and 4 Sept 2013 (La); 16 June 2014 (Ot); 29 June 2014, and 7 and 13 Aug 2014 (La) | 9 and 11 June 2014 (Ot); 27 July 2014 (La) | 27 Aug 2013 (La); 19 June 2014 (Ot); 4 Aug 2014 (La) | 29 May 2014 (Ot) |
| n fish collected | Ot: 75 | Ot: 38 | Ot: 72 | Ot: 56 |
| La: 18§ and 31^ | La: 40^ | La: 24§ and 47^ | ||
| Mean (SD) salmon wt. (g) | 5.4 (2.4) | 13.4 (8.2) | 6.8 (1.5) | 5.4 (0.9) |
| Mean (SD) sculpin wt. (g) | La§: 60.7 (29.4) | La^: 18.8 (6.6) | La§: 36.7 (14.3) | N/A |
| La^: 22.7 (20.5) | La^: 16.1 (5.0) | |||
| % hatchery chinook | 70% | 71% | 100% | 100% |
| Salmon CF mean (sd) | 0.94 (0.14) | 0.90 (0.19) | 0.96 (0.12) | 1.09 (0.12) |
| Sample dates water | 21 Aug 2013 (EW); 17 Sept 2014 (EF) | 22 July 2014 (EW); 9 Sept 2014 (EF) | 27 Aug 2013 (EW) | N/A |
| Chemistry composites | ||||
| N Fish/chem composite, lipids % | Ot A: 10, 4.3% Ot B: 12, 3.2% La§: 3, 1.6% La^: 5, 1.9% |
Ot A: 3, 3.3% Ot B: 3, 1.5% La^: 3, 1.7% |
Ot: 9, 2.5% La§: 4, 2.1% La^: 3, 1.6% |
Ot: 12, 5.1% |
| Mean (SD) salmon wt. (g) | Ot A: 5.5 (1.3) | Ot A: 14.1 (4.7) | 5.6 (0.7) | 5.4 (0.9) |
| Ot B: 4.1 (0.6) | Ot B: 16.9 (9.0) | |||
| Mean (SD) sculpin wt. (g) | La§: 47.5 (50.2) | La^: 30.9 (6.2) | La§: 48.1 (31.8) | N/A |
| La^: 9.4 (1.6) | La^: 16.8 (2.8) | |||
| Estuary parameters | ||||
| pH | 8.04 | 8.45 | 7.62 | — |
| Salinity (ppt) | 23.5 | 27 | 15.5 | 0 |
| Temp (°C) | 12.5 | 12.5 | 13.5 | 10 |
| Oxygen (mg/L) | 8.2 | 15 | 10.6 | 12 |
EW= estuary water, EF=effluent, Ot= Oncorhynchus tshawytscha (Chinook salmon), La= Leptocottus armatus (staghorn sculpin).
=2013 sampling year sculpin,
=2014 sampling year sculpin. CF is condition factor (=weight (g)2/length (mm)3). Percent hatchery fish based on the presence of an adipose fin. Estuary parameters determined at time of water sampling. SD is standard deviation.
2.2. Sampling for CEC analytes in WWTP effluents and water
The effluent from Bremerton West WWTP was sampled on 9 September 2014 and the effluent flow was 13.2 MLD. The maximum monthly design flow from October – April is stated to be 58.7 MLD and permitted at 86 MLD (Bremerton Westside Factsheet, 2013). The effluent from Tacoma Central WWTP, Tacoma, WA was collected on 17 September 2014 and the flow on that day was 56.8 MLD. The maximum month design flow for wet weather is listed as 143.8 MLD (Tacoma Central WWTP Factsheet, 2004) and the permitted capacity is 228 MLD (Pierce County, 2010). These values do not include secondary treatment bypass during high volume flows or peak flows, which may exceed average flows by 2-fold. For the two week period prior to sampling, Tacoma experienced 2.03 inches of rain and Bremerton received 2.89 inches of rain (Weatherunderground, 2015).
At each WWTP, a total of 11 one-liter amber glass bottles were filled with effluent sampled at the final stage of processing, just before discharge into the outfall leading to the estuary. Similarly, at each field site a total of 11 one-liter amber glass bottles were filled with estuarine water at a depth of 2 m below the surface with a swing-sampling pole designed to collect water below the surface. We generally followed Washington Department of Ecology (2006) for obtaining water samples. Estuary water quality parameters including dissolved oxygen, conductivity, salinity, and temperature of the water column were measured at a depth of 2 m below the surface using the YSI Model 85 handheld probe (YSI Incorporated, Yellow Springs, OH). Similarly, the pH of the water column was measured using the Eutech Multi-Parameter PCSTestr 35 (Oakton Instruments, Vernon Hills, IL). One water sample was taken at each site and the estuary parameters were measured within minutes of water collection. No field blanks were collected.
2.3. Fish sampling
Juvenile Chinook salmon were obtained at each field site with a beach seine and were categorized as wild or hatchery origin based on the presence of an adipose fin. Artificially reared salmon are marked by removal of the adipose fin by each hatchery. Staghorn sculpin were also obtained by beach seining; however 4 – 5 individuals from the Puyallup estuary were obtained by shrimp traps set at 8 – 9 m below the surface. Each species was collected as close as possible to the outfall area (Figure 1), which in most cases was several hundred meters away. Fish were kept alive after collection in the field and transported to the laboratory for processing. Fish were transported in site water that was aerated and temperature was maintained at 11 °C with ice packs. Samples were taken approximately 3 – 6 hours after capture and whole bodies of all fish were frozen at −80 °C after processing.
Fish were euthanized with tricaine methanesulfonate (MS-222; Argent Chemical Laboratories, Redmond, WA) for processing. To avoid analysis of stomach contents that were considered external to the fish, the entire alimentary canal and stomach contents of all fish analyzed for chemistry were cleaned of material by rinsing with distilled water. The contents were discarded and the cleaned tissue included with the whole bodies for analysis. Chemical analyses for CEC analytes were conducted on composite samples consisting of 3 – 12 whole-body salmon or 3 – 5 whole-body sculpin.
Juvenile Chinook salmon from the nearby Gorst Creek rearing ponds that empty directly into the head of Sinclair Inlet (far west end) were released unusually early in the year (Mike Huff, hatchery manager, personal communication) and were probably out of the area at the time of sampling. As a result, the juvenile salmon sampled were likely from outside the area, as noted in previous studies of this local estuary (Fresh et al., 2006), but were nonetheless exposed to WWTP effluent while residing in Sinclair Inlet. All collected fish were scanned for the presence of coded wire tags (CWTs) by personnel from the U.S. Fish and Wildlife Service (USFWS). Heads of fish with detected CWTs were removed and read by USFWS personnel. Only four CWTs were found in Chinook salmon obtained from Sinclair Inlet and all were from nearby Grover’s Creek Hatchery. Two CWTs were detected in Chinook salmon obtained from Puyallup estuary and both were from the White River Hatchery. Two CWTs were also detected in Chinook salmon from the Nisqually estuary, which indicated the Kalama Creek and Clear Creek Hatcheries as the source.
2.4. Analytical methods
Concentrations of CEC analytes were determined by AXYS Analytical, Ltd. (Sidney, British Columbia, Canada) using LC/MS/MS techniques. Table S1 gives a complete list of the 150 CEC analytes with their analytical methods and reporting limits (RLs). Of the 150 analytes, 147 were analyzed in water samples and 122 were analyzed in fish tissue. Based on the low RL values obtained in these samples, the analytical methods employed were generally highly sensitive for most compounds and represent the state-of-the-art approaches in quantitating this diverse group of compounds in environmental media.
All analytes were measured in water and tissue, except hormones, hexabromocyclododecanes (HBCDDs), and phthalate esters. Hormones were only determined in water because many of these compounds occur naturally in tissue and the available phthalate ester method was developed for water. Because phthalates are difficult to quantify in various matrices due to high control and analytical blank values, we opted to analyze ester metabolites of these compounds, which are less problematic. HBCDDs were analyzed in tissue only. Two of the compounds (bisphenol A and triclosan) were determined by two different analytical methods, once as part of a general analytical method and again by a compound-specific method (Table S1). No corrections were applied to the analytical values (e.g. percent recovery). All sampling objectives and quality control parameters outlined in Yeh et al. (2013) were achieved in this study. Many of the quality assurance and quality control parameters for the chemical analyses can be found in U.S. Environmental Protection Agency (2007), which have improved post-publication of this document.
3. Results
Of the 150 targeted analytes for this study, 92 (61%) were detected in effluent, estuarine water, or fish and only 58 (39%) were not detected in any of these matrices (Tables 2, S2, and S3). Additional information and data highlighting chemical output rates from effluent, physical-chemical properties, known half-lives, available partition coefficients, undetected compounds, and reporting limits can be found in Appendix A (Tables S1 – S4). Site and fish data are listed in Table 1. The available data for partitioning as determined by the bioconcentration factor (BCF) and organic-carbon normalized sediment-water partition coefficient (Koc) and listed in Table S4. Most values in this table are estimated based on various schemes, many of which are based on water solubility and an octanol-water partition coefficient (Kow) dependent regression. These approximations likely underestimate actual values for ionizable organic compounds.
Table 2.
Range of observed concentrations for CECs detected in water or fish.
| Analytes | Range for effluent (ng/L) | Range for estuary water (ng/L) | Range for salmon (ng/g) | Range for sculpin (ng/g) | WWTP output (g/d)# | Percentile ranking for effluent | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Bremerton | Tacoma | ||||||
| Albuterol | 36 – 41 | 12 | 0.54 | 2.03 | > 90th | ||
| Alprazolam | 3.0 – 4.0 | 0.38 | 0.04 | 0.23 | > 95th | ||
| Amitriptyline | 88 – 119 | 0.58 – 0.68 | 1.58 | 4.97 | > 99th | ||
| 10-OH-amitriptyline | 43 – 60 | 0.19 – 0.21 | 0.09 | 0.13 | 0.80 | 2.43 | *> 99th |
| Amlodipine | 9.7 – 26 | 0.62 – 1.0 | 0.13 | 1.49 | > 99th | ||
| Amphetamine | 67 – 164 | 2.2 – 29 | 3.4 – 25 | 7.3 – 25 | 2.17 | 3.81 | > 99th |
| Androstenedione | 8.4 | 0.11 | |||||
| Atenolol | 1,700 – 2,440 | 3 – 22 | 22.5 | 138.5 | > 95th | ||
| Atorvastatin | 68 | 3.87 | *> 99th | ||||
| Azithromycin | 261 – 629 | 2.2 | 1.7 | 8.33 | 14.8 | ||
| Benzoylecgonine | 151 – 293 | 0.50 – 0.80 | 3.88 | 8.57 | |||
| Benztropine | 0.57 – 0.93 | 0.20 | 0.01 | 0.03 | *> 99th | ||
| Bisphenol A | 350 – 4,290 | 2.8 – 4.3 | 3.3 – 41 | 3.6 – 4.5 | 4.64 | 243 | |
| Caffeine | 152 – 1170 | 18 | 13 | 15.5 | 8.63 | ||
| Carbamazepine | 510 – 735 | 1.9 | 6.76 | 41.7 | > 99th | ||
| Cimetidine | 194 | 11.0 | > 99th | ||||
| Ciprofloxacin | 158 – 192 | 7.3 | 17 | 2.54 | 8.97 | > 80th | |
| Clarithromycin | 52 – 181 | 0.69 | 10.3 | ||||
| Cocaine | 9 – 59 | 0.30 | 0.78 | 0.48 | |||
| Codeine | 290 – 178 | 2.36 | 16.5 | ||||
| Cotinine | 115 – 340 | 4.50 | 6.53 | ||||
| DEET | 23.3 – 684 | 2.4 – 5.3 | 0.39 – 1.6 | 0.41 – 2.2 | 9.06 | 1.32 | |
| Diazepam | 1.5 – 2.2 | 0.39 | 0.25 | 0.03 | 0.09 | ||
| Dehydronifedipine | 13 – 15 | 0.20 | 0.73 | ||||
| Diltiazem | 390 – 425 | 0.52 – 0.75 | 1.4 – 1.6 | 5.17 | 24.1 | > 99th | |
| Diltiazem desmethyl | 82 – 148 | 0.06 – 1.5 | 0.07 – 0.08 | 1.96 | 4.64 | > 99th | |
| Dimethylxanthine 1,7 | 873 – 2060 | 27.3 | 49.6 | ||||
| Diphenhydramine | 1030 – 1240 | 0.96 – 1.5 | 0.24 – 2.7 | 0.28 | 16.4 | 58.5 | |
| Enalapril | 5.9 | 1.2 | 0.34 | > 80th | |||
| Erythromycin | 87 – 138 | 3.3 | 0.90 | 1.83 | 4.96 | ||
| Estrone | 4.5 – 58 | 0.77 | 0.25 | > 85th | |||
| Fluocinonide | 6.5 | ||||||
| Fluoxetine | 57 – 60 | 4.9 | 0.75 | 3.38 | > 99th | ||
| Furosemide | 994 – 1290 | 17.1 | 56.4 | > 95th | |||
| Gemfibrozil | 1360 – 1640 | 3.4 – 4.5 | 1.3 | 21.7 | 77.2 | > 90th | |
| Glipizide | 22 – 23 | 0.29 | 1.24 | *> 99th | |||
| Glyburide | 7.6 – 11 | 0.14 | 0.43 | *> 99th | |||
| α-HBCDD | 0.10 – 0.20 | ||||||
| γ-HBCDD | 0.42 | ||||||
| Hydrochlorothiazide | 411 – 578 | 7.66 | 23.3 | ≈ 5 th | |||
| Hydrocodone | 69 – 74 | 0.98 | 3.93 | > 80th | |||
| Ibuprofen | 116 – 1060 | 14.0 | 6.59 | > 80th | |||
| 2-OH-ibuprofen | 1,160 – 4,550 | 60.3 | 65.9 | > 95th | |||
| Lincomycin | 27 | 1.55 | *> 99th | ||||
| MBP | 289 – 491 | ||||||
| MEHP | 0.40 | 0.02 | |||||
| Meprobamate | 513 – 623 | 8.25 | 29.1 | ||||
| Metformin | 29,300 – 82,700 | 105 – 832 | 28 | 388 | 4695 | ||
| Metoprolol | 805 – 835 | 10.7 | 47.4 | > 90th | |||
| Miconazole | 4.9 | 1.8 | 0.28 | ||||
| Naproxen | 106 –701 | 9.29 | 6.02 | ||||
| Norfluoxetine | 17 – 28 | 0.68 – 3.2 | 0.37 | 0.97 | > 99th | ||
| Norverapamil | 13 – 14 | 0.12 – 0.47 | 0.20 – 0.30 | 0.17 | 0.77 | > 95th | |
| 4-NP | 506 – 1690 | 41 | 30 – 76 | 7.7 – 35 | 6.70 | 95.9 | |
| NP1EO | 1,220 – 1,760 | 1.3 – 60 | 3 – 4.9 | 23.3 | 69.3 | ||
| NP2EO | 1,690 – 2,610 | 1.4 – 51 | 1.9 – 17 | 34.6 | 95.9 | ||
| Ofloxacin | 108 – 387 | 5.13 | 6.13 | > 90th | |||
| Ormetoprim | 44 – 1,600 | ||||||
| Oxycodone | 158 – 231 | 2.09 | 13.1 | > 95th | |||
| Paroxetine | 6.6 – 42 | 0.56 | 0.37 | *> 99th | |||
| PFBA | 6.7 | 0.38 | |||||
| PFBS | 13 | 0.17 | |||||
| PFDA | 0.78 | ||||||
| PFHpA | 3 – 7.5 | 0.10 | 0.17 | ||||
| PFHxA | 15 – 53 | 0.71 | 0.86 | ||||
| PFHxS | 55 | 0.73 | 0.00 | ||||
| PFNA | 2 | 0.11 | |||||
| PFOA | 7.6 – 12 | 0.16 | 0.43 | ||||
| PFOS | 461 | 1.2 – 34 | 1.1 – 1.4 | 6.11 | 0.00 | ||
| PFOSA | 0.82 – 2.2 | ||||||
| PFPeA | 3.4 – 4.7 | 0.06 | 0.19 | ||||
| Promethazine | 3.8 | 0.21 | *> 99th | ||||
| Propoxyphene | 0.7 – 1.9 | 0.02 | 0.04 | > 80th | |||
| Propranolol | 76 – 109 | 1.00 | 6.19 | > 95th | |||
| Ranitidine | 494 | 0.75 | 0.82 – 1.1 | 0.97 | 28.1 | > 95th | |
| Roxithromycin | 3.8 | 0.22 | |||||
| Sertraline | 89 – 116 | 17 | 0.20 | 1.54 | 5.05 | > 95th | |
| Simvastatin | 34 | 1.95 | *> 99th | ||||
| Sulfadiazine | 0.88 | ||||||
| Sulfadimethoxine | 8.2 | 0.46 | 0.34 – 17 | 0.47 | *> 99th | ||
| Sulfamerazine | 0.51 | ||||||
| Sulfamethoxazole | 1380 | 1.5 – 4.2 | 78.4 | > 90th | |||
| Testosterone | 1.9 | ||||||
| Thiabendazole | 24 – 27 | 0.36 | 1.35 | ||||
| Triamterene | 151 – 156 | 2.00 | 8.86 | > 95th | |||
| Triclocarban | 12 – 17 | 6.5 | 0.16 | 0.96 | |||
| Triclosan | 250 – 538 | 5.2 | 26 | 7.13 | 14.2 | ||
| Trimethoprim | 742 – 852 | 2.3 | 9.83 | 48 | > 99th | ||
| Valsartan | 2010 – 3000 | 5.4 | 26.6 | 170 | > 80th | ||
| Verapamil | 40 – 44 | 0.30 – 0.60 | 0.07 – 0.27 | 0.54 | 2.52 | > 80th | |
| Virginiamycin M1 | 10 | 8 – 34 | |||||
| Warfarin | 6.2 | 0.35 | *> 99th | ||||
| Detected analytes | 81 | 25 | 37 | 21 | |||
| Sum kg/d for sample flow | 0.82 | 6.66 | |||||
| kg/d at maximum flow | 3.5 | 17 | |||||
All blank values indicate a concentration <RL. Range shows minimum and maximum for each matrix (effluent, estuary water, or fish tissue) and type (sculpin or salmon). All single values indicate at least one site with a quantifiable concentration. Tissue concentrations are whole-body wet weight. Grams/day (g/d) for each analyte shown based on measured concentration (Table S4) and flow rate on the date of collection (personal communication from plant operators). Also shown is predicted kg/d for flow at the time of sampling and maximum flow. Our effluent concentrations expressed as percentile ranking compared to Kostich et al. (2013, 2014) who analyzed 56 active pharmaceutical ingredients in the 50 largest WWTPs in the U.S.
= all values from Kostich et al. (2014) below detection but detected in the present study. See Table S1 for all analyte abbrevations and text for details.
3.1. Occurrence and concentrations of CECs in WWTP effluents
We detected 81 analytes in WWTP effluent (Table S4) representing 55% of the total analyzed. Several of these analytes (15) were detected at concentrations greater than 1,000 ng/L (low ppb range) and 8 of those analytes were detected in estuarine water. A few compounds were observed in estuarine waters but not effluent, including sulfadimethoxine, sulfamethoxazole, testosterone, and mono-n-butyl phthalate, the latter a metabolite of dibutyl phthalate. In general, the detection frequency and concentrations were similar for a given type of media (e.g. effluent or estuary water) among impacted sites, although there were several notable differences (Table S4). For effluent, 77 analytes were detected in the Tacoma effluent, with 15 being unique for this type of matrix and location. The Bremerton WWTP effluent contained 66 detected compounds, with 4 (PFOS, PFBS, PFHxS, and androstenedione) being unique to this effluent. Several of the 15 analytes detected in Tacoma effluent and not the Bremerton effluent were observed at elevated concentrations (>20 ng/L). For the 62 compounds detected in both WWTP effluents there was no clear pattern of dominance with respect to concentration. However, comparing between the Bremerton and Tacoma effluent we found substantially higher concentrations in the Bremerton effluent compared to the Tacoma effluent for DEET (684 v. 23 ng/L), caffeine (1,170 v. 152 ng/L), BPA (350 v. 4,290 ng/L), and estrone (58 v. 4.5 ng/L) (Table S4), which may indicate regional differences in usage.
3.2. Occurrence and concentrations of CECs in estuary waters
In the present study, we detected 25 CEC analytes in estuarine waters (Table S4). The estuary samples from both Sinclair Inlet and the Puyallup estuary contained 16 – 17 analytes with 5 or 6 analytes unique to each estuary. The Nisqually reference site contained 10 detectable analytes, including comparatively high concentrations of 4-nonlyphenol (4-NP), and monobutyl phthalate (Table S4). All analytes detected in effluent were considered as a source to estuarine waters in terms of mass per day. Based on both the effluent flow rate at the time of collection and measured concentrations, the total amount of detected analytes flowing into their respective estuarine waters ranged from 0.8 and 6.6 kg/d for the Bremerton Westside and Tacoma Central WWTPs (Table 2). During “maximum design flows” occurring October – April, CEC inputs from these WWTP could be substantially higher at 3.5 and 16.8 kg/d, which is based on flow data obtained from Bremerton Westside Factsheet (2013) and Tacoma Central WWTP Factsheet (2004). These values would not account for episodic releases of influent during peak flows that bypass secondary treatment. Based on the data presented in Lubliner et al. (2010), influent concentrations can be 1 – 2 orders of magnitude higher than effluent concentrations for many PPCPs.
3.3. CECs in sculpin and salmon tissues
A number of compounds were found in fish and not in effluent or estuary water. These include PFDA, PFOSA, enalapril, benztropine, fluocinonide, sulfadaizine, sulfamerazine, virginiamycin M1, and ormetoprim (Table 2). Interestingly, ormetoprim is widely used in hatcheries to treat fish under the trade name Romet™, and likely was in some hatchery fish at the time of release. Sulfadimethoxine is also a component of Romet™ and was found only in salmon; however it was detected in effluent and estuary water, and therefore it is not known if tissue levels were due to estuarine or hatchery exposure. The compounds HBCDD (not analyzed in water), PFDA, and PFSOA have been detected in fish or WWTP effluent in Puget Sound or its watershed (Washington Department of Ecology, 2010; Johnson and Friese, 2009) and are likely from industrial sources in the area. Conversely, even though phthalate ester metabolites were not analyzed for tissue samples they likely occurred in whole-body fish because of their relatively high Kow and elevated concentrations in estuary water. Sulfadiazine has been reported in effluent by Verlicchi et al. (2012). The source of the remaining compounds sulfamerazine, fluocinonide, and virginiamycin is unclear. Virginiamycin is an antibiotic approved for large animal use and may occur in estuaries from runoff. A review of the literature did not reveal any studies reporting detectable concentrations for these compounds in either effluent or fish tissue.
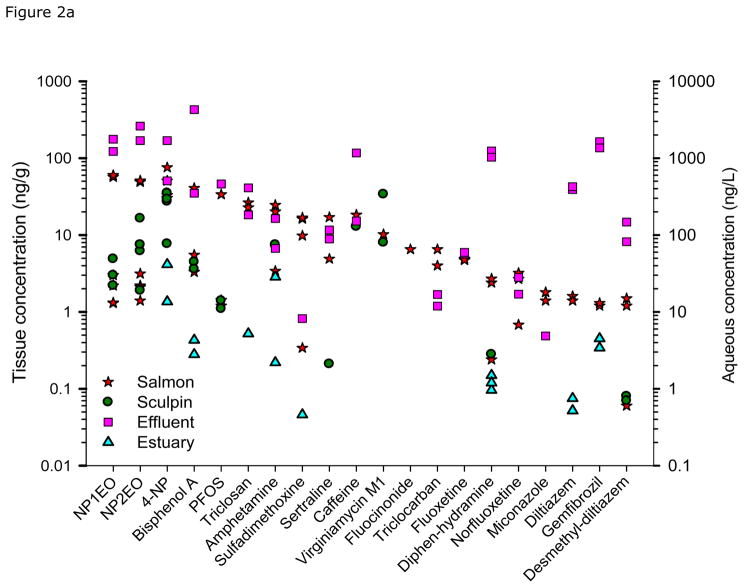
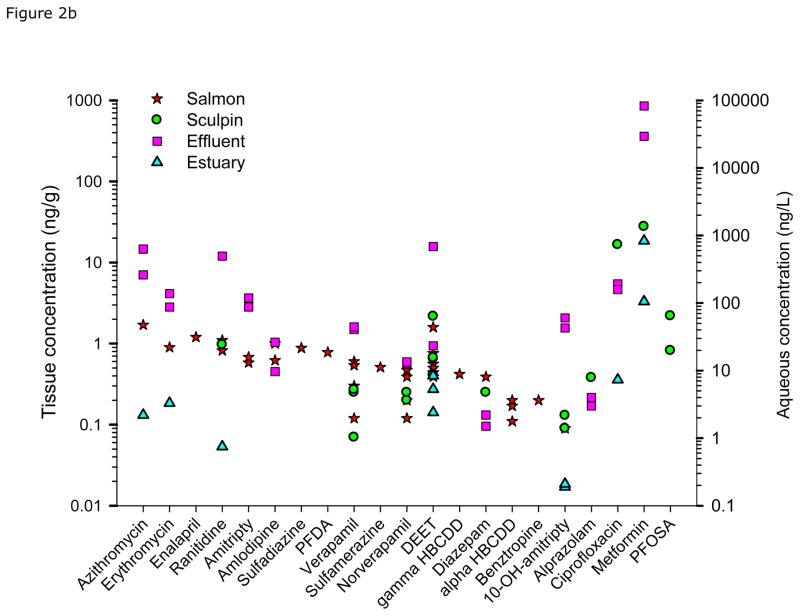
Collectively, we detected 42 compounds in whole-body fish (Tables 2 and S5). CECs in juvenile Chinook salmon were detected at greater frequency and higher concentrations compared to staghorn sculpin. Figure 2 shows the concentrations of detected analytes in fish, estuary water, and effluent sorted by occurrence from high to low concentrations in salmon tissue. In general, juvenile Chinook salmon from the Puyallup estuary contained a greater frequency of detected analytes (25) and higher concentrations (most > 1 ng/g) than that observed for Chinook collected in Sinclair Inlet. Notable compounds occurring at comparatively high concentrations in juvenile Chinook from the Puyallup estuary include amphetamine, azithromycin, diltiazam, diphenhydramine, fluoxetine, gemfibrozil, miconazole, norfluoxetine, sertraline, sulfadimethoxine, triclosan, triclocarban, virginiamycin, and nonylphenol and its metabolites. Chinook collected in Sinclair inlet contained 19 detected analytes and most were lower in concentrations compared to Puyallup Chinook with some exceptions (e.g., PFOS, caffeine, and fluocinonide). Nisqually Chinook salmon contained 13 detected analytes; however most exhibited low concentrations, except for nonylphenol. Chinook salmon from both effluent sites contained several CECs at concentrations substantially higher than those observed for Nisqually Chinook. We detected 7 analytes in juvenile Chinook collected from the Voight’s Creek Hatchery. Two of these analytes (benztropine and enalapril) were found only in these fish, which have been detected in WWTP effluent or lake water in other studies (Verlicchi et al., 2012; Ferrey, 2013). Three of the other detected compounds (BPA, nonlyphenol, and DEET) were unusually elevated in these fish. Values for nonylphenol and bisphenol A in fish tissue were as high or higher than levels found in estuary fish and may have come from leaky septic systems in the area or other discharge upstream.
Figure 2.
Plot showing occurrence of detected analytes in fish, estuary water, and effluent. Data are ordered from high to low concentrations in juvenile chinook. All replicate data shown for each matrix.
Among sculpin, concentrations of detected analytes were relatively similar between the 2 effluent sites, both in terms of chemical concentrations and frequency of occurrence. Sculpin from the Nisqually estuary reference site contained 9 detected analytes, including several at comparatively high concentration. The predominant analytes in sculpin harvested from the Nisqually included nonylphenol, caffeine, ciprofloxacin, and metformin. However, based on water concentrations, sculpin from the effluent sites were exposed to higher numbers and concentrations of contaminants than those collected in the Nisqually estuary, many of which were likely not bioaccumulated to levels above the analytical detection limit.
Based on the relatively rapid half-life for several of the compounds tested for (Table S2) and the lag time between capturing fish in the field and sacrifice in the lab (3 – 6 h), many of the analytes examined in this study may have been higher, some substantially, in feral fish. Therefore the reported concentrations may underestimate, sometimes by a large margin, the concentrations accumulated by fish in these estuaries or even fall below detection after capture if elimination is particularly rapid.
4. Discussion
The greater Puget Sound area contains 106 publicly owned WWTPs that discharge at an average total flow about 1,347 MLD (Washington Department of Ecology, 2010). Our study examined 2 of these with a combined total of 71 MLD. The output for these 2 WWTPs alone was on the order of kg quantities of detected CECs per day into estuarine waters of Puget Sound. Considering the low percentage of commercially available PPCPs analyzed in this study and the amount of effluent discharged to Puget Sound waters, it is apparent that a substantial load of potentially harmful chemicals are introduced into streams and nearshore marine waters daily. If the concentrations from the 2 studied effluents are representative of that from other WWTPs in Puget Sound, then it is reasonable to assume that inputs to streams and nearshore waters are substantial and likely on the order of 100 kg/d (≈ 36,500 kg annually) and even higher if secondary treatment bypass, permitted flows, maximum outputs, unmeasured compounds, septic system contributions, and transboundary contributions are considered.
Based on our water and fish data, the Nisqually estuary was more contaminated than expected, which highlights the difficulties of establishing suitable non-polluted reference sites for these ubiquitously distributed CECs (Ferguson et al., 2013). It is noteworthy that for all 3 estuaries investigated in the present study, a few analytes (e.g., cocaine, ciprofloxacin, and ranitidine) were found only in estuary water at our reference site, even though compounds were present in effluent from the contaminated sites. Although the source of these compounds to the Nisqually estuary is unknown, the Nisqually River, Nisqually Reach, and McAllister Creek are all included on the 303(d) list of water bodies that do not meet water quality standards for fecal coliform bacteria, which may be caused by leaking septic systems (Washington Department of Ecology, 2007; Washington Department of Ecology, 2015). Even though a number of analytes were surprisingly elevated in water and tissue (e.g., nonylphenol, diphenhydramine, ciprofloxacin, DEET, and metformin), overall the frequency of occurrence and concentrations of these contaminants in the Nisqually estuary were generally low relative to the effluent-impacted sites. While it is unknown if these chemicals alone or in combination are sufficiently elevated to result in adverse effects, we are conducting other studies that link exposure to CECs with adverse physiological outcomes in sculpin and salmon.
4.1. CECs in water and fish tissue
Compared to other marine studies, our results for effluent were generally similar to those reported by Vidal-Dorsch et al. (2012) and Hedgespeth et al. (2012) for the few overlapping analytes. As for surface waters, our values for the few analytes in common for each study were generally greater than the reported values in Vidal-Dorsch et al. (2012), but lower than the values observed in Charleston Harbor, San Francisco Bay, and the Gulf of Mexico estuaries (Hedgespeth et al., 2012; Klosterhaus et al., 2013; Scott et al., 2015). For the 16 effluent CECs in common between Lubliner et al. (2010) and the present study for Puget Sound, most of the analytes reported in the present study were observed at higher concentrations, which could be a result of increased rates of usage for these CECs or differences in the treatment processes among plants.
As discussed, our results indicate a large number of analytes in effluent were below their respective limits of analytical detection in estuarine waters. These chemicals may have been present at extremely low levels in water and fish but could not be quantified; however, this does not imply the absence of potential toxic effects as noted by Schlenk et al. (2012) for mixtures of CECs. It is noteworthy that our estuarine water samples were collected several hundred meters from the effluent outfalls and at a depth of only 2 meters, thus reported concentrations likely underestimate those occurring in deeper water and closer to outfalls. The effluent plume is expected to move horizontally with currents before substantial mixing occurs (Environment Canada, 2003).
To better understand the characteristics of our WWTP effluents relative to those in other locations, we compared our effluent concentrations to those reported by Kostich et al. (2014) for the 50 largest WWTPs in the U.S., none of which discharged to marine waters or were located in the Pacific Northwest. The Kostich et al. (2014) data for 53 pharmaceuticals and 7 metabolites were summarized statistically and compared to our measured values in the two effluents. As observed in Table 2, the results of our comparison to the Kostich et al. (2014) data overlapped on 45 compounds. The CEC analyte concentrations observed in our study were generally higher than most values for a given compound measured in the 50 WWTP effluents, which is reflected in the percentile ranking of our values to those presented in Kostich et al. (2014). Our concentrations were greater than the 90th percentile for values from all 50 WWTPs (most >99th percentile) for 34 of those 45 analytes. For 10 of the common analytes, all 50 effluent values in that study were below their reporting limits, but were detected in our study (OH-amitriptyline, atorvastatin, benztropine, lincomycin, paroxetine, promethazine, simvastatin, sulfadimethoxine, testosterone, and warfarin). Conversely, we report non-detectable concentrations for acetaminophen, sulfamethazine, and theophylline in effluent whereas detectable concentrations were reported in the Kostich et al. (2014) dataset. While our observed concentrations were among the highest reported for effluent in the United States, higher concentrations have been reported in secondary effluent in other countries (Verlicchi et al., 2012).
The concentrations obtained in our one-time sampling event for each estuary are likely representative of samples taken for other time points throughout the year and not expected to exhibit substantial temporal variability. One study on CECs in the marine environment examined temporal variability of effluent and receiving water concentrations and observed little difference among the 4 seasons for the 56 analytes examined (Vidal-Dorsch et al., 2012). Some seasonality was observed by Hedgespeth et al. (2012) in their study of 19 CEC compounds in effluent and surface water, who noted higher frequencies of detection in winter compared to summer, a similar phenomenon observed by Daneshvar et al. (2010). Higher frequency of detection and greater concentrations during winter months are likely due to colder temperatures inhibiting bacterial metabolism and reduced photolysis (Vieno et al., 2005; Daneshvar et al., 2010; Hedgespeth et al., 2012), which may offset any dilution due to potential stormwater inputs. Additionally, for some PPCPs there is likely a seasonal component for usage rates by consumers. For example, some chemicals such as antihistamines may be more prevalent during spring and summer months, whereas others such as DEET, are expected to be lower during winter.
Despite the widespread occurrence of CECs and importance whole-body tissue concentration in risk assessment and regulatory frameworks (Sappington et al. 2011), we found no comprehensive studies reporting on whole-body tissue concentrations for these compounds in field-collected fish. Clearly, this is an important data gap in assessing the environmental risk of CECs. Choosing one representative tissue for assessing toxic effects and bioaccumulation is generally more problematic than analyzing whole bodies. Whole-body concentrations are likely a better surrogate for toxic dose and bioaccumulation compared to individual organ concentrations due to greater comparability among species toxicity metrics and bioaccumulation factors and because of the inherent variability for target-organ specificity and lipid content, in addition to confounding effects and seasonal differences (Meador et al., 2008). Many studies provide data on organ-specific concentrations, which are generally higher than reported for whole-body concentrations. Ramirez et al. (2009) examined PPCPs in fish tissue from 5 effluent-dominated streams and one recent review (Daughton and Brooks, 2011) summarized the known data for wild fish. The report of Ramirez et al. (2009) and the present study have 5 analytes in common that were detected in fish tissue (norfluoxetine, sertraline, diphenhydramine, diltiazem, and triclosan). Ramirez et al. (2009) detected carbamazepine in tissue, whereas we detected this compound only in effluent and estuary water. The 2 studies are not directly comparable because Ramirez et al. (2009) reported concentrations for fish fillets and liver. Another interesting comparison is the San Francisco Bay data for co-located water and mussel tissue concentrations (Klosterhaus et al., 2013). Even though most of their estuarine water concentrations were higher than our values, our fish tissue concentrations were higher, sometimes substantially, compared to mussel tissue, with notable exceptions for carbamazepine, DEET, and NP2EO.
4.2. CEC physicochemical characteristics and bioaccumulation
Compounds with log10Kow value > 2 were more likely to bioaccumulate in fish; however, compounds with relatively short half-lives (less than 24 h) would not be expected to appreciably bioaccumulate due to elevated rates of clearance and/or metabolism. Unfortunately, a review of the literature revealed few values for chemical half-lives in fish. For most compounds with elimination data for both humans and fish, the reported half-life for humans was much shorter than that observed for fish (Table S2). It should be noted that human half-life values are for plasma and they may be representative of whole-body half-life only if the compounds moved freely among tissues and were not sequestered or stored in other tissues. Therefore human plasma half-lives are likely not directly comparable to whole-body half-lives for fish, but may be a reasonable estimation for relative persistence in tissue.
4.2.1. Bioaccumulation of CECs in sculpin and salmon
As discussed by Daughton and Brooks (2011), pharmaceuticals are generally more polar and less hydrophobic than most environmental contaminants considered in risk assessments and therefore do not preferentially associate with sediment or tissue. While these compounds remain mostly dissolved in water, they can be bioaccumulated by organisms through ventilation, ingested water, and prey and therefore may interact with receptor targets resulting in pharmacological effects if concentrations are high enough. Even though predicted bioaccumulation and bioconcentration factors estimated with Kow values are relatively low, it is well known that many ionic compounds do not bioaccumulate according to these predicted values (Meador 2000; Fu et al., 2009; Daughton and Brooks 2011). One study that measured plasma bioconcentration factors in fish found large variation among sites that was not attributed to aqueous concentration, pH, exposure time, or temperature (Brown et al., 2007), indicating the difficulty of predicting tissue concentrations.
Of the 69 PPCPs detected in water or fish in the present study, 70% are ionizable organic compounds. Bioaccumulation of polar and ionizable compounds is generally not predictable with the current target lipid model (Di Toro et al., 2000) that is premised solely on hydrophobic partitioning to organismal lipid. Instead of passive diffusion across membranes that can be easily modeled, predictions of bioaccumulation for many CECs demand an evaluation based on toxicokinetics, passive diffusion, and active transport, which can vary widely among species (Daughton and Brooks 2011; Meredith-Williams et al., 2012). Active transport is likely an important mechanism to consider because a large number of drugs are known to be taken up across biological membranes by one of several known transporters (Dobson and Kell 2008).
Various estimates for the percentages of commercially available drugs that are ionizable range from 63 – 95% (Manallack 2007) indicating this as an important factor for determining bioaccumulation, toxicity, and environmental fate. Specifically, organic compounds with pKa values several units above or below the pH of seawater (pH ≈ 8 – 8.1) are expected to be ionic and may not readily accumulate in fish, unless there is active transport across gill or gastrointestinal membranes. Wells (1988) estimated that 75% of pharmaceuticals are weak bases, indicating that pKa is a crucial factor for assessing bioaccumulation and toxicity in marine waters especially when pH – pKa > −3 to 1 (Rendal et al., 2011).
A number of compounds in Table S2 have relatively high log10Kow values (>3) and pKa values similar to seawater (pH approx. 8.0), indicating a high potential for bioaccumulation in aquatic organisms. It is not known if these high Kow compounds would exhibit even higher bioaccumulation as a result of active transport over that predicted based on thermodynamics (e.g., the target lipid model). In the present study, most of the compounds that were detected in fish are characterized by high Kow values (Table S2), with the exception of amphetamine, caffeine, ciprofloxacin, DEET, ranitidine, and sulfadimethoxine. It is unknown if pKa would play a role in bioaccumulation for these low Kow compounds. It should be noted that BCF values may be a poor estimator of bioaccumulation for some of these compounds in the field. For example, the steady-state BCF values for caffeine, carbamazepine, and diphenhydramine determined in the laboratory for mosquito fish (Gambusia holbrooki) were 2, 1.4, and 16, respectively, whereas the BCF values for this species naturally exposed to these compounds in a pond were 29, 108, and 821 (15 – 77× greater), indicating that dietary exposure is likely important for bioaccumulation (Wang and Gardinali, 2012).
As noted by Rendal et al., (2011), organic bases such as fluoxetine, norfluoxetine, propanolol, lidocaine, sertraline, and trimipramine, exhibit increasing toxicity for algae and fish with rising pH, with large differences between pH 6.5 and 8.5. As shown for fluoxetine (pKa = 9.8) each unit increase in pH from 7 – 9 caused both the log10Kow and levels of unionized fluoxetine to increase 10-fold (Nakamura et al., 2008). These data indicate a much greater potential for bioaccumulation in aquatic environments with greater than neutral pH, such as marine systems. This was confirmed by Nakamura et al. (2008) who showed a substantial increase in the fluoxetine BCF for fish (30-fold) in addition to a 28-fold decrease in the LC50 (more toxic) as pH increased from 7 to 9.
Even though observed and predicted BCFs for many CECs are relatively low (e.g., 3 – 10, Table S4), salmon and sculpin collected in the present study contained higher than expected concentrations when based on analytes detected in estuary water. These higher than predicted tissue concentrations could be due to additional sources, such as upriver inputs or foodweb magnification. One study demonstrated large differences in bioconcentration factors among invertebrates exposed to a number of pharmaceuticals with species varying 10 – 100 fold (Meredith-Williams et al., 2012). Notably, these authors reported a BCF of 185,900 for fluoxetine in the amphipod (Gammarus pulex), which may contribute to higher than expected fish tissue concentrations. Such differences are often due to variable uptake and elimination kinetics among species, similar to those described for invertebrates exposed to tributyltin, which is both polar and ionizable (Meador 1997). The unexpectedly large differences in tissue concentrations for juvenile Chinook salmon and staghorn sculpin in this study are unknown; however such differences noted above for invertebrate prey, in addition to variability in ventilation and ingestion rates between fish species, potential metabolic differences, and degree of mobility may explain the disparity. As noted in Meador (2014), Chinook salmon can exhibit high rates of ingestion and gill ventilation.
4.4. Classes of compounds
Noteworthy groups of compounds are highlighted due to the high frequency of occurrence and potential to cause adverse effects in fish.
4.4.1. Pharmaceuticals
4.4.1.1. Hormones
Many pharmaceuticals are considered endocrine disrupting (ED) compounds affecting reproductive function (Diamanti-Kandarakis et al., 2009). Hormones are the most potent EDs affecting fish at low ng/L concentrations and several were detected in effluent or estuarine waters (androstenedione, estrone, and testosterone). Estrone (E1) was elevated in the Bremerton effluent and the measured value (58 ng/L) is in the 85th percentile of all measured effluent values from U.S. WWTPs as summarized by Kostich et al. (2013). Dammann et al. (2011) reported increased levels of vitellogenin, altered secondary sexual characteristics, and enhanced aggression in male fish exposed at aqueous concentrations of estrone ranging from 15 – 54 ng/L, exhibiting a similar potency as 17-α-ethinylestradiol (EE2). Dietary uptake may be a substantial source of these compounds for fish species. The predicted E1 BCF for fish is 54 (Table S4); however Daphnia magna exhibited a BCF for E1 of 228 (Gomes et al., 2004), which may be representative of bioaccumulation in other invertebrates and could lead to enhanced tissue concentrations in fish.
4.4.1.2. Antibiotics
In our study, 16 antibiotic compounds were detected in water and fish tissue. Excess antibiotics in the water may affect the natural composition of bacteria externally and internally in fish (Daughton and Brooks 2011, Carlson et al., 2015). Possible effects include the suppression of beneficial bacteria and enhancement of pathogenic bacterial resistance to antibiotics. A number of authors have raised the possibility that continuous discharge of antibiotics into surface waters may increase the occurrence of antibiotic resistant strains of bacteria (Kristiansson et al., 2011, Berglund 2015). Several macrolide antibiotics (-mycins, Table S2), were detected and they summed to approximately 500 – 980 ng/L in effluent, 5 ng/L in estuarine water, and 13 – 34 ng/g in whole-body fish. Because a number of these antibiotics work by the same MoA (e.g., macrolide antibiotics at a specific site on subunit 50S of the bacterial ribosome), their effect concentration may be considered together through dose addition (Meador 2006).
4.4.1.3. Central nervous system agents
A large number (25) of detected compounds in this study are used to modulate neurological function in humans. These include serotonin selective re-uptake inhibitors (SSRIs) in addition to central nervous system stimulants, narcotics, and analgesics. These compounds have been widely prescribed to treat anxiety, epilepsy, and hypertension in humans (Table S2). Many of these chemicals may also affect behavioral function in fish and invertebrates, even at the relatively low concentrations found in contaminated receiving waters (Painter et al., 2009; Brooks, 2014). Surprisingly, algal growth was very sensitive to fluoxetine (Brooks et al., 2003).
Two of the antidepressants, sertraline and fluoxetine, are especially noteworthy because these were observed in juvenile Chinook (Table 2) at concentrations higher than those reported by Brooks et al. (2005) for 3 species of fish from an effluent-dominated stream. A number of studies have examined effects of sertraline and fluoxetine in fish and report a large range in aqueous concentrations causing adverse effects. For example, Schultz et al. (2011) reported increased mortality and histological alterations in the testes for sertraline and fluoxetine and increased vitellogenin production in male fathead minnows exposed to very low concentrations (1.6 – 5.2 ng/L of sertraline and 28 ng/L for fluoxetine), which are substantially lower than effluent concentrations reported in the present study. In Schultz et al. (2011), reported concentrations of these compounds in brain tissue were very low (0.17 ng/g for fluoxetine and 0.02 – 0.06 ng/g for sertraline). While the concentrations of these SSRIs were below detection limits in estuarine water in the present study, our whole body concentrations for these compounds were elevated (5 – 17 ng/g) for juvenile Chinook salmon. Because neural tissue preferentially accumulates sertraline and fluoxetine and exhibits concentrations that are higher than other tissues (Brooks et al., 2005; Schultz et al., 2010), whole-body concentrations are likely lower than that expected for brain tissue, suggesting that brain tissue of juvenile Chinook salmon in our study contained very high levels of these antidepressants. Additionally, the metabolite norfluoxetine binds the serotonin reuptake transporter with a similar affinity as fluoxetine and is considered as potent as the parent compound. Based on these characteristics, it is reasonable to sum the concentrations of these compounds to determine the potential for adverse effects for this MoA (Daughton and Brooks, 2011).
4.4.1.4. Metabolic regulators
A number of compounds that target metabolic abnormalities (e.g. metabolic regulators) such as diabetes, elevated lipids, and hyperglycemia were observed in effluent, estuarine water, and fish tissue. These include atorvastatin, gemfibrozil, glipizide, glyburide, metformin, and simvastatin and they have the potential to act as metabolic disruptors affecting growth, lipid homeostasis, and energy balance in nontarget organisms when introduced to the environment (Casals-Casas and Desvergne, 2011). Other chemicals that are known metabolic disruptors were also detected at high concentrations in the present study, including bisphenol A, nonylphenols, phthalates, and perfluorinated compounds.
Metformin, a medicine to treat diabetes, was the analyte detected at the highest concentration in effluent (29,300 – 82,700 ng/L) with very high concentrations in estuary water (up to 832 ng/L). The high metformin concentration observed in sculpin from the Nisqually estuary (27.8 ng/g) was surprising given the very low Kow for this compound. A recent study (Niemuth and Klaper 2015) demonstrated reduced growth in male fathead minnow (Pimephales promelas) and extensive disruption of reproductive parameters in both sexes of this species exposed to metformin at 40,000 ng/L. Another recent study demonstrated significant increases in mRNA transcripts for vitellogenin, estrogen receptor-alpha, gonadotropin releasing hormone 3, and cytochrome P450 3A4-like isoform in juvenile fathead minnow exposed to concentrations in water as low as 1 ng/mL (Crago et al., 2016).
4.4.2. Personal care products
Triclosan and triclocarban were detected in effluent and salmon tissue. Only triclosan was detected in estuary water (Sinclair Inlet) and was present at 5.2 ng/L, which would theoretically result in a fish tissue concentration of 0.47 ng/g, given the observed fish BCF of 90 for this compound. A high concentration was observed in salmon tissue from the Puyallup estuary (mean = 24.4 ng/g), which may be due to foodweb magnification from algae and invertebrate species that exhibit relatively high bioaccumulation factors (500 – 1,000) (Hontela and Habibi, 2014). High tissue concentrations are also expected in higher trophic level species such as marine mammals (Hontela and Habibi, 2014). Given the observed BCF for triclosan, our reported tissue concentration in salmon would be equivalent to a water exposure concentration of 271 ng/L. Triclosan is weakly estrogenic in fish (Hontela and Habibi, 2014), but has been shown to significantly increase aggressive behavior in fathead minnows when exposed to a mixture of triclosan (560 ng/L) and triclocarban (179 ng/L) (Schultz et al., 2012). These aforementioned concentrations are only about 2-fold higher than the modeled exposure concentration (271 ng/L) expected to result in the observed salmon tissue concentration.
4.4.3. Industrial chemicals
Nonylphenol (NP) was one of the more ubiquitous compounds in our study and was observed in every sample (except Sinclair Inlet estuary water) at relatively high concentrations in water (14 – 41 ng/L) and tissue 8 – 76 ng/g). The ethoxylates of nonylphenol (NP1EO and NP2EO) were also detected in most effluent and tissue samples. The U.S. Environmental Protection Agency (2005) chronic water quality criterion (WQC) for nonylphenol in marine systems is 1.7 ng/mL, a value that approximates the observed effluent concentration for the Tacoma WWTP reported here. Also, the U.S. Environmental Protection Agency (2010) provides toxic equivalency factors (TEFs) for aquatic species exposed to nonylphenol ethoxalates and these are considered to be about 50% as potent as NP (NP = 1; NP1EO and NP2EO = 0.5). When these TEFs are applied to the observed effluent concentrations, the combined concentrations of NP and these 2 ethoxylates exceed the WQC approximately 2-fold. No toxicity data for alkylphenols in fish tissue could be found for comparison to our observed values.
Several studies indicate adverse effects for fish exposed to alkylphenols at environmentally-relevant concentrations. One study reported severe reductions in growth for rainbow trout (O. mykiss) exposed separately to 1 ng/mL of NP and NP2EO at concentrations as low as 1 ng/mL that persisted for several weeks to months after exposure was terminated (Ashfield et al., 1998). Our measured concentrations for each of these compounds in effluent was higher than this growth impairment concentration and combined were approximately 3-fold higher. The second study observed a negative correlation between catch data for Atlantic salmon and the application of a pesticide to various tributaries within a river basin during smolt development for a one year period (Fairchild et al., 1999). Based on the analysis of Fairchild et al. (1999), the authors concluded that NP (an adjuvant for the pesticide application) was responsible for excess mortality during this life stage. Similar effects were also noted by Fairchild et al. (1999) for spray events over several years for another anadromous species (Blueback herring, Alosa aestivalis).
4.5. Implications for potential adverse ecological effects in Puget Sound
As discussed, the observed water and tissue concentrations of numerous analytes detected in the two effluent impacted estuaries in Puget Sound have the potential to cause adverse effects in both fish species in this study. Endocrine and metabolic disruption may have important impacts on adult fish, such as staghorn sculpin examined here; however, metabolic disruption is even more critical for actively growing juvenile salmonids. A recent study concluded that juvenile Chinook salmon migrating through contaminated estuaries in Puget Sound exhibited a two-fold reduction in survival compared to those migrating through uncontaminated estuaries (Meador, 2014). Some of the lowest survival rates for juvenile Chinook salmon were seen for estuaries that have WWTPs discharging into the estuary or nearshore areas where this species rears before heading into open water.
Some of the compounds observed in Chinook salmon and staghorn sculpin tissue may also accumulate in larger fish that prey on these species, in addition to aquatic-dependent wildlife including birds and marine mammals (Diehl et al., 2012). Although a few studies have examined potential bioaccumulation, biomagnification, or potential adverse effects for these higher trophic-level aquatic predators (Arnold et al., 2014; Gaw et al., 2014), these are relatively uncommon. Another relatively unexplored aspect concerns the bioaccumulation and adverse effects of these compounds on estuarine invertebrates and algae, which are an important component of the foodweb for fish. In addition to enhanced bioaccumulation via dietary uptake, reductions in prey species could impact growth rates of fish residing in these estuaries.
A noteworthy outcome of the present study is the occurrence of several compounds in water and tissue that have the potential to affect fish growth, behavior, reproductive impairment, immune function, and antibiotic resistance. One recent review provides a summary of studies on the effects of endocrine disruptors on immune system in fish (Milla et al., 2011). Many of these agents, such as metformin, may impact multiple systems such as growth and reproductive pathways. It is expected that few, if any, of these compounds would result in direct mortality to estuarine organisms; however, all of the above mentioned responses could lead to indirect mortality or reduced population fitness. As noted by Spromberg and Meador (2005) and Meador (2014) even a minor inhibition in juvenile salmonid immune function or growth likely results in a major impact on survivability during their first year in marine waters.
5. Conclusions
The CECs investigated in the current study were selected based upon their widespread use, in addition to the likelihood of continued use and potential for increased contamination in the future. Accordingly, regulation and assessment of the ecological and human health risks of these compounds continue to warrant high interest as human populations increase. It should be noted that the results of the present study represent a snapshot of concentrations that exist at our sites and that may vary day-to-day and seasonally. Surprisingly, a large percentage of the chemicals detected in Puget Sound effluents are among the highest concentrations reported in the U.S., which may be a function of per capita usage of these compounds or the treatment processes used at these WWTPs. The fact that we observed multiple pharmaceuticals capable of interacting with a variety of molecular targets in our two fish species, leads to the potential for mixture interactions on critical physiological processes. These interactions can be additive, synergistic, or inhibitory, which are difficult to assess in the field or laboratory. Future work developing and applying mechanism-based biomarkers linked to physiological outcomes resulting from exposure to CECs would help close this data gap and lead to better predictions of adverse ecological impacts.
Supplementary Material
Highlights.
Provides data on a broad range of contaminants of emerging concern in marine waters and whole-body fish, which is uncommon in the literature.
Detected analytes in effluent higher than most concentrations reported for large wastewater treatment plants in the United States.
Whole-body fish tissue concentrations of CECs elevated for some analytes and occur at greater than expected concentrations.
Several compounds were observed at concentrations that may result in adverse responses in biota.
Loading of CEC analytes to estuaries considered substantial and expected to increase with human population growth.
Acknowledgments
This work was supported in part by a grant from the Washington Department of Ecology (G1300089), as well as the National Institute of Environmental Health Sciences Superfund Research Program [P42- ES004696], and the University of Washington Sea Grant Program, pursuant to National Oceanic and Atmospheric Administration Project R/OCEH-8 [NA10OAR4170057]. The authors gratefully acknowledge review comments from Dale Norton (Washington Department of Ecology) and the technical assistance in the laboratory and field provided by University of Washington personnel Richard Ramsden, Christopher Monson, Stuart Munsch, Yasushi Shibata, Anna Bute, Chase R. Williams, Monica Chang, Nancy Huizar, Margaret G. Mills, Jeff Cordell, Michael Caputo, and Jason Toft. Additional thanks to Georgina Brooks, Candice Navaroli, Cynthia Tomey, Sean Campbell, Richard Grace, and other laboratory staff at AXYS Analytical Services, Ltd, for their excellent analytical skills. We also appreciate assistance from Jay Davis, Steve Damm, and Howard Gearns of the US Fish and Wildlife Service for CWT assessment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold KE, Brown AR, Ankley GT, Sumpter JP, Arnold KE. Medicating the environment : assessing risks of pharmaceuticals to wildlife and ecosystems. Phil Trans R Soc B. 2014;369:20130569. doi: 10.1098/rstb.2013.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfield LA, Pottinger TG, Sumpter JP. Exposure of female juvenile rainbow trout to alkylphenolic compounds results in modifications to growth and ovosomatic index. Environ Toxicol Chem. 1998;17:679–686. [Google Scholar]
- Berglund B. Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infect Ecol Epidemiol. 2015;5:28564. doi: 10.3402/iee.v5.28564. http://dx.doi.org/10.3402/iee.v5.28564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremerton Westside Factsheet. [last accessed on 16.01.16];Fact Sheet for NPDES Permit WA0029289. 2013 https://fortress.wa.gov/ecy/wqreports/public/f?p=110:1000:0::NO:RP:P1000_FACILITY_ID,P1000_FACILITY_NAME:79261235,Bremerton%20Westside%20WWTP.
- Brooks BW. Fish on Prozac (and Zoloft): Ten years later. Aquat Toxicol. 2014;151:61–67. doi: 10.1016/j.aquatox.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem. 2005;24:464–469. doi: 10.1897/04-081r.1. 0730-7268/05. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Turner PK, Stanley JK, Weston JJ, Glidewell Ea, Foran CM, Slattery M, La Point TW, Huggett DB. Waterborne and sediment toxicity of fluoxetine to select organisms. Chemosphere. 2003;52:135–142. doi: 10.1016/S0045-6535(03)00103-6. [DOI] [PubMed] [Google Scholar]
- Brown AR, Gunnarson L, Kristiansson E, Tyler CR. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Phil Trans R Soc B. 2014:576. doi: 10.1098/rstb.2013.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JN, Paxeus N, Forlin L, Larsson DGJ. Variations in bioconcentration of human pharmaceuticals from sewage effluents into fish blood plasma. Environ Toxicol Pharmacol. 2007;24:267–274. doi: 10.1016/j.etap.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Hyde ER, Petrosino JF, Manage ABW, Primm TP. The host effects of Gambusia affinis with an antibiotic-disrupted microbiome. Comp Biochem Physiol Part C Toxicol Pharmacol. 2015;178:163–168. doi: 10.1016/j.cbpc.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- Crago J, Bui C, Grewal S, Schlenk D. Age-dependent effects in fathead minnows from the anti-diabetic drug metformin. Gen Compar Endocrinol. 2016 doi: 10.1016/j.ygcen.2015.12.030. http://dx.doi.org/10.1016/j.ygcen.2015.12.030. [DOI] [PubMed]
- Dammann AA, Shappell NW, Bartell SE, Schoenfuss HL. Comparing biological effects and potencies of estrone and 17β-estradiol in mature fathead minnows, Pimephales promelas. Aquat Toxicol. 2011;105:559–68. doi: 10.1016/j.aquatox.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Daneshvar A, Svanfelt J, Kronberg L, Prevost M, Weyhenmeyer GA. Seasonal variations in the occurrence and fate of basic and neutral pharmaceuticals in a Swedish river-lake system. Chemosphere. 2010;80:301–9. doi: 10.1016/j.chemosphere.2010.03.060. [DOI] [PubMed] [Google Scholar]
- Daughton CG, Brooks BW. Active pharmaceutical ingredients and aquatic organisms. In: Beyer WN, Meador JP, editors. Environmental Contaminants in Biota: Interpreting Tissue Concentrations. Taylor and Francis; Boca Raton, Fla: 2011. pp. 287–347. [Google Scholar]
- Di Toro DM, McGrath JA. Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. II Mixtures and sediments. Environ Toxicol Chem. 2000;19:1971–1982. doi: 10.1897/08-364.1. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J, Johnson SE, Xia K, West A, Tomanek L. The distribution of 4-nonylphenol in marine organisms of North American Pacific Coast estuaries. Chemosphere. 2012;87:490–7. doi: 10.1016/j.chemosphere.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7:205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- Environment Canada. [last accessed 14.01.16];Revised technical guidance on how to conduct effluent plume delineation studies. 2003 https://www.ec.gc.ca/esee-eem/default.asp?lang=En&n=E93AE5BC-1&printfullpage=true.
- Fairchild WL, Swansburg EO, Arsenault JT, Brown SB. Does an association between pesticide use and subsequent declines in catch of Atlantic salmon (Salmo salar) represent a case of endocrine disruption? Environ Health Perspect. 1999;107:349–357. doi: 10.1289/ehp.99107349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson PJ, Bernot MJ, Doll JC, Lauer TE. Detection of pharmaceuticals and personal care products (PPCPs) in near-shore habitats of southern Lake Michigan. Sci Total Environ. 2013;458–460:187–196. doi: 10.1016/j.scitotenv.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Ferrey M. Pharmaceuticals and endocrine active chemicals in Minnesota Lakes. Minnesota Pollution Control Agency; 2013. [last accessed 7.10.15]. Document number: tdr-g1-1 http://www.pca.state.mn.us/index.php/view-document.html?gid=19427. [Google Scholar]
- Fresh KL, Small DJ, Kim H, Waldbilling C, Mizell M, Carr MI, Stamatiou L. Juvenile salmon use of Sinclair Inlet, Washington in 2001 and 2002. Washington Department of Fish and Wildlife; Olympia, WA: 2006. [last accessed 7.10.15]. Technical Report No. FPT 05-08. http://wdfw.wa.gov/publications/00184/wdfw00184.pdf. [Google Scholar]
- Fu W, Franco A, Trapp S. Methods for estimating the bioconcentration factor of ionizable organic chemicals. Env Toxicol Chem. 2009;28:1372–1379. doi: 10.1897/08-233.1. 08-233 [pii]\r. [DOI] [PubMed] [Google Scholar]
- Gaw S, Thomas KV, Hutchinson TH, Gaw S. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Phil Trans R Soc B. 2014:20130572. doi: 10.1098/rstb.2013.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RL, Deacon HE, Lai KM, Birkett JW, Scrimshaw MD, Lester JN. An assessment of the bioaccumulation of estrone in Daphnia magna. Environ Toxicol Chem. 2004;23:105–108. doi: 10.1897/02-613. [DOI] [PubMed] [Google Scholar]
- Healey MC. Life history of Chinook salmon (Oncorhynchus tshawytscha) In: Groot C, Margolis L, editors. Pacific Salmon Life Histories. UBC Press; Vancouver, B.C., Canada: 1991. pp. 313–393. [Google Scholar]
- Hedgespeth ML, Sapozhnikova Y, Pennington P, Clum A, Fairey A, Wirth E. Pharmaceuticals and personal care products (PPCPs) in treated wastewater discharges into Charleston Harbor, South Carolina. Sci Total Environ. 2012;437:1–9. doi: 10.1016/j.scitotenv.2012.07.076. [DOI] [PubMed] [Google Scholar]
- Hontela A, Habibi HR. Personal care products in the aquatic environment: a case study on the effects of triclosan in fish. In: Tierney KB, Farrell AP, Brauner CJ, editors. Fish Physiology: Organic Chemical Toxicology of Fishes. Vol. 33. Elsevier; 2014. pp. 411–437. http://dx.doi.org/10.1016/B978-0-12-398254-4.00008-X. [Google Scholar]
- Johnson A, Friese M. [last accessed 14.01.16];PBTs analyzed in bottom fish from four Washington rivers and lakes. 2009 https://fortress.wa.gov/ecy/publications/summarypages/1203042.html.
- Klosterhaus SL, Grace R, Hamilton MC, Yee D. Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ Int. 2013;54:92–99. doi: 10.1016/j.envint.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Kostich M, Flick R, Martinson J. Comparing predicted estrogen concentrations with measurements in US waters. Environ Pollut. 2013;178:271–277. doi: 10.1016/j.envpol.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Batt AL, Lazorchak JM. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ Pollut. 2014;184:354–359. doi: 10.1016/j.envpol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Kristiansson E, Fick J, Janzon A, Grabic R, Rutgersson C, Weijdegard B, Soderstrom H, Larsson DGJ. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS One. 2011;6:1–7. doi: 10.1371/journal.pone.0017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubliner B, Redding M, Ragsdale D. [last accessed 14.01.16];Pharmaceuticals and personal care products in municipal wastewater and their removal by nutrient treatment technologies. 2010 www.ecy.wa.gov/biblio/1003004.html.
- Manallack DT. The pK(a) Distribution of drugs: application to drug discovery. Perspect Medicin Chem. 2007;1:25–38. [PMC free article] [PubMed] [Google Scholar]
- Meador JP. Comparative toxicokinetics of tributyltin in five marine species and its utility in predicting bioaccumulation and acute toxicity. Aquat Toxicol. 1997;37:307–326. doi: 10.1016/S0166-445X(96)00827-2. [DOI] [Google Scholar]
- Meador JP. Predicting the fate and effects of tributyltin in marine systems. Rev Environ Contam Toxicol. 2000;166:1–48. [PubMed] [Google Scholar]
- Meador JP. Rationale and procedures for using the tissue-residue approach for toxicity assessment and determination of tissue, water, and sediment quality guidelines for aquatic organisms. Hum Ecol Risk Assess. 2006;12:1018–1073. doi: 10.1080/10807030600801535. [DOI] [Google Scholar]
- Meador JP. Do chemically contaminated river estuaries in Puget Sound (Washington, USA) affect the survival rate of hatchery-reared Chinook salmon? Can J Fish Aquat Sci. 2014;71:162–180. doi: 10.1139/cjfas-2013-0130. [DOI] [Google Scholar]
- Meador JP, McCarty LS, Escher BI, Adams WJ. 10th Anniversary Critical Review: The tissue-residue approach for toxicity assessment: concepts, issues, application, and recommendations. J Environ Monit. 2008;10:1486–1498. doi: 10.1039/b814041n. [DOI] [PubMed] [Google Scholar]
- Meredith-Williams M, Carter LJ, Fussell R, Raffaelli D, Ashauer R, Boxall ABA. Uptake and depuration of pharmaceuticals in aquatic invertebrates. Environ Pollut. 2012;165:250–258. doi: 10.1016/j.envpol.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Milla S, Depiereux S, Kestemont P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: a review. Ecotoxicology. 2011;20:305–319. doi: 10.1007/s10646-010-0588-7. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto H, Sekizawa J, Kondo T, Hirai N, Tatarazako N. The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): Acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere. 2008;70:865–873. doi: 10.1016/j.chemosphere.2007.06.089. [DOI] [PubMed] [Google Scholar]
- Niemuth NJ, Klaper RD. Emerging wastewater contaminant metformin causes intersex and reduced fecundity in fish. Chemosphere. 2015;135:38–45. doi: 10.1016/j.chemosphere.2015.03.060. [DOI] [PubMed] [Google Scholar]
- Oulton RL, Kohn T, Cwiertny DM. Pharmaceuticals and personal care products in effluent matrices: A survey of transformation and removal during wastewater treatment and implications for wastewater management. J Environ Monit. 2010;12:1956–1978. doi: 10.1039/c0em00068j. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–9966. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Painter MM, Buerkley Ma, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas) Environ Toxicol Chem. 2009;28:2677–2684. doi: 10.1897/08-556.1. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Chalmers AT, Gray JL, Kolpin DW, Foreman WT, Wall GR. Combined sewer overflows: an environmental source of hormones and wastewater micropollutants. Environ Sci Technol. 2012;46:5336–5343. doi: 10.1021/es3001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce County. [last accessed on 1.10.15];Pierce County Public Works and Utilities – Sewer Utility Unified Sewer Plan Update. 2010 http://www.co.pierce.wa.us/index.aspx?NID=3108&ART=6573&ADMIN=1.
- Ramirez AJ, Brain Ra, Usenko S, Mottaleb Ma, O’Donnell JG, Stahl LL, Wathen JB, Snyder BD, Pitt Jennifer L, Perez-Hurtado P, Dobbins LL, Brooks BW, Chambliss CK. Occurrence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environ Toxicol Chem. 2009;28:2587–2597. doi: 10.1897/08-561.1. [DOI] [PubMed] [Google Scholar]
- Rendal C, Kusk KO, Trapp S. Optimal choice of pH for toxicity and bioaccumulation studies of ionizing organic chemicals. Environ Toxicol Chem. 2011;30:2395–2406. doi: 10.1002/etc.641. [DOI] [PubMed] [Google Scholar]
- Roos V, Gunnarsson L, Fick J, Larsson DGJ, Ruden C. Prioritising pharmaceuticals for environmental risk assessment: towards adequate and feasible first-tier selection. Sci Total Environ. 2012;421–422:102–110. doi: 10.1016/j.scitotenv.2012.01.039. [DOI] [PubMed] [Google Scholar]
- Sappington KG, Bridges TS, Bradbury SP, Erickson RJ, Hendriks AJ, Lanno RP, Meador JP, Mount DR, Salazar MH, Spry DJ. Application of the tissue residue approach in ecological risk assessment. Integr Environ Assess Manag. 2011;7:116–140. doi: 10.1002/ieam.116. [DOI] [PubMed] [Google Scholar]
- Schlenk D, Lavado R, Loyo-Rosales JE, Jones W, Maryoung L, Riar N, Werner I, Sedlak D. Reconstitution studies of pesticides and surfactants exploring the cause of estrogenic activity observed in surface waters of the San Francisco Bay Delta. Environ Sci Technol. 2012;46:9106–11. doi: 10.1021/es3016759. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Bartell SE, Schoenfuss HL. Effects of triclosan and triclocarban, two ubiquitous environmental contaminants, on anatomy, physiology, and behavior of the fathead minnow (Pimephales promelas) Arch Environ Contam Toxicol. 2012;63:114–124. doi: 10.1007/s00244-011-9748-x. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Furlong ET, Kolpin DW, Werner SL, Schoenfuss HL, Barber LB, Blazer VS, Norris DO, Vajda AM. Antidepressant pharmaceuticals in two U.S. effluent-impacted streams: occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ Sci Technol. 2010;44:1918–25. doi: 10.1021/es9022706. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Painter MM, Bartell SE, Logue A, Furlong ET, Werner SL, Schoenfuss HL. Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat Toxicol. 2011;104:38–47. doi: 10.1016/j.aquatox.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Scott WC, Bowen D, Haddad SP, Breed CS, Saari GN, Kelly M, Broach L, Chambliss CK, Brooks BW. Predicted and observed therapeutic dose exceedences of ionizable pharmaceuticals in fish plasma from urban coastal systems. Environ Toxicol Chem. 2015 doi: 10.1002/etc.3236. [DOI] [PubMed] [Google Scholar]
- South Kitsap Water Reclamation Facility. [last accessed 9.10.15];Fact sheet for NPDES permit WA0020346, South Kitsap Water Reclamation Facility. 2013 https://fortress.wa.gov/ecy/wqreports/public/WQPERMITS.document_pkg.download_document?p_document_id=94793.
- Spromberg JA, Meador JP. Relating results of chronic toxicity responses to population-level effects: modeling effects on wild Chinook salmon populations. Integr Environ Assess Manag. 2005;1:9–21. doi: 10.1897/IEAM_2004a-005.1. [DOI] [PubMed] [Google Scholar]
- [last accessed on 29.01.14];Tacoma Central WWTP Factsheet. 2004 https://fortress.wa.gov/ecy/wqreports/public/f?p=110:304:0::NO:RP.
- Tasto RN. Aspects of the biology of pacific staghorn sculpin, Leptocottus armatus Girard, in Anaheim Bay. Lane ED, Hill CW, editors. [last accessed 14.01.16];The Marine Resources of Anaheim Bay, Fish Bulletin 165. 1976 http://escholarship.org/uc/item/5vt2q1wk.
- U.S. Environmental Protection Agency. [last accessed 14.01.16];Aquatic life ambient water quality criteria – nonylphenol, final. 2005 http://www.epa.gov/waterscience/criteria/nonylphenol/final-doc.pdf.
- U.S. Environmental Protection Agency. [last accessed on 14.01.16];Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS. 2007 http://www.epa.gov/cwa-methods/contaminants1105emerging-concern-methods-documents.
- U.S. Environmental Protection Agency. [last accessed on 14.01.16];Nonylphenol (NP) and nonylphenol ethoxylates (NPEs) action plan. 2010 http://www.epa.gov/assessing-and-managing-chemicals-under-tsca/nonylphenol-and-nonylphenol-ethoxylates.
- U.S. Food and Drug Administration. [last accessed on 14.01.16];Approved drug products with therapeutic equivalence evaluations (orange book) 2015 http://www.fda.gov/Drugs/InformationOnDrugs/ucm129662.htm.
- Verlicchi P, Al Aukidy M, Zambello E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment - A review. Sci Total Environ. 2012;429:123–155. doi: 10.1016/j.scitotenv.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Vidal-Dorsch DE, Bay SM, Maruya K, Snyder SA, Trenholm RA, Vanderford BJ. Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ Toxicol Chem. 2012;31:2674–2682. doi: 10.1002/etc.2004. [DOI] [PubMed] [Google Scholar]
- Vieno NM, Tuhkanen T, Kronberg L. Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environmantal Sci Technol. 2005;39:8220–8226. doi: 10.1021/es051124k. [DOI] [PubMed] [Google Scholar]
- Wang J, Gardinali PR. Analysis of selected pharmaceuticals in fish and the fresh water bodies directly affected by reclaimed water using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2012;404:2711–2720. doi: 10.1007/s00216-012-6139-8. [DOI] [PubMed] [Google Scholar]
- Washington Department of Ecology. [last accessed on 14.01.16];Standard Operating Procedure for Manually Obtaining Surface Water Samples. 2006 http://www.ecy.wa.gov/programs/eap/qa/docs/ECY_EAP_SOP_ManuallyObtainingSurfaceWaterSamples_v1_2EAP015.pdf.
- Washington Department of Ecology. Nisqually River basin fecal coliform bacteria and dissolved oxygen total maximum daily load water quality. [last accessed on 14.01.16];Implementation plan. 2007 www.ecy.wa.gov/biblio/0710016.html.
- Washington Department of Ecology. [last accessed on 14.01.16];Phase 3: Loadings of toxic chemicals to Puget Sound from POTW discharge of treated wastewater. 2010 https://fortress.wa.gov/ecy/publications/summarypages/1010057.html.
- Washington Department of Ecology. [last accessed on 14.01.16];Water quality assessment (303[d]) & water quality improvement. 2015 http://www.ecy.wa.gov/programs/wq/links/wq_assessments.html.
- Weatherunderground. 2015 http://www.wunderground.com/history/
- Wells JI. Pharmaceutical preformulation : the physicochemical properties of drug substances. Halsted Press; New York: 1988. p. 227. [Google Scholar]
- Yeh A, Gallagher EP, Meador JP. Quality assurance project plan for grant - integrated biomonitoring for emerging contaminants. 2013. Prepared for Washington State Department of Ecology. Available upon request. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.