SUMMARY
The receptor for advanced glycation endproducts (RAGE) is a pro-inflammatory pattern recognition receptor (PRR) that has been implicated in the pathogenesis of numerous inflammatory diseases. It was discovered in 1992 on endothelial cells and was named for its ability to bind advanced glycation endproducts and promote vascular inflammation in the vessels of patients with diabetes. Further studies revealed that RAGE is most highly expressed in lung tissue and spurred numerous explorations into RAGE’s role in the lung. These studies have found that RAGE is an important mediator in allergic airway inflammation (AAI) and asthma, pulmonary fibrosis, lung cancer, chronic obstructive pulmonary disease (COPD), acute lung injury, pneumonia, cystic fibrosis, and bronchopulmonary dysplasia. RAGE has not yet been targeted in the lungs of paediatric or adult clinical populations, but the development of new ways to inhibit RAGE is setting the stage for the emergence of novel therapeutic agents for patients suffering from these pulmonary conditions.
Keywords: Receptor for advanced glycation, endproducts (RAGE), Pulmonary disease, Asthma, Bronchopulmonary dysplasia, Cystic fibrosis
INTRODUCTION TO RAGE BIOLOGY
Structure
RAGE is a 35 kDa protein from the immunoglobulin superfamily [1] and exists in the body in two main forms: membrane-bound RAGE (mRAGE) and soluble RAGE (sRAGE) [2]. Membrane-bound RAGE has three domains: an extracellular domain that recognizes and binds RAGE ligands, a hydrophobic transmembrane domain, and a charged cytoplasmic domain that functions in intracellular signaling. Soluble RAGE contains only the extracellular domain and is a product of either alternative splicing events [2,3] or the proteolytic cleavage of mRAGE [4] by ADAM10 or matrix metalloproteinase 9 [5,6]. Because it can bind ligands but cannot transduce signals intracellularly, sRAGE is a decoy receptor that sequesters RAGE ligands and prevents inflammatory responses.
Expression
Twenty splice forms of RAGE have been identified in the human body, and seventeen exist in mice; tissue type dictates what splice form is expressed [7–9]. RAGE is highly expressed in many tissues of the developing embryo, but this expression decreases in all tissues except the lung as the organism enters adult life [10]. In adult tissues at baseline, RAGE is constitutively highly expressed in the lung, while other tissues show little to no RAGE expression at all [11–14]. Subsequent studies have localized RAGE expression to the basal membrane of type 1 alveolar epithelial (AT1) cells, and RAGE has been defined as a specific marker of AT1 cells [15–18]. Some reports have also shown that RAGE may also be expressed in type 2 alveolar epithelial (AT2) cells [12]. In addition to expression in the lung epithelium, RAGE expression has also been noted in vascular smooth muscle cells [11], airway smooth muscle cells [19], endothelial cells [11], neurons [11,20], and immune cells such as macrophages [11], dendritic cells [21], eosinophils [22], T cells [23–26], and B cells [23]. Many of these cells and tissues induce RAGE expression only when they are activated to do so, such as by local expression of RAGE ligands [10]. Notably, RAGE expression is upregulated on various cell types in pathological inflammatory states such as diabetes mellitus, vascular disease, cancer, and neurodegenerative diseases [27]. However, decreased RAGE expression in the lung has been associated with lung cancer and pulmonary fibrosis [13,28].
Ligands
Although RAGE was first identified as a receptor for advanced glycation endproducts (AGEs) [1], it can bind a large variety of endogenous ligands, leading to its classification as a pattern recognition receptor [29,30]. RAGE identifies ligands based on their three-dimensional structure rather than a specific amino acid sequence [29,31]. While RAGE has the ability to bind many ligands, its ligands also can bind to other receptors, making RAGE-ligand binding a complex process to study.
Common RAGE ligands include AGEs, S100/calgranulin proteins, and high mobility group box 1 protein (HMGB1). AGEs are the result of a non-enzymatic Maillard reaction between the carbonyl group on an aldose sugar (commonly glucose) and amino groups on proteins [32]. AGEs are found at increased levels in patients with diabetes due to high blood glucose levels. Age and oxidative stress also elevate AGE levels.
S100 proteins are small calcium-binding proteins that are often elevated in inflammatory states and were first found to interact with RAGE on the endothelium [33]. They usually localize to sites of inflammation since they are released by activated inflammatory cells. Numerous S100 proteins can activate RAGE in a variety of tissues to initiate an inflammatory response [34]. These include S100A4 in pulmonary artery smooth muscle cells [35], S100A12 in airway epithelial cells, [36], and S100A9 in keratinocytes. [37],
HMGB1 was discovered as a novel RAGE binding partner that played a role in neurite outgrowth in developing embryos [20]. Since then, HMGB1 has been studied as a nuclear protein that is important in chromatin remodeling. However, it can also be passively released from damaged cells as a proinflammatory alarmin [38]. Additionally, macrophages, natural killer cells, and dendritic cells can actively secrete HMGB1 [39]. In addition to binding RAGE, HMGB1 also binds to and activates toll-like receptors [40], demonstrating the promiscuity of this molecule and highlighting the potential for HMGB1 to activate non-specific targets when administered to cells or animals in experimental models.
Other RAGE binding partners include collagen I, collagen IV, and laminin in the extracellular matrix [41]. RAGE’s ability to bind extracellular matrix components such as collagen have been shown to be important for its role in the spreading of AT1 cells in the lung [42]. In Alzheimer’s disease, RAGE has the ability to bind soluble amyloid-β, resulting in oxidative stress, release of inflammatory cytokines, and plaque formation in the central nervous system [43,44]. Through electrostatic interactions between a positive cavity on RAGE and the negative charges on the backbone of nucleic acids, RAGE has been shown to bind DNA and RNA to facilitate their uptake into the cell to promote inflammatory responses [45,46]. Finally, RAGE has also been shown to bind to Mac-1 integrin (αMβ2, CD11b/CD18) on leukocytes to facilitate their recruitment to inflamed tissue [47].
The downstream signaling pathway that is stimulated by RAGE-ligand binding depends on the identity of the ligand, how the ligand is bound to RAGE, the tissue type where inflammation is occurring, and the ligand oligomerization state. The presence of RAGE ligands in the extracellular environment has been shown to frequently induce RAGE expression, which leads to further amplification of inflammatory signaling cascades (see Section “Functions and signaling pathways”) [48–50]. Importantly, RAGE ligands are not degraded or altered to prevent further signaling when they bind and signal through RAGE. Therefore, as ligands accumulate, they continuously amplify the inflammatory response by pooling in the inflamed region.
Functions and signaling pathways
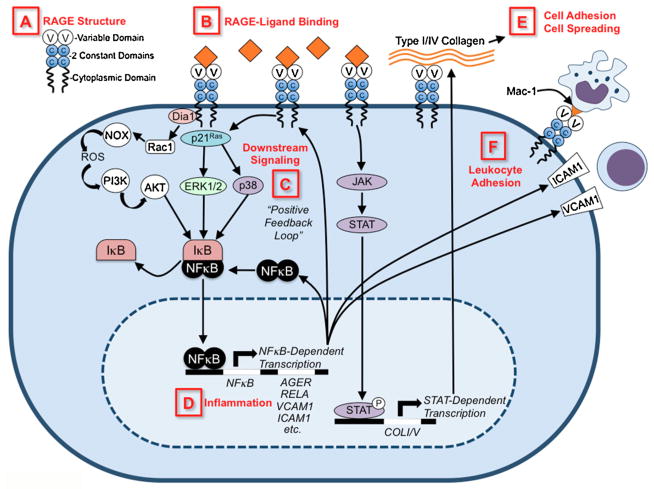
RAGE has been shown to have a myriad of functions, but its most well-studied role is in the amplification of cellular inflammatory responses (Figure 1). Activation of RAGE causes a sustained nuclear factor kappa B (NF-κB) response by maintaining a steady pool of newly synthesized NF-κBp65 mRNA and protein [51]. A large byproduct of RAGE signaling is the formation reactive oxygen species (ROS), which can also activate NF-κB and promote other inflammatory mechanisms such as increased vascular cell adhesion molecule 1 (VCAM-1) expression or cellular apoptosis [52–54]. Further perpetuation of the inflammatory response comes from the fact that NF-κB can directly bind to the gene encoding RAGE to promote RAGE expression [48]. This positive feedback loop between RAGE and NF-κB contributes to chronic, pathological inflammation in many diseases.
Figure 1. Schematic diagram of RAGE signaling pathways.
A) RAGE structure is depicted showing its variable domain (V), two constant domains (C), and cytoplasmic tail. B) RAGE ligands are depicted as orange diamonds, and include AGEs, HMGB1, S100/calgranulins, amyloid β, etc. C) Upon ligand binding, intracellular signaling cascades are initiated (see Section “Functions and signaling pathways” for detailed description), which leads to D) transcriptional activation of NF-κB- and STAT-dependent gene transcription. RAGE-dependent activation of NF-κB induces a positive feedback loop by inducing RAGE and NF-kB gene transcription. E) RAGE binds type I and IV collagen, resulting in cell adhesion and cell spreading. F) RAGE binds Mac-1 integrins on leukocytes and RAGE-signaling induces ICAM1/VCAM1 expression, all of which promote leukocyte adhesion.
The specific signaling pathway that becomes activated by RAGE and ultimately leads to NF-κB activation is dependent on the identity of the RAGE ligand and the tissue type where the receptor is expressed. Studies have identified several of these intermediary players in the RAGE signaling cascade and they include extracellular-regulated kinase (ERK) 1/2 mitogen-activated protein (MAP) kinases [55], p38 MAP kinase [56], Rho GTPases [57,58], phosphatidylinositol-3 kinase [54], and JAK/STAT [59], among others. Signaling through RAGE is dependent on expression of full-length RAGE containing the cytoplasmic tail [57,60]. Interestingly, the C-terminus of RAGE has no tyrosine kinase activity, suggesting that RAGE must interact with other molecules in the cytoplasm to transduce extracellular signals [58]. Indeed, studies have shown that the cytoplasmic domain of RAGE contains binding sites for ERK 1/2 [60] and diaphanous-1 (a Rho effector protein) [58]. Activation of either of these proteins leads to activation of Rac/Cdc42 small GTPases that then control cellular migration.
Interestingly, RAGE is only expressed in mammals and its protein composition is closely related to that of cellular adhesion molecules (CAMs), suggesting that it evolved to play a role in cell-to-cell adhesion [61]. Indeed, multiple studies have shown that RAGE plays both a direct and indirect role in leukocyte adhesion and recruitment to inflamed tissues. In an animal model of acute peritonitis and subsequent in vitro studies, RAGE was shown to directly bind and recruit leukocytes via interactions with Mac-1 on the white blood cell surface [47]. In a follow-up study, endothelial RAGE was found to bind Mac-1 in conjunction with intercellular adhesion molecule 1 (ICAM-1) to recruit leukocytes into the tissue [62,63]. RAGE was also crucial for leukocyte adhesion in studies using blood cells from preterm infants, suggesting a role for RAGE in young, developing animals that have high receptor expression [64]. RAGE signaling also indirectly promotes adhesion and recruitment of inflammatory cells by inducing the expression of VCAM-1 on vascular endothelial cells [65] and peritoneal mesothelial cells [66].
RAGE has long been recognized as an important molecule in the initiation and maintenance of innate immune responses, but it also has roles in the adaptive immune system [67]. Specifically, dendritic cell maturation, T helper 1 (Th1) cell polarization of CD4+ cells, T cell priming, and T cell proliferation have all been shown to be dependent on RAGE signaling [21,24,25,68,69]. Interestingly, human T cells express RAGE not on the extracellular surface as in murine cells, but intracellularly in endosomes [26]. The reasons for this altered cellular location are currently unknown.
RAGE IN PULMONARY DISEASES
Asthma
Two genome-wide association studies suggest that RAGE is important in asthma pathogenesis in humans. In patients with decreased forced expiratory volume in one second (FEV1), an association with a single nucleotide polymorphism (SNP) (rs2070600) in the RAGE ligand-binding domain was found [70,71]. This sequence variant results in a glycine to serine substitution at amino acid 82 (G82S), which increases RAGE’s affinity for ligands, leading to amplified inflammatory responses when compared to wild-type RAGE [72,73]. Endogenous and soluble RAGE levels were also increased in sputum samples of adult and paediatric asthma patients and correlated with disease severity in some cases [74–76].
Additional studies have also shown associations between RAGE ligands and asthma. One report showed that HMGB1 promotes the recruitment of eosinophils to the lungs in asthma, and that levels of HMGB1 positively correlate with the expression of TNF-α, IL-5, and IL-13 in human sputum samples [77]. This same study demonstrated that HMGB1 levels are elevated in the sputum of asthmatics and positively correlate with the severity of the disease and the number of inflammatory cells in the lungs. This result was confirmed in a second study that showed elevated HMGB1 levels in the sputum of asthmatic patients when compared to healthy controls [74]. S100A8/A9, a heterodimer complex that binds to RAGE, has also been implicated in airway remodeling and inflammation in asthma [78]. Additionally, S100A12 from eosinophils signals through RAGE to promote mast cell degranulation and IgE-mediated responses in the lung [79]. Notably, asthmatic patient sputum contains elevated levels of S100A12, and asthmatic patient lungs possess greater numbers of S100A12+ eosinophils when compared to non-asthmatic controls [79].
In the past few years, mouse models of asthma have been utilized to better understand the molecular mechanisms by which RAGE promotes asthma pathogenesis [80–83]. In one study, wild-type and RAGE knockout (KO) mice were treated with house dust mite (HDM) extract. This led to airway eosinophilia, goblet cell hyperplasia, and impaired pulmonary function after methacholine challenge in wild-type mice [80]. In the absence of RAGE, however, the mice were completely protected against this asthma-like phenotype, as the airway was normal both physiologically and histologically. IL-4, which is important for activation of T cells, was increased normally in both wild-type and RAGE KO mice treated with HDM. IL-5 and IL-13, which direct eosinophil recruitment and mucus secretion, respectively, were increased in wild-type mice, but remained at baseline levels in RAGE KO mice, suggesting that RAGE is important for the production of these type 2 cytokines. Eotaxins also were not elevated in RAGE KO mice, but were increased in wild-type mice. All of these studies were recapitulated using an ovalbumin (OVA) model with similar results [80]. These studies demonstrated that RAGE is necessary for AAI.
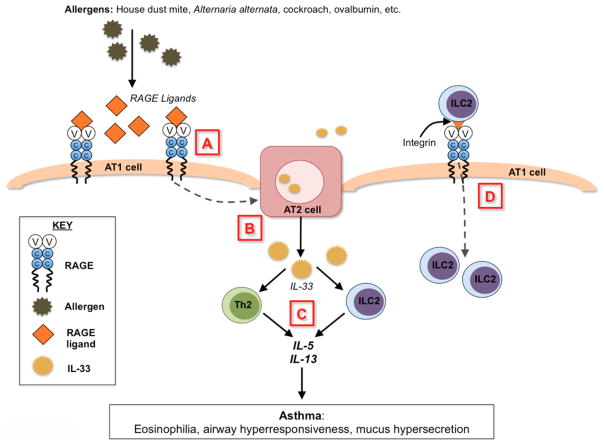
Work is ongoing to determine the exact molecular mechanism by which RAGE promotes type 2 immune responses in the lung. Because RAGE KO mice produced normal amounts of IL-4 in response to HDM, but had attenuated IL-5 and IL-13 responses [80], attention turned toward group 2 innate lymphoid cells (ILC2s). ILC2s have emerged as important new players in the pathogenesis of allergic asthma [84–86] due to their ability to secrete large amounts of IL-5 and IL-13 after activation by the epithelial-derived cytokines IL-25, IL-33, and TSLP [87,88]. RAGE was recently shown to be necessary for the accumulation of ILC2s in the lung of mice in response to allergens [83]. More work is needed to determine if RAGE is involved in the recruitment of ILC2s to the lung or if it is important in the expansion and growth of resident ILC2 populations within the lung itself during an allergic airway response. Further studies using RAGE KO mice also showed that RAGE promotes expression of IL-33 in the lung and is important in orchestrating IL-33’s downstream inflammatory signaling effects [83]. Therefore, RAGE appears to be an important player in the early initiation of AAI (Figure 2).
Figure 2. Summary of RAGE’s known and hypothetical roles in allergic airway inflammation.
A) Allergens trigger release of RAGE ligands, which bind to and activate RAGE on type 1 alveolar (AT1) epithelial cells. B) This signaling is suspected to trigger release of IL-33 from type 2 alveolar (AT2) epithelial cells (dotted line). C) IL-33 can activate resident immune cells in the lung and is also released into the circulation to activate ILC2s in the bone marrow. Activated pulmonary Th2 cells and ILC2s produce large amounts of IL-5 and IL-13 to exacerbate allergic airway inflammation and airway hyperresponsiveness. D) RAGE may potentially bind to and recruit ILC2s directly into the lung via interactions with integrins on ILC2s (not yet studied).
Further research into the timing of RAGE’s role in asthma pathogenesis has shown that it can act as early as the sensitization phase of allergic airway responses. Ullah et al. found that, during HDM- or cockroach-induced AAI, airway sensitization to allergens relied on intact RAGE signaling and was mediated by the RAGE ligand, HMGB1 [81]. Another group demonstrated a role for RAGE in T cell activation in OVA-induced allergic airway sensitization [82], suggesting that RAGE is important in both the early innate and adaptive immune responses to allergens.
More studies are needed to fully characterize all of RAGE’s effects in the complex pathways that contribute to asthma pathogenesis, but at this point in time, RAGE does appear to be a key early mediator of allergic airway responses in both mouse models and human patients.
Cystic fibrosis
Polymorphisms of the AGER gene have been associated with increased disease severity in patients with cystic fibrosis (CF). AGER −429T/C is associated with decreased FEV1 and increased AGER promoter activity, leading to increased RAGE expression and worsening lung function in CF patients [89]. The AGER −374T/A variant was also associated with increased RAGE expression and resulted in increased IgE levels in the lungs of CF patients [90]. The authors hypothesized that CF patients with this RAGE-mediated increase in allergic airway inflammation were more susceptible to sensitization by environmental allergens and pathogens such as
Aspergillus
RAGE-mediated inflammation in the CF lung creates an environment that is prone to infection, and these damaging cycles of opportunistic infections lead to further activation of RAGE inflammatory pathways. Interestingly, CF lungs are also deficient in the anti-inflammatory decoy receptor sRAGE [91], leaving ligands free to bind and signal through RAGE. Patients with CF were found to have higher levels of the neutrophil-secreted RAGE ligand S100A12 in their sputum than healthy controls, and CF patients with acute exacerbations of their disease had the highest levels of the ligand [92]. Treatment with antibiotics resulted in a significantly decreased S100A12 level in the sputum of CF patients, demonstrating that eliminating inciting bacteria can temper RAGE-mediated inflammatory signaling. S100B-RAGE signaling was also upregulated in CF mice suffering from acute fungal infections [90]. This increased expression of s100 B and RAGE was thought to be triggered by hypoxia, and both fungal burden and inflammation were decreased after administration of exogenous sRAGE. Therefore, overexpression of RAGE and RAGE ligands in combination with defective sRAGE production appear to drive inflammatory responses and worsen lung function in patients with cystic fibrosis.
ALI/ARDS
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are characterized by epithelial barrier disruption, endothelial permeability, and impaired alveolar fluid clearance. One of the major hallmarks of ALI/ARDS is alveolar epithelial cell injury, for which RAGE has been suggested as a biomarker [18]. Because RAGE is highly expressed on AT1 cells, it was thought that damage to alveolar cells would result in release of sRAGE into pulmonary fluid. Indeed, in multiple mouse models of ALI and in patients with ALI/ARDS, sRAGE levels were increased in bronchoalveolar lavage fluid and correlated with the degree of lung injury [18].
Interestingly, mice lacking RAGE were protected from hyperoxia-induced ALI and mortality [93], suggesting that intact RAGE signaling promotes lung inflammation and destruction in ALI. RAGE KO mice were also partially protected from injury following gram-negative (Escherichia coli)[94] or gram-positive (Streptococcus pneumoniae)[95] bacterial challenges. However, lipopolysaccharide-induced ALI was similar in both wild-type and RAGE KO mice, suggesting that RAGE-mediated inflammation is induced by bacterial-associated molecular pattern molecules other than lipopolysaccharide [94].
In humans, systemic and alveolar levels of HMGB1, S100A12, and sRAGE from damaged AT1 cells are increased in patients with ARDS [96], and plasma sRAGE levels correlate with severity of lung injury and increased mortality [97,98]. As the ALI/ARDS resolves in patients, plasma sRAGE levels also fall, suggesting that sRAGE can act not only as a marker of disease severity and prognosis but also as a marker of resolving disease [99,100].
Bronchopulmonary dysplasia (BPD)
While the pathogenesis of BPD remains poorly understood, it is thought that pulmonary inflammation, oxygen toxicity, and mechanical stress/volume trauma disrupt proper lung development, leading to under-developed airways and vasculature [101]. A number of developmental studies have shown that RAGE expression is crucial to proper lung development. Comparison of neonatal and adult rat lungs shows that pulmonary RAGE expression increases with development, and this increase can be hindered by exposure to chronic hyperoxia [102]. The induction of RAGE expression in the lung is a delicate process: overexpression of pulmonary RAGE too early in development is lethal due to airway hypoplasia [103] and overexpression of RAGE at the time of weaning leads to airspace enlargement with characteristics of COPD in mice [104]. High levels of the RAGE ligand, HMGB1, in tracheal aspirates of neonates were correlated with an increased incidence of BPD, suggesting that RAGE signaling may play a role in the pathogenesis of this disease [105].
One can hypothesize that ventilation-induced pulmonary stress may activate RAGE signaling, leading to increased RAGE expression, disruption of lung development, and BPD. Alternatively, hyperoxia may inhibit RAGE expression, thus preventing AT1 cell differentiation and alveolarization. While RAGE’s role in BPD is still being investigated, its critical role in early lung development and susceptibility to inflammatory signals make it an attractive target for future research studies.
Interstitial lung disease
Interstitial lung disease (ILD) is rare in the paediatric population, but instances of parenchymal disease causing impaired gas exchange, usually from congenital disorders, do exist [106–108]. While RAGE has not been specifically studied in paediatric ILD, it has been shown to play a role in adult populations with ILD. It is likely that similar mechanisms are at play in paediatric patients.
Pulmonary fibrosis results from improper remodeling, repair, and regenerative processes in response to tissue injury. In pulmonary fibrosis, contrary to other pulmonary inflammatory diseases, RAGE protein levels are decreased in human lung homogenates [13,109,110] and bronchoalveolar lavage fluid [111] when compared to levels in healthy controls. RAGE gene expression is also attenuated in lungs from patients with idiopathic pulmonary fibrosis and during acute exacerbations of the disease. [13,112] In aged mice, the absence of RAGE leads to spontaneous fibrosis-like alterations in the lungs [13], suggesting a role for RAGE in protection against pulmonary fibrosis.
RAGE expression is significantly reduced in the lungs of mice in multiple experimental models of pulmonary injury/fibrosis including bleomycin [14], asbestos [13], and silica [113]. However, the role of RAGE in the development of fibrosis appears to be divergent among these models. Lack of RAGE was found to have no effect in silica-induced fibrosis [113], an adverse, worsening effect on asbestos-induced fibrosis [13], and a protective effect on bleomycin-induced fibrosis [114,115].
It is unclear why there are differences in the role of RAGE among these models, but it may have to do with RAGE’s functions in cellular adhesion and regeneration. Invitro, RAGE KO alveolarcellsare able to re-epithelialize better than wild-type cells after bleomycin-induced injury, likely due to improved migration and reparative processes in RAGE KO cells [115]. Aspreviously discussed, RAGE on AT1 cells binds to collagen to facilitate cell spreading[42]; lack of RAGE impairs alveolar adherence to the basement membrane and may allow for increased alveolar cell migration. RAGE is also involved in the trans-differentiation of AT1 cells from AT2 cells[17] and is found at decreased levels in AT2 cells isolated from fibrotic human and murine lungs [109]. Lack of RAGE in alveolar epithelial cells impaired cellular adhesion and promoted cell proliferation and migration in vitro [109]. These changes may lead to cellular dysfunction or improved repair processes, and whether lack of RAGE is harmful or protective for the cell may be dependent upon the type of injury the cell is exposed to.
RAGE has also been suggested to play a role in epithelial to mesenchymal transition (EMT), which has emerged as a key mechanism in ILD for promoting a profibrotic environment [116]. RAGE KO mice are protected from bleomycin-induced injury, and AT2 cells from RAGE KO mice do not undergo HMGB1-induced EMT as wild-type cells do [114]. On the other hand, stimulation of AT2-like cells (A549) with TGF-β and proinflammatory cytokines, induced cytoskeletal rearrangements characteristic of EMT, and decreases RAGE expression [110,117]. The role of RAGE in EMT remains a question, as it is unclear if it is required for EMT or if its deficiency promotes EMT. Data suggest this may depend on the driving factors associated with experimental models used, namely, HMGB1 or TGF-β.
In summary, RAGE expression is down-regulated in humans with and in experimental animal models of pulmonary fibrosis. The decrease in RAGE in the lungs may be due to down-regulation of the gene and/or a result of trans-differentiation of AT2 cells to AT1 cells or loss of AT1 cells secondary to a lack of proper adherence. Such cellular detachments and changes in cellular differentiation patterns may be fueled by processes such as EMT, which promote improper repair mechanisms leading to matrix deposition and impaired epithelial regeneration.
Lung cancer and COPD
While the focus of this review is on pulmonary diseases affecting the paediatric population, it is important to at least mention RAGE’s emerging role in lung cancer and chronic obstructive pulmonary disease (COPD). Mechanisms at play in these disease states may have important implications for the treatment of the aforementioned paediatric diseases.
Several studies have shown that RAGE signaling contributes to chronic inflammation, impaired cell communication, as well as aberrant activation of cell survival pathways, thus promoting tumorigenesis [27,118,119]. Interestingly, RAGE seems to play an opposite role in lung malignancies. Studies have found that RAGE expression is significantly down-regulated in lung cancers, namely non-small cell carcinomas and adenocarcinomas [28,120,121]. Moreover, it has been demonstrated that over-expression of RAGE in lung cancer cells reduces proliferation and tumor growth [28,122]. Paradoxically, although RAGE is decreased in lung cancer, its ligands, which are often associated with proliferative effects, are increased [123–126] and are predictors of poor prognosis [127]. This may be due in part to the fact that the predominant cell-type in lung cancers is the bronchial epithelial cell, which does not typically express RAGE. The apparent decrease in RAGE expression is likely due to the expansion of these non-RAGE expressing cells. Therefore, a bronchogenic tumor may appear to have less RAGE when compared to an entire healthy lung with numerous ATI cells with high RAGE expression, but there may actually be an increase in RAGE expression in the tumor cells compared to non-malignant bronchial epithelial cells. Thus, RAGE ligands may actually still promote tumorigenic processes in lung cancer similar to other non-pulmonary tumors. Further studies may solidify the potential use of RAGE expression as a biomarker in diagnosis and prognosis of lung cancers. In addition, targeting RAGE ligands may represent a promising avenue for new potential lung cancer therapeutics.
COPD is a heterogeneous respiratory disease associated with exposure to various pollutants, most notably cigarette smoke, resulting in a chronic inflammatory response leading to airflow obstruction. The RAGE axis appears to play a role in the development of COPD. Enhanced expression of RAGE and its ligands [19,128–135], and decreased sRAGE expression in the lungs [135–140] suggests RAGE signaling contributes to the chronic inflammatory response in COPD. Activation of RAGE signaling in response to smoke likely initially evolved as a protective mechanism to promote an inflammatory response against inhaled pollutants; in smokers who are constantly exposed to smoke, however, there is a “tipping point,” at which this response goes unchecked, possibly due to genetic susceptibility factors [141,142].
DIRECTIONS FOR FUTURE RESEARCH
RAGE is clearly an important inflammatory mediator in many pulmonary diseases and is an attractive therapeutic target.
sRAGE normally circulates in the blood at low levels, and sRAGE levels increase in patients with inflammatory diseases, highlighting a potential role for sRAGE as a biomarker [143]. Administration of sRAGE as a therapeutic agent to block RAGE signaling has shown promising results in studies of asthma [80], chronic hypoxia [144], and cystic fibrosis [90]. Further work here in clinical trials is warranted.
Other methods of blocking RAGE specifically in the lung have not yet been tested. These other inhibitors, such as anti-RAGE antibodies and small molecule inhibitors of RAGE (pyrazole-5-carboxamides; TTP488, azeliragon from Trans-Tech Pharma, LLC; FPS-ZM1) have shown promise in other tissues and disease models [24,145–149] and are just beginning to make their way into human clinical trials for treatment of Alzheimer’s disease [150,151] (ClinicalTrials.gov Identifier: NCT02080364).
Use of sRAGE, a RAGE blocking antibody, or a small molecule inhibitor to interfere with RAGE signaling may become a novel therapeutic approach to inhibit RAGE-mediated inflammation in patients suffering from pulmonary disease.
EDUCATIONAL AIMS.
The reader will come to appreciate:
The structure and function of the receptor for advanced glycation endproducts (RAGE).
How RAGE has been implicated in numerous paediatric and adult respiratory diseases.
How RAGE research may steer clinical care in the next decade.
Acknowledgments
FUNDING
EAO is funded by an NIEHS NRSA1F30ES024045. TNP is funded by an NHLBI NRSA1T32HL129949-01A1. TDO is funded by the American Heart Association15GRNT25150004.
Abbreviations
- AAI
allergic airway inflammation
- AGE
advanced glycation end-product
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- AT1/2
type 1/2 alveolar epithelial cells
- BPD
bronchopulmonary dysplasia
- CAM
cellular adhesion molecule
- COPD
chronic obstructive pulmonary disease
- CF
cystic fibrosis
- EMT
epithelial to mesenchymal transition
- FEV1
forced expiratory volume in one second
- HDM
house dust mite
- HMGB1
high mobility group box 1 protein
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- ILC2
group 2 innate lymphoid cell
- ILD
interstitial lung disease
- KO
knockout
- mRAGE
membrane receptor for advanced glycation endproducts
- NFκ-B
nuclear factor kappa B
- OVA
ovalbumin
- PRR
pattern recognition receptor
- RAGE
receptor for advanced glycation endproducts
- SNP
single nucleotide polymorphism
- sRAGE
soluble receptor for advanced glycation endproducts
- TGFβ
transforming growth factor beta
- Th1/2
T helper 1/2
- TLR
toll-like receptor
- VCAM-1
vascular cell adhesion molecule 1
References
- 1.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(Jul (21)):14998–5004. [PubMed] [Google Scholar]
- 2.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370(Mar (Pt 3)):1097–109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harashima A, Yamamoto Y, Cheng C, Tsuneyama K, Myint KM, Takeuchi A, et al. Identification of mouse orthologue of endogenous secretory receptor for advanced glycation end-products: structure, function and expression. Biochem J. 2006;396(May (1)):109–15. doi: 10.1042/BJ20051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, et al. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2004;279(Nov (48)):50019–24. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22(Oct (10)):3716–27. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Bukulin M, Kojro E, Roth A, Metz VV, Fahrenholz F, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283(Dec (51)):35507–16. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 7.Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, et al. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22(May (5)):1572–80. doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- 8.Gefter JV, Shaufl AL, Fink MP, Delude RL. Comparison of distinct protein isoforms of the receptor for advanced glycation end-products expressed in murine tissues and cell lines. Cell Tissue Res. 2009;337(Jul (1)):79–89. doi: 10.1007/s00441-009-0791-0. [DOI] [PubMed] [Google Scholar]
- 9.Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23(Jun (6)):1766–74. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, et al. Understanding RAGE, the receptor for advanced glycation end products. Journal of molecular medicine. 2005;83(Nov (11)):876–86. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 11.Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143(Dec (6)):1699–712. [PMC free article] [PubMed] [Google Scholar]
- 12.Katsuoka F, Kawakami Y, Arai T, Imuta H, Fujiwara M, Kanma H, et al. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem Biophys Res Commun. 1997;238(Sep (2)):512–6. doi: 10.1006/bbrc.1997.7263. [DOI] [PubMed] [Google Scholar]
- 13.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, et al. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008;172(Mar (3)):583–91. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanford LE, Fattman CL, Shaefer LM, Enghild JJ, Valnickova Z, Oury TD. Regulation of receptor for advanced glycation end products during bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2003;29(Sep (3 Suppl)):S77–81. [PubMed] [Google Scholar]
- 15.Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisyle-grand) 1998;44(Nov (7)):1147–57. [PubMed] [Google Scholar]
- 16.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, et al. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31(Sep (3)):309–16. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 17.Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes to cells: devoted to molecular & cellular mechanisms. 2004;9(Feb (2)):165–74. doi: 10.1111/j.1356-9597.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 18.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(May (9)):1008–15. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferhani N, Letuve S, Kozhich A, Thibaudeau O, Grandsaigne M, Maret M, et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(May (9)):917–27. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- 20.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270(Oct (43)):25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 21.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174(Jun (12)):7506–15. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 22.Curran CS, Bertics PJ. Human eosinophils express RAGE, produce RAGE ligands, exhibit PKC-delta phosphorylation and enhanced viability in response to the RAGE ligand, S100B. International immunology. 2011;23(Dec (12)):713–28. doi: 10.1093/intimm/dxr083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Yan SS, Colgan J, Zhang HP, Luban J, Schmidt AM, et al. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol. 2004;173(Jul (2)):1399–405. doi: 10.4049/jimmunol.173.2.1399. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Akirav EM, Chen W, Henegariu O, Moser B, Desai D, et al. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181(Sep (6)):4272–8. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moser B, Desai DD, Downie MP, Chen Y, Yan SF, Herold K, et al. Receptor for advanced glycation end products expression on T cells contributes to antigen-specific cellular expansion in vivo. J Immunol. 2007;179(Dec (12)):8051–8. doi: 10.4049/jimmunol.179.12.8051. [DOI] [PubMed] [Google Scholar]
- 26.Akirav EM, Preston-Hurlburt P, Garyu J, Henegariu O, Clynes R, Schmidt AM, et al. RAGE expression in human T cells: a link between environmental factors and adaptive immune responses. PLoS One. 2012;7(4):e34698. doi: 10.1371/journal.pone.0034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(Oct (7)):949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartling B, Hofmann HS, Weigle B, Silber RE, Simm A. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis. 2005;26(Feb (2)):293–301. doi: 10.1093/carcin/bgh333. [DOI] [PubMed] [Google Scholar]
- 29.Lin L. RAGE on the Toll Road? Cellular & molecular immunology. 2006;3(Oct (5)):351–8. [PubMed] [Google Scholar]
- 30.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11(Jul (7)):615–28. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Reverdatto S, Frolov A, Hoffmann R, Burz DS, Shekhtman A. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE) J Biol Chem. 2008;283(Oct (40)):27255–69. doi: 10.1074/jbc.M801622200. [DOI] [PubMed] [Google Scholar]
- 32.Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47(Aug (Suppl 1)):3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(Jun (7)):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 34.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793(Jun (6)):993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. Journal of the American Heart Association. 2013;2(1):e005157. doi: 10.1161/JAHA.112.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang JH, Hwang SM, Chung IY. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-kappaB pathways. Immunology. 2015;144(Jan (1)):79–90. doi: 10.1111/imm.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin S, Park CO, Shin JU, Noh JY, Lee YS, Lee NR, et al. DAMP molecules S100A9 and S100A8 activated by IL-17A and house-dust mites are increased in atopic dermatitis. Experimental dermatology. 2014;23(Dec (12)):938–41. doi: 10.1111/exd.12563. [DOI] [PubMed] [Google Scholar]
- 38.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(Jul (6894)):191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 39.Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Frontiers in immunology. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(Apr (4)):331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 41.Milutinovic PS, Englert JM, Crum LT, Mason NS, Ramsgaard L, Enghild JJ, et al. Clearance Kinetics and Matrix Binding Partners of the Receptor for Advanced Glycation End Products. PLoS One. 2014;9(3):e88259. doi: 10.1371/journal.pone.0088259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323(Mar (3)):475–88. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 43.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(Aug (6593)):685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 44.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9(Jul (7)):907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 45.Park H, Adsit FG, Boyington JC. The 1.5 A crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J Biol Chem. 2010;285(Dec (52)):40762–70. doi: 10.1074/jbc.M110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirois CM, Jin T, Miller AL, Bertheloot D, Nakamura H, Horvath GL, et al. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J Exp Med. 2013;210(Oct (11)):2447–63. doi: 10.1084/jem.20120201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198(Nov (10)):1507–15. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272(Jun (26)):16498–506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Qu X, Schmidt AM. Sp1-binding elements in the promoter of RAGE are essential for amphoterin-mediated gene expression in cultured neuroblastoma cells. J Biol Chem. 1998;273(Nov (47)):30870–8. doi: 10.1074/jbc.273.47.30870. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275(Aug (33)):25781–90. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 51.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50(Dec (12)):2792–808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 52.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269(Apr (13)):9889–97. [PubMed] [Google Scholar]
- 53.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25(Jul (7)):1401–7. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 54.Vincent AM, Perrone L, Sullivan KA, Backus C, Sastry AM, Lastoskie C, et al. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148(Feb (2)):548–58. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 55.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272(Jul (28)):17810–4. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 56.Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, Bjercke RJ, et al. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50(Jun (6)):1495–504. doi: 10.2337/diabetes.50.6.1495. [DOI] [PubMed] [Google Scholar]
- 57.Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274(Jul (28)):19919–24. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- 58.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283(Dec (49)):34457–68. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. Journal of cellular biochemistry. 2001;81(1):102–13. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 60.Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003;550(Aug (1–3)):107–13. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]
- 61.Sessa L, Gatti E, Zeni F, Antonelli A, Catucci A, Koch M, et al. The receptor for advanced glycation end-products (RAGE) is only present in mammals, and belongs to a family of cell adhesion molecules (CAMs) PLoS One. 2014;9(1):e86903. doi: 10.1371/journal.pone.0086903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frommhold D, Kamphues A, Hepper I, Pruenster M, Lukic IK, Socher I, et al. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood. 2010;116(Aug (5)):841–9. doi: 10.1182/blood-2009-09-244293. [DOI] [PubMed] [Google Scholar]
- 63.Frommhold D, Kamphues A, Dannenberg S, Buschmann K, Zablotskaya V, Tschada R, et al. RAGE and ICAM-1 differentially control leukocyte recruitment during acute inflammation in a stimulus-dependent manner. BMC immunology. 2011;12:56. doi: 10.1186/1471-2172-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buschmann K, Tschada R, Metzger MS, Braach N, Kuss N, Hudalla H, et al. RAGE controls leukocyte adhesion in preterm and term infants. BMC immunology. 2014;15(Nov (1)):53. doi: 10.1186/s12865-014-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96(Sep (3)):1395–403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boulanger E, Wautier MP, Wautier JL, Boval B, Panis Y, Wernert N, et al. AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney international. 2002;61(Jan (1)):148–56. doi: 10.1046/j.1523-1755.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- 67.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113(Jun (11)):1641–50. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moser B, Szabolcs MJ, Ankersmit HJ, Lu Y, Qu W, Weinberg A, et al. Blockade of RAGE suppresses alloimmune reactions in vitro and delays allograft rejection in murine heart transplantation. American journal of transplantation: offficial journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(Feb (2)):293–302. doi: 10.1111/j.1600-6143.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 69.Manfredi AA, Capobianco A, Esposito A, De Cobelli F, Canu T, Monno A, et al. Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J Immunol. 2008;180(Feb (4)):2270–5. doi: 10.4049/jimmunol.180.4.2270. [DOI] [PubMed] [Google Scholar]
- 70.Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M, et al. Genome-wide association study identifies five loci associated with lung function. Nature genetics. 2010;42(Jan (1)):36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nature genetics. 2010;42(Jan (1)):45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes and immunity. 2002;3(May (3)):123–35. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 73.Osawa M, Yamamoto Y, Munesue S, Murakami N, Sakurai S, Watanabe T, et al. De-N-glycosylation or G82S mutation of RAGE sensitizes its interaction with advanced glycation endproducts. Biochim Biophys Acta. 2007;1770(Oct (10)):1468–74. doi: 10.1016/j.bbagen.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105(Apr (4)):519–25. doi: 10.1016/j.rmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 75.El-Seify MY, Fouda EM, Nabih ES. Serum level of soluble receptor for advanced glycation end products in asthmatic children and its correlation to severity and pulmonary functions. Clinical laboratory. 2014;60(6):957–62. doi: 10.7754/clin.lab.2013.130418. [DOI] [PubMed] [Google Scholar]
- 76.Bediwy AS, Hassan SM, El-Najjar MR. Receptor of advanced glycation end products in childhood asthma exacerbation. Egyptian Journal of Chest Diseases and Tuberculosis. 2016;65:15–8. [Google Scholar]
- 77.Shim EJ, Chun E, Lee HS, Bang BR, Kim TW, Cho SH, et al. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clinical and Experimental Allergy: journal of the British Society for Allergy and Clinical Immunology. 2012;42(Jun (6)):958–65. doi: 10.1111/j.1365-2222.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- 78.Halayko AJ, Ghavami S. S100A8/A9: a mediator of severe asthma pathogenesis, morbidity? Canadian journal of physiology pharmacology. 2009;87(Oct (10)):743–55. doi: 10.1139/Y09-054. [DOI] [PubMed] [Google Scholar]
- 79.Yang Z, Yan WX, Cai H, Tedla N, Armishaw C, Di Girolamo N, et al. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol. 2007;119(Jan (1)):106–14. doi: 10.1016/j.jaci.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 80.Milutinovic PS, Alcorn JF, Englert JM, Crum LT, Oury TD. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis. Am J Pathol. 2012;181(Oct (4)):1215–25. doi: 10.1016/j.ajpath.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ullah MA, Loh Z, Gan WJ, Zhang V, Yang H, Li JH, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014;134(Aug (2)):440–50. doi: 10.1016/j.jaci.2013.12.1035. [DOI] [PubMed] [Google Scholar]
- 82.Akirav EM, Henegariu O, Preston-Hurlburt P, Schmidt AM, Clynes R, Herold KC. The receptor for advanced glycation end products (RAGE) affects T cell differentiation in OVA induced asthma. PLoS One. 2014;9(4):e95678. doi: 10.1371/journal.pone.0095678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oczypok EA, Milutinovic PS, Alcorn JF, Khare A, Crum LT, Manni ML, et al. Pulmonary receptor for advanced glycation end-products promotes asthma pathogenesis through IL-33 and accumulation of group 2 innate lymphoid cells. J Allergy Clin Immunol. 2015;136(Sep (3)):747–56. e4. doi: 10.1016/j.jaci.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(Jan (7534)):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 85.Kim BS, Artis D. Group 2 Innate Lymphoid Cells in Health and Disease. Cold Spring Harbor perspectives in biology. 2015;7(Jan (5)) doi: 10.1101/cshperspect.a016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doherty TA. At the bench: understanding group 2 innate lymphoid cells in disease. Journal of leukocyte biology. 2015;97(Mar (3)):455–67. doi: 10.1189/jlb.5BT0814-374R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31(Dec):31–7. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Lloyd CM, Saglani S. Epithelial cytokines and pulmonary allergic inflammation. Curr Opin Immunol. 2015;34(Jun):52–8. doi: 10.1016/j.coi.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Beucher J, Boelle PY, Busson PF, Muselet-Charlier C, Clement A, Corvol H, et al. AGER -429T/C is associated with an increased lung disease severity in cystic fibrosis. PLoS One. 2012;7(7):e41913. doi: 10.1371/journal.pone.0041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iannitti RG, Casagrande A, De Luca A, Cunha C, Sorci G, Riuzzi F, et al. Hypoxia promotes danger-mediated inflammation via receptor for advanced glycation end products in cystic fibrosis. Am J Respir Crit Care Med. 2013;188(Dec (11)):1338–50. doi: 10.1164/rccm.201305-0986OC. [DOI] [PubMed] [Google Scholar]
- 91.Makam M, Diaz D, Laval J, Gernez Y, Conrad CK, Dunn CE, et al. Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc Natl Acad Sci U S A. 2009;106(Apr (14)):5779–83. doi: 10.1073/pnas.0813410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Foell D, Seeliger S, Vogl T, Koch HG, Maschek H, Harms E, et al. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax. 2003;58(Jul (7)):613–7. doi: 10.1136/thorax.58.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reynolds PR, Schmitt RE, Kasteler SD, Sturrock A, Sanders K, Bierhaus A, et al. Receptors for advanced glycation end-products targeting protect against hyperoxia-induced lung injury in mice. Am J Respir Cell Mol Biol. 2010;42(May (5)):545–51. doi: 10.1165/rcmb.2008-0265OC. [DOI] [PubMed] [Google Scholar]
- 94.Ramsgaard L, Englert JM, Manni ML, Milutinovic PS, Gefter J, Tobolewski J, et al. Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in mice. PLoS One. 2011;6(5):e20132. doi: 10.1371/journal.pone.0020132. Epub 2011/06/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Zoelen MA, Schouten M, de Vos AF, Florquin S, Meijers JC, Nawroth PP, et al. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol. 2009;182(Apr (7)):4349–56. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 96.Jabaudon M, Blondonnet R, Roszyk L, Pereira B, Guerin R, Perbet S, et al. Soluble Forms and Ligands of the Receptor for Advanced Glycation End-Products in Patients with Acute Respiratory Distress Syndrome: An Observational Prospective Study. PLoS One. 2015;10(8):e0135857. doi: 10.1371/journal.pone.0135857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63(Dec (12)):1083–9. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem. 2011;44(Jun (8–9)):601–4. doi: 10.1016/j.clinbiochem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 99.Patel BV, Wilson MR, Takata M. Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J. 2012;39(May (5)):1162–70. doi: 10.1183/09031936.00093911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jabaudon M, Blondonnet R, Roszyk L, Bouvier D, Audard J, Clairefond G, et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2015;192(Jul (2)):191–9. doi: 10.1164/rccm.201501-0020OC. [DOI] [PubMed] [Google Scholar]
- 101.Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Current opinion in pediatrics. 2014;26(Jun (3)):306–14. doi: 10.1097/MOP.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lizotte PP, Hanford LE, Enghild JJ, Nozik-Grayck E, Giles BL, Oury TD. Developmental expression of the receptor for advanced glycation end-products (RAGE) and its response to hyperoxia in the neonatal rat lung. BMC Dev Biol. 2007;7:15. doi: 10.1186/1471-213X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynolds PR, Stogsdill JA, Stogsdill MP, Heimann NB. Up-Regulation of RAGE by Alveolar Epithelium Influences Cytodifferentiation and Causes Severe Lung Hypoplasia. Am J Respir Cell Mol Biol. 2011;(Jun) doi: 10.1165/rcmb.2011-0170OC. Epub 2011/06/21. Eng. [DOI] [PubMed] [Google Scholar]
- 104.Stogsdill MP, Stogsdill JA, Bodine BG, Fredrickson AC, Sefcik TL, Wood TT, et al. Conditional overexpression of receptors for advanced glycation end-products in the adult murine lung causes airspace enlargement and induces inflammation. Am J Respir Cell Mol Biol. 2013;49(Jul (1)):128–34. doi: 10.1165/rcmb.2013-0013OC. [DOI] [PubMed] [Google Scholar]
- 105.Aghai ZH, Saslow JG, Meniru C, Porter C, Eydelman R, Bhat V, et al. High-mobility group box-1 protein in tracheal aspirates from premature infants: relationship with bronchopulmonary dysplasia and steroid therapy. Journal of perinatology: official journal of the California Perinatal Association. 2010;30(Sep (9)):610–5. doi: 10.1038/jp.2010.16. [DOI] [PubMed] [Google Scholar]
- 106.Fan LL, Deterding RR, Langston C. Pediatric interstitial lung disease revisited. Pediatric pulmonology. 2004;38(Nov (5)):369–78. doi: 10.1002/ppul.20114. [DOI] [PubMed] [Google Scholar]
- 107.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188(Aug (3)):376–94. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soares JJ, Deutsch GH, Moore PE, Fazili MF, Austin ED, Brown RF, et al. Childhood interstitial lung diseases: an 18-year retrospective analysis. Pediatrics. 2013;132(Oct (4)):684–91. doi: 10.1542/peds.2013-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Queisser MA, Kouri FM, Konigshoff M, Wygrecka M, Schubert U, Eickelberg O, et al. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am J Respir Cell Mol Biol. 2008;39(Sep (3)):337–45. doi: 10.1165/rcmb.2007-0244OC. [DOI] [PubMed] [Google Scholar]
- 110.Buckley ST, Medina C, Kasper M, Ehrhardt C. Interplay between RAGE, CD44, and focal adhesion molecules in epithelial-mesenchymal transition of alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(Apr (4)):L548–59. doi: 10.1152/ajplung.00230.2010. [DOI] [PubMed] [Google Scholar]
- 111.Bargagli E, Penza F, Bianchi N, Olivieri C, Bennett D, Prasse A, et al. Controversial role of RAGE in the pathogenesis of idiopathic pulmonary fibrosis. Respir Physiol Neurobiol. 2009;165(Feb (2–3)):119–20. doi: 10.1016/j.resp.2008.10.017. author reply 21–2. [DOI] [PubMed] [Google Scholar]
- 112.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180(Jul (2)):167–75. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramsgaard L, Englert JM, Tobolewski J, Tomai L, Fattman CL, Leme AS, et al. The role of the receptor for advanced glycation end-products in a murine model of silicosis. PLoS One. 2010;5(3):e9604. doi: 10.1371/journal.pone.0009604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, et al. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;293(dec (6)):L1427–36. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 115.Englert JM, Kliment CR, Ramsgaard L, Milutinovic PS, Crum L, Tobolewski JM, et al. Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. Int J Clin Exp Pathol. 2011;4(Mar (3)):241–54. Epub 2011/04/14. eng. [PMC free article] [PubMed] [Google Scholar]
- 116.Willis BC, Borok Z. TGF-beta-induced EMT. mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(Sep (3)): L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 117.Ding H, Ji X, Chen R, Ma T, Tang Z, Fen Y, et al. Antifibrotic properties of receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2015;35(Dec):34–41. doi: 10.1016/j.pupt.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 118.Arumugam T, Simeone DM, Schmidt AM, Logsdon CD. S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE) J Biol Chem. 2004;279(Feb (7)):5059–65. doi: 10.1074/jbc.M310124200. [DOI] [PubMed] [Google Scholar]
- 119.Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis. 2010;31(Mar (3)):334–41. doi: 10.1093/carcin/bgp322. [DOI] [PubMed] [Google Scholar]
- 120.Schraml P, Bendik I, Ludwig CU. Differential messenger RNA and protein expression of the receptor for advanced glycosylated end products in normal lung and non-small cell lung carcinoma. Cancer Res. 1997;57(Sep (17)):3669–71. [PubMed] [Google Scholar]
- 121.Stav D, Bar I, Sandbank J. Usefulness of CDK5RAP3, CCNB2, and RAGE genes for the diagnosis of lung adenocarcinoma. Int J Biol Markers. 2007;22(Apr–Jun (2)):108–13. doi: 10.1177/172460080702200204. [DOI] [PubMed] [Google Scholar]
- 122.Kalea AZ, See F, Harja E, Arriero M, Schmidt AM, Hudson BI. Alternatively spliced RAGEv1 inhibits tumorigenesis through suppression of JNK signaling. Cancer Res. 2010;70(Jul (13)):5628–38. doi: 10.1158/0008-5472.CAN-10-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Li Y, Yu W, Ma L, Ji X, Xiao W. Expression of the receptor for advanced glycation end-products and frequency of polymorphism in lung cancer. Oncology letters. 2015;10(Jul (1)):51–60. doi: 10.3892/ol.2015.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8(Aug (8)):816–24. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 125.Diederichs S, Bulk E, Steffen B, Ji P, Tickenbrock L, Lang K, et al. S100 family members and trypsinogens are predictors of distant metastasis and survival in early-stage non-small cell lung cancer. Cancer Res. 2004;64(Aug (16)):5564–9. doi: 10.1158/0008-5472.CAN-04-2004. [DOI] [PubMed] [Google Scholar]
- 126.Hsieh HL, Schafer BW, Sasaki N, Heizmann CW. Expression analysis of S100 proteins and RAGE in human tumors using tissue microarrays. Biochem Biophys Res Commun. 2003;307(Jul (2)):375–81. doi: 10.1016/s0006-291x(03)01190-2. [DOI] [PubMed] [Google Scholar]
- 127.Miyazaki N, Abe Y, Oida Y, Suemizu H, Nishi M, Yamazaki H, et al. Poor outcome of patients with pulmonary adenocarcinoma showing decreased E-cadherin combined with increased S100A4 expression. International Journal of Oncology. 2006;28(6):1369–74. [PubMed] [Google Scholar]
- 128.Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Modern pathology: an official journal of the United States and Canadian Academy of Pathology Inc. 2006;19(Nov (11)):1437–45. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 129.Sambamurthy N, Leme AS, Oury TD, Shapiro SD. The Receptor for Advanced Glycation End Products (RAGE) Contributes to the Progression of Emphysema in Mice. PLoS One. 2015;10(3):e0118979. doi: 10.1371/journal.pone.0118979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Waseda K, Miyahara N, Taniguchi A, Kurimoto E, Ikeda G, Koga H, et al. Emphysema requires the receptor for advanced glycation end-products triggering on structural cells. Am J Respir Cell Mol Biol. 2015;52(Apr (4)):482–91. doi: 10.1165/rcmb.2014-0027OC. [DOI] [PubMed] [Google Scholar]
- 131.Reynolds PR, Kasteler SD, Schmitt RE, Hoidal JR. Receptor for advanced glycation end-products signals through Ras during tobacco smoke-induced pulmonary inflammation. Am J Respir Cell Mol Biol. 2011;45(Aug (2)):411–8. doi: 10.1165/rcmb.2010-0231OC. [DOI] [PubMed] [Google Scholar]
- 132.Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, et al. High mobility group protein B1 (HMGB1) in Asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011;17(7–8):807–15. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu L, Ma L, Nicholson LF, Black PN. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir Med. 2011;105(Mar (3)):329–36. doi: 10.1016/j.rmed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 134.Reimann S, Fink L, Wilhelm J, Hoffmann J, Bednorz M, Seimetz M, et al. Increased S100A4 expression in the vasculature of human COPD lungs and murine model of smoke-induced emphysema. Respiratory research. 2015;16:127. doi: 10.1186/s12931-015-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gopal P, Reynaert NL, Scheijen JL, Schalkwijk CG, Franssen FM, Wouters EF, et al. Association of plasma sRAGE, but not esRAGE with lung function impairment in COPD. Respiratory research. 2014;15:24. doi: 10.1186/1465-9921-15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gopal P, Rutten EP, Dentener MA, Wouters EF, Reynaert NL. Decreased plasma sRAGE levels in COPD: influence of oxygen therapy. Eur J Clin Invest. 2012;42(Aug (8)):807–14. doi: 10.1111/j.1365-2362.2012.02646.x. [DOI] [PubMed] [Google Scholar]
- 137.Miniati M, Monti S, Basta G, Cocci F, Fornai E, Bottai M. Soluble receptor for advanced glycation end products in COPD: relationship with emphysema and chronic cor pulmonale: a case-control study. Respiratory research. 2011;12:37. doi: 10.1186/1465-9921-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iwamoto H, Gao J, Pulkkinen V, Toljamo T, Nieminen P, Mazur W. Soluble receptor for advanced glycation end-products and progression of airway disease. BMC pulmonary medicine. 2014;14:68. doi: 10.1186/1471-2466-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng DT, Kim DK, Cockayne DA, Belousov A, Bitter H, Cho MH, et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(Oct (8)):948–57. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 140.Smith DJ, Yerkovich ST, Towers MA, Carroll ML, Thomas R, Upham JW. Reduced soluble receptor for advanced glycation end-products in COPD. Eur Respir J. 2011;37(Mar (3)):516–22. doi: 10.1183/09031936.00029310. [DOI] [PubMed] [Google Scholar]
- 141.Sukkar MB, Ullah MA, Gan WJ, Wark PA, Chung KF, Hughes JM, et al. RAGE: a new frontier in chronic airways disease. British journal of pharmacology. 2012;167(Nov (6)):1161–76. doi: 10.1111/j.1476-5381.2012.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378(Sep (9795)):1015–26. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 143.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Farmer DG, Ewart MA, Mair KM, Kennedy S. Soluble Receptor for Advanced Glycation End Products (sRAGE) Attenuates Haemodynamic Changes to Chronic Hypoxia in the Mouse. Pulm Pharmacol Ther. 2014;(Jan) doi: 10.1016/j.pupt.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Tekabe Y, Anthony T, Li Q, Ray R, Rai V, Zhang G, et al. Treatment effect with anti-RAGE F(ab')2 antibody improves hind limb angiogenesis and blood flow in Type 1 diabetic mice with left femoral artery ligation. Vascular medicine. 2015;(Mar) doi: 10.1177/1358863X14568337. [DOI] [PubMed] [Google Scholar]
- 146.Brederson JD, Strakhova M, Mills C, Barlow E, Meyer A, Nimmrich V, et al. A monoclonal antibody against the receptor for advanced glycation end products attenuates inflammatory and neuropathic pain in the mouse. European journal of pain. 2016;20(Apr (4)):607–14. doi: 10.1002/ejp.775. [DOI] [PubMed] [Google Scholar]
- 147.Han YT, Kim K, Choi GI, An H, Son D, Kim H, et al. Pyrazole-5-carboxamides, novel inhibitors of receptor for advanced glycation end products (RAGE) European journal of medicinal chemistry. 2014;79(May):128–42. doi: 10.1016/j.ejmech.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 148.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122(Apr (4)):1377–92. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Christaki E, Opal SM, Keith JC, Jr, Kessimian N, Palardy JE, Parejo NA, et al. A monoclonal antibody against RAGE alters gene expression and is protective in experimental models of sepsis and pneumococcal pneumonia. Shock. 2011;35(May (5)):492–8. doi: 10.1097/SHK.0b013e31820b2e1c. [DOI] [PubMed] [Google Scholar]
- 150.Burstein AH, Grimes I, Galasko DR, Aisen PS, Sabbagh M, Mjalli AM. Effect of TTP488 in patients with mild to moderate Alzheimer's disease. BMC neurology. 2014;14:12. doi: 10.1186/1471-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Walker D, Lue LF, Paul G, Patel A, Sabbagh MN. Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer's disease. Expert opinion on investigational drugs. 2015;24(Mar (3)):393–9. doi: 10.1517/13543784.2015.1001490. [DOI] [PMC free article] [PubMed] [Google Scholar]