Abstract
Background
The gene ABCB1 encodes p-glycoprotein, a xenobiotic efflux pump capable of transporting certain opioids, including fentanyl. ABCB1 genotype has been previously associated with patient opioid requirements and may influence fentanyl dosing requirements in critically ill children.
Methods
A diagnostically diverse cohort of 61 children who received a fentanyl infusion while admitted to the pediatric intensive care unit (PICU) were included in this study. We examined associations between fentanyl requirements, pain and sedation scores, serum fentanyl levels and ABCB1 genotype.
Results
Patients with the AA allele at ABCB1 locus rs1045642 received less fentanyl compared to patients with the AG or GG allele. A multivariable model demonstrated that patients with the AA allele received 18.6 mcg/kg/day less fentanyl than patients with either the AG or GG allele (95% Confidence Interval −33.4 to −3.8 mcg/kg/day; p = 0.014). Incorporating race in this model demonstrated a similar association but did not reach the threshold for multiple testing.
Conclusion
ABCB1 genotype rs1045642 AA is associated with fentanyl administration in this cohort of children admitted to the PICU, likely because of decreased expression and activity of p-glycoprotein. Prospective evaluation of the influence of ABCB1 in sedative-analgesia administration in critically ill children is warranted.
Introduction
Invasive life support strategies and interventions in the intensive care unit are frequently painful and psychologically distressing (1,2). The use of opioid medications is ubiquitous in pediatric intensive care units (PICU) for ensuring patient safety and mitigating the psychological impact of critical illness. A number of approaches have been described to guide titration of opioids; however, considerable variation exists amongst both practitioners and centers regarding optimal dosing strategies (3). Insufficient evidence exists to champion any single approach to administering sedation and analgesia in the PICU. A recent cluster-randomized, controlled trial conducted across 31 PICUs in the United States failed to demonstrate benefit measured in mechanical ventilation days amongst patients treated with a sedation protocol versus those who received usual care (4).
The current standard of care for providing sedation and analgesia relies on an individualized clinical assessment of a patient’s propensity for experiencing pain and psychological distress. Variables such as the presence of mechanical ventilation, recent surgery, age, objective rating scales and clinical intuition factor into the initiation of a sedative-analgesic regimen. Subsequent adjustments are made post hoc based on a patient’s response, with restrictive dosing typically used to minimize iatrogenic dependence and subsequent withdrawal (5). Intrinsic to this reactive approach are episodes of inadequate pain control and inappropriate wakefulness that contribute to adverse events and outcomes such as prolonged mechanical ventilation, unplanned extubation, post-extubation airway edema, delirium, and post-traumatic stress disorder (3,6,7).
Clarification of individual patient characteristics including genotypes deterministic of sedative and analgesic requirements would improve the precision of drug administration during the care of seriously ill children and potentially reduce related adverse events. ABCB1 codes for p-glycoprotein, a xenobiotic transporter present in multiple tissues, including the liver, intestines, kidney, and the blood-brain barrier (BBB) (8). As such, it is known to be a key determinant of systemic drug exposure and central nervous system disposition for several medications, including some opioids (9). Single nucleotide polymorphisms (SNPs) in ABCB1 have been associated with peri-procedural opioid requirements, though the influence of ABCB1 genotype on sedative-analgesia requirements in seriously and critically ill children has not been examined (10–13). Accordingly, we undertook a retrospective analysis of a cohort of diagnostically diverse patients admitted to a quaternary PICU to determine associations between fentanyl requirements and genotypes for SNPs in the ABCB1 gene.
Materials and Methods
Study Design and Setting
A retrospective cohort study was undertaken involving 61 diagnostically diverse patients admitted to the PICU at our quaternary children’s hospital that received a fentanyl infusion. Approval was granted by the University of Pittsburgh Institutional Review Board.
Participants and Data Collection
Serum samples and DNA were prospectively collected as part of a cohort study of 104 randomly enrolled PICU patients with inclusion criteria that included only predicted PICU length of stay (LOS) ≥ 3 days, presence of an indwelling central venous or arterial line for blood sampling, and obtainment of informed consent from the parent or legal guardian, and were otherwise unrestricted. Of these 104 patients, 61 received a fentanyl infusion and were therefore included in this study. Blood was obtained from the indwelling catheter, and serum samples and blood pellets were stored at −80°C. DNA was extracted using the Qiamp DNA extraction kit (Qiagen Inc; Valencia, CA).
Relevant demographics and clinical characteristics were extracted from an electronic health record database by our organization’s data warehouse and de-identified for this study. The primary admission diagnosis for each patient was categorized as neurologic, respiratory, sepsis, gastrointestinal/transplant, post-operative, or other. Record review of sedative requirements noted patients who received an opioid infusion (fentanyl, morphine, or hydromorphone), midazolam infusion, dexmedetomidine infusion, or neuromuscular blocker infusion (cisatracurium or vecuronium). Clinical parameters of interest included whether the patient had tracheal intubation, had received mechanical ventilation while in the PICU, had underwent surgery during the hospitalization, or had received a vasopressor infusion while in the PICU.
Genotyping
The TaqMan allelic discrimination assay was used to analyze genotypes using a candidate gene approach. Three SNPs involving the ABCB1 transporter gene were selected on the basis of both previously reported and hypothesized associations with opioid requirements: rs1045642 (C3435T), rs1128503 (C1236T), and rs2229109 (G1692A) (10–13). Allele frequencies in this cohort were compared to population distributions reported in the 1000 genome project (14).
Medication Dosing and Clinical Outcome Metrics
The main outcome of interest was opioid responsiveness according to genotype as determined by fentanyl dose requirements during PICU admission. Fentanyl was the most common sedative-analgesic medication administered to the cohort. Total fentanyl exposure was calculated for patients who received a fentanyl infusion for at least 6 hours while in the PICU. Sedative-analgesic dose titration and infusion initiation practices in the study center PICU vary by clinical circumstances and ordering clinician (either physician or nurse practitioner), with some patients receiving several boluses of fentanyl prior to initiation of an infusion. Fentanyl exposure was therefore calculated as a sum of all fentanyl administered to the patient while receiving an infusion, inclusive of all boluses delivered concomitant with the infusion. Boluses were also included if administered within 12 hours prior to the start of the infusion or for as long as the bolus administration frequency equaled or exceeded 1 bolus per hour. This approach was taken to maximize inclusion of administered fentanyl that was directed towards ongoing sedation-analgesia efforts while excluding sporadic, isolated doses such as a single bolus given for brief procedural or operative sedation-analgesia.
Other metrics for opioid responsiveness included the Face, Legs, Activity, Cry, Consolability (FLACC) scale score and the University of Michigan Sedation Scale score. Both of these metrics are recorded hourly for all patients on continuous sedative infusions in the study center’s PICU. The FLACC scale ranges from 0 to 10, with higher scores indicating more pain (15). The Michigan Sedation scale ranges from 0 to 4 with higher scores indicating deeper sedation (16). Averages for each score were calculated by multiplying each scale’s scores by the proportion of measurements attributed to that score for each patient. Other sedative-analgesic infusions, neuromuscular blocker infusions, and methadone prescriptions were recorded if they were administered or prescribed during the patient’s hospital stay.
Fentanyl Levels
Fentanyl levels were measured in samples obtained from patients who had received at least 12 hours of preceding fentanyl infusion, in order to optimize the likelihood that a relative steady state had been achieved. Plasma fentanyl concentrations were determined by ultra-high performance-tandem mass spectrometry (UPLC-MS/MS). Sample preparation of 100 μL plasma involved protein precipitation with 2% phosphoric acid, addition of d5-fentanyl (Cerilliant, Round Rock, TX) as an internal standard, solid phase extraction using Oasis MCX cation-exchange columns (Waters, Milford, MA), and sample reconstitution in 90% 0.15% formic acid/10% acetonitrile. Separation of 7.5 μL of the injected sample was achieved via reverse phase UPLC using a BEH C18 1.7 μm, 2.1×100 mm column (Acquity, Waters, Milford, MA). Mobile phase consisted of 0.15% formic acid (solution A) and acetonitrile (solution B) to provide a 0.2 mL/min gradient elution as follows: initially 90% A/10% B for 1 min, then linearly increasing to 100% B over 3min and holding for 1min before finally returning to initial conditions over 0.5 min and holding for 2min to achieve a total run time of 7.5 min. Mass spectrometric detection of fentanyl was performed using a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo, Waltham, MA) operated in positive electrospray ionization mode with optimized instrument parameters. Calibration curves were linear from 0.125–200 ng/mL (r2=0.999) with a variation of <14.8%.
Statistical Analysis
Hardy-Weinberg equilibrium was tested for the individual SNPs. The chi-squared test was used to examine dichotomous outcomes. The binomial probability test was used to compare cohort allele frequency with population allele frequency. The Mann-Whitney test and student’s t test were used to compare nonparametric and parametric continuous variables, respectively. Univariable and multivariable linear regression was used to identify associations between SNPs and fentanyl exposure and levels. Biallelic loci were coded in both additive and dichotomized models. Bivariate univariable regression analysis was used to examine associations between fentanyl exposure and the clinical characteristics of alternative sedative infusions, mechanical ventilation, tracheal intubation, any surgical intervention during the hospital stay, administration of vasopressors, gender and race. Age and body-mass-index (BMI) were tested as continuous variables. Multivariable linear regression models were constructed incorporating independent variables that demonstrated a statistically significant association with fentanyl exposure in univariable analysis (P < 0.05). Models were limited to a maximum of 5 independent variables, to allow at least 10 to 15 degrees of freedom for each variable. It was expected that the cohort would be relatively racially homogenous based on experience with the demographics of the study center. Accordingly, we questioned whether this cohort was sufficiently diverse to adequately distinguish whether race was a significant predictor of fentanyl exposure. Two multivariable models differing only in the inclusion/exclusion of race as an independent variable were constructed in recognition of the relatively small sample size and racial homogeneity of the cohort. To account for multiple testing across 3 SNPs, alpha was set 0.027 according to the Benjamini-Yekutieli (B-Y) method (17,18). Multicollinearity was evaluated by examining variance inflation factors for the multivariable model. Analysis was performed using Stata 14.0 (StatCorp, College Station, TX).
Results
Demographics and Clinical Characteristics
Of the 61 patients in the study cohort, primary admission diagnoses were categorized as neurologic for 19 (31.1%), respiratory for 13 (21.3%), gastrointestinal/transplant for 8 (13.1%), other for 8 (13.1%), sepsis for 7 (11.5%), and post-operative for 6 (9.8%). Table 1 displays demographics and clinical characteristics of the cohort, including stratification by race. Black patients were younger than white patients in this cohort. Of the entire cohort of 104 patients, those who received a fentanyl infusion were younger, more likely to be female, and more likely to require tracheal intubation, mechanical ventilation, a vasopressor infusion or a muscle relaxant infusion compared to patients who did not receive a fentanyl infusion (Supplementary Table S1).
Table 1.
Clinical characteristics
| Fentanyl Infusion N = 61 | Racea White n = 53 | Black n = 6 | |
|---|---|---|---|
| Age (mo) (median [IQR]) | 72 [19 – 132] | 84 [18 – 144] | 30 [24–108] |
| BMI (mean ± SD) | 20.4 ± 7.3 | 20.2 ± 7.0 | 23.1 ± 9.5 |
| Female, n (%) | 30 (49.2) | 27 (50.9) | 3 (50) |
| Tracheal Intubation | 55 (90.2) | 47 (88.7) | 6 (100) |
| Mechanical Ventilation | 57 (93.4) | 49 (92.5) | 6 (100) |
| Muscle Relaxant Infusion | 28 (45.9) | 25 (47.2) | 2 (33.3) |
| Surgery | 34 (55.7) | 30 (56.6) | 3 (50) |
| Tracheostomy Dependence | 7 (11.5) | 7 (13.2) | 0 (0) |
| Vasopressor infusion | 26 (42.6) | 24 (45.3) | 2 (33.3) |
Race undocumented for 2 patients; Abbreviation: mo, months; BMI, Body Mass Index
Allele Frequencies
Table 2 displays the frequencies of the individual alleles at each tested loci with the respective disequilibrium coefficients. All SNPs were in Hardy-Weinberg equilibrium. Minor alleles at rs2229109 were low frequency in this cohort, with only one patient with a minor allele receiving a fentanyl infusion, precluding further analysis of this genotype. There were no significant differences in allele frequencies between the population of patients receiving fentanyl and the population reported in the 1000 genome project or our previous study of adults with traumatic brain injury (TBI) hospitalized in this catchment area (Supplementary Tables S2 and S3) (14,19).
Table 2.
ABCB1 allele details
| SNP | |||||||
|---|---|---|---|---|---|---|---|
| Alias | Function | Location | Allele | Frequency | P (HWE) | D’ | |
| rs1045642 | C3435T | Synonymous | 87138645 | A | 0.490 | 0.232 | 0.0299 |
| G | 0.510 | ||||||
| rs1128503 | C1236T | Synonymous | 87179601 | A | 0.405 | 0.561 | −0.0140 |
| G | 0.595 | ||||||
| rs2229109 | G1692A | Missense | 87179809 | C | 0.975 | 0.799 | −0.0006 |
| T | 0.025 | ||||||
ABCB1 and Fentanyl Exposure
Fentanyl exposure in the cohort ranged from an aggregate of 13.8 μg/kg/day to 142.8 μg/kg/day. Univariable regression demonstrated a significant association between the rs1045642 genotype and fentanyl exposure, with less fentanyl administered to the AA versus AG/GG genotypes (Table 3). The average fentanyl exposure for the AA group was 49.4 ± 24.6 versus 66.5 ± 30.0 μg/kg/day (P = 0.041) for the AG/GG genotypes. There was no significant difference between the rs1045462 AA and AG/GG genotypes with regards to median duration of fentanyl infusion (Table 4).
Table 3.
Univariable analysis of association between ABCB1 alleles and fentanyl exposure
| SNP (n) | Allele | n | β (μg/kg/day) [95% CI] | P* |
|---|---|---|---|---|
| rs1045642 (61) | AA | 17 | −17.1 [−35.5 − −0.7] | 0.041 |
| AG | 25 | |||
| GG | 19 | 2.3 [−14.1 – 18.7] | 0.782 | |
| Add G | 6.7 [−3.1 – 16.4] | 0.177 | ||
|
| ||||
| rs1128503 (61) | AA | 11 | −6.6 [−26.3 – 13.1] | 0.503 |
| AG | 26 | |||
| GG | 24 | 2.7 [−12.9 – 18.2] | 0.730 | |
| Add G | 3.1 [−7.4 – 13.5] | 0.558 | ||
|
| ||||
| rs2229109 (60) | CC | 59 | -- | |
| TC | 1 | |||
Univariable linear regression
Table 4.
Characteristics of patients according to rs1045462 genotype
| rs1045462 | |||
|---|---|---|---|
| AA n = 17 | AG/GG n = 44 | P* | |
| Fentanyl Exposure (μg/kg/day)a | 49.4 ± 24.6 | 66.5 ± 30.0 | 0.041 |
| Fentanyl Infusion Duration (days)b | 2.7 [1.7 – 6.2] | 4.4 [1.7 – 10.5] | 0.326 |
| PICU LOS (days)b | 7.99 [5.5 – 14.9] | 9.9 [6.6 – 16.1] | 0.385 |
| Age (mo)b | 60 [18.8 – 132] | 72 [36 – 168] | 0.596 |
| Female, n (%) | 9 (52.9%) | 21 (47.7%) | 0.715 |
| BMIa | 20.6 ± 9.0 | 20.4 ± 6.7 | 0.898 |
| Average Sedation Scorea | 1.6 ± 0.8 | 1.5 ± 0.6 | 0.585 |
| Average FLACC Scorea | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.946 |
| Black Race, n (%) | 0 (0) | 6 (13.6) | 0.115 |
mean ± SD;
edian [IQR];
Mann-Whitney for medians, Student’s t test for averages;
Abbreviations: SD, standard deviation; IQR, interquartile range; LOS, length of stay; mo, month; PICU, Pediatric Intensive Care Unit; BMI, Body Mass Index; FLACC, Face Legs Activity Cry Consolability
Clinical Covariates, rs1045642 and Fentanyl Exposure
Regression analysis examining clinical covariates and fentanyl exposure are shown in Table 5. No significant association was observed for patients also receiving infusions of dexmedetomidine, morphine, and/or midazolam. Only one patient in the cohort who received fentanyl also received a hydromorphone infusion. Patients who received infusions of either cisatracurium or vecuronium received significantly more fentanyl, as did patients who had tracheal intubation and those who required mechanical ventilation. No significant association was observed between fentanyl exposure and BMI, age, gender, vasopressor infusion, PICU LOS, or surgery during admission. After adjusting for the presence of a neuromuscular blocker infusion (cisatracurium or vecuronium), intubation, and mechanical ventilation, rs1045642 and neuromuscular blocker infusion remained significantly associated with fentanyl exposure (Table 6). A multivariable model incorporating race and 59 patients who received fentanyl (excluding 2 of unknown race) also demonstrated a nominal association between rs1045642 and fentanyl exposure, but did not reach the B-Y threshold for significance. Race remained significantly associated with fentanyl exposure after adjustment. Variance inflation factors for all variables in the multivariable models were <1, indicating low multi-collinearity.
Table 5.
Univariable analysis of clinical covariates
| Covariate | n | β (μg/kg/day) [95% CI] | P* |
|---|---|---|---|
| Age | 61 | −0.1 [−0.2 – 0.1] | 0.379 |
| Any Neuromuscular Blocker | 28 | 21.4 [7.2 – 35.6] | 0.004 |
| Black Racea | 6 | 35.4 [11.4 – 59.5] | 0.005 |
| Dexmedetomidine | 20 | 6.8 [−9.3 – 22.9] | 0.399 |
| Tracheal Intubation | 55 | 30.1 [5.8 – 54.4] | 0.016 |
| Female | 30 | −4.1 [−19.3 – 11.1] | 0.591 |
| Mechanical Ventilation | 57 | 31.3 [1.7 – 60.9] | 0.039 |
| Midazolam | 16 | 3.9 [−13.4 – 21.2] | 0.652 |
| Morphine | 6 | −1.3 [−26.9 – 24.2] | 0.917 |
| PICU LOS | 61 | 0.2 [−0.02 – 0.5] | 0.07 |
| Surgery | 34 | 0.9 [−14.4 – 16.2] | 0.905 |
| Tracheostomy Dependence | 7 | −18.8 [−42.2 – 4.5] | 0.112 |
| Vasopressor Infusion | 26 | −3.1 [−18.4 – 12.3] | 0.690 |
Univariable linear regression;
59 patients included, 2 patients of unknown race excluded;
Abbreviations: PICU, Pediatric Intensive Care Unit
Table 6.
Multivariable regression model
| Covariate | β (μg/kg/day) [95% CI] | P* | β (μg/kg/day)*[95% CI] | P* |
|---|---|---|---|---|
| rs1045642 (AA) | −18.6 [−33.4 − −3.8] | 0.014 | −15.0 [−29.8 − −0.2] | 0.047 |
| Any Neuromuscular Blocker | 21.4 [8.1 – 34.8] | 0.002 | 22.8 [9.8 – 35.8]0.001 | |
| Tracheal Intubation | 22.4 [−14.8 – 59.5] | 0.232 | 19.2 [−16.2 – 54.7] | 0.281 |
| Mechanical Ventilation | 5.4 [−39.3 – 50.2] | 0.808 | 4.2 [−38.4 – 46.8] | 0.844 |
| Black Racea | -- | -- | 31.6 [9.9 – 53.3] | 0.005 |
Multivariable linear regression;
59 patients included, 2 patients of unknown race excluded
SNPs, Fentanyl Levels and Patient Comfort
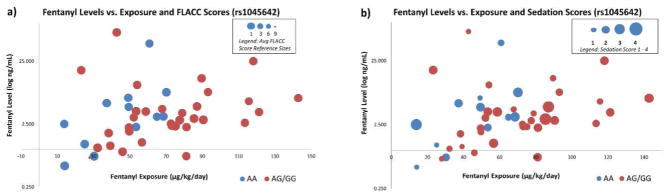
Blood samples obtained at least 12 hours following initiation of a fentanyl infusion were available for 44 patients. Regression analysis did not reveal any significant associations between fentanyl blood levels and rs1045642 or rs1128503 genotype. Median fentanyl levels for the rs1045642 genotypes AA and AG/GG were 3.3 [IQR 2.0 – 5.7] and 3.0 [IQR 2.1 – 5.0] ng/mL (P = 0.958), respectively. There was no association between rs1045642 genotype and either average sedation score or average FLACC score. Figure 1 displays the relationship between fentanyl exposure, fentanyl levels, average sedation score and average FLACC score according to rs1045642 genotype. Among patients who received neuromuscular blockade, there was no significant difference in average FLACC score between those with the AA versus AG/GG genotypes.
Figure 1.
The relationship between fentanyl level (log ng/mL), fentanyl exposure (mcg/kg/day), average
Discussion
Continuous sedative-analgesic infusions are commonly necessary to facilitate invasive life support strategies during critical illness and in the peri-operative period. Fentanyl is the preferred medicine for this purpose at our institution and many other centers (20). Fentanyl’s potency and potential for dose-related toxicities mandate careful titration, yet anticipating a patient’s response frequently involves heuristic risk stratification. This same estimative approach also typically guides the decision to add-in or substitute other sedative-analgesic medications.
Foreknowledge of an individual patient’s response to sedative-analgesics would promote greater precision in optimizing sedation. Such an individualized approach relies on predicting dose-response relationships of commonly used medications such as fentanyl through an understanding of pharmacokinetics and pharmacodynamics. Each patient’s metabolic profile, opioid receptor structure and expression, drug transporter structure and expression, and clinical circumstances should be appreciated. Recognizing pharmacogenenomic factors that predict drug response across a range of clinical scenarios represents an initial step in this regard. We identified one ABCB1 SNP, rs1045642 genotype AA, that is associated with reduced fentanyl requirements in a diagnostically diverse sample of PICU patients, but other genes related to opioid metabolism, transport, and elimination including cytochrome P450 family proteins, mu-opioid receptor, and rhomboid family members, among others, warrant evaluation in future studies.
As a xenobiotic efflux transporter with multiple substrates, p-glycoprotein (the protein product of ABCB1) has been associated with opioid requirements in several clinical settings. Inhibition of p-glycoprotein with quinidine has been shown to potentiate the effects the opiate loperamide. When administered together to a small study cohort of 8 subjects, quinidine and loperamide resulted in a significant degree of respiratory depression compared with control patients who received loperamide and placebo (21). In mice with variable levels of brain ABCB1 expression, those mice with lower expression had more pronounced responses to morphine (22). Prospective evaluation of morphine requirements in 263 children undergoing tonsillectomy revealed an additive effect of a minor allele (G) at ABCB1 locus rs9282564 that was associated with increased post-operative respiratory depression (13). Examination of rs1045642 did not reveal any association with either post-operative respiratory depression or post-operative morphine requirements in this tonsillectomy cohort. Two other, prospective studies evaluating the influence of ABCB1 SNPs controlled for ethnicity in their inclusion criteria. In a study of 126 Koreans who underwent spinal anesthesia with fentanyl, patients with rs1045642 genotype AA and rs2032582 genotype AA experienced a significant decrease in respiratory rate within minutes of fentanyl administration compared with other genotypes (12). A similar study of 83 Turkish patients undergoing spinal anesthesia with a single dose of intravenous fentanyl did not demonstrate relationships between ABCB1 SNPs (including rs1045462) and respiratory depression (10). The authors did point out a nonsignificant downtrend in respiratory rate in patients with rs1045642 genotype AA.
Individuals with rs1045462 genotype AA have lower levels of ABCB1 expression in duodenal tissue (23). A comparable reduction in expression in the liver, kidney and the BBB would provide a mechanistic basis for the reduced fentanyl requirements in patients with the AA genotype. P-glycoprotein is present in the canalicular membrane of bile duct cells, the tubular membrane of the proximal renal tubule, and in the endothelium of the BBB. Reduced expression of p-gylcoprotein may result in decreased excretion of substrate opioids from the brain into plasma and decreased excretion from plasma into bile and urine. Despite receiving different amounts of fentanyl, plasma levels and the FLACC pain scores amongst patients with the rs1045642 AA genotype were similar relative to the remaining cohort. This may be expected as fentanyl is dosed to effect (sedation/pain scores); it suggests that patients with rs1045642 genotype AA require lower fentanyl doses to achieve desired clinical effect and equivalent plasma levels.
rs1045462 was independently associated with fentanyl exposure controlling for covariates associated with fentanyl requirements in this cohort. In determining fentanyl exposure, the duration of fentanyl infusion was accounted for by calculating the total dose administered (μg/kg) by the duration over which it was given (days); however, children in the PICU are known to develop tolerance to opioids over time that is typically matched with escalating doses until the patient is ready to wean (24). Importantly, there was no difference in the duration of fentanyl infusion in relation to rs1045462 genotype. Race was also significantly associated with fentanyl exposure in both univariable and multivariable analyses, with White patients receiving less fentanyl per day compared with Black patients. This finding is compatible with previous reports of African American patients exhibiting lower pain tolerance compared to Caucasians, though the genetic determinants of this differential response remain to be clarified (25).
This study is limited by its retrospective design. As this study made use of a convenience sample and was not sufficiently powered to clarify the relationship between race and fentanyl exposure, conclusions regarding this association must be curbed, though warrant further investigation in a larger population. More robust population pharmacokinetic modeling utilizing detailed pharmacokinetic sampling from a larger cohort is planned to validate the relationship observed between genotype and fentanyl exposure in the present cohort (26). While the present study represents the largest pharmacogenomic evaluation of ABCB1 in critically ill children to-date, the relatively small sample size raises the possibility that the findings were driven by a few outliers; however, as Figure 1 displays, fentanyl exposure, fentanyl levels, sedation scores and FLACC scores were generally well-distributed across the cohort.
Patients in this cohort represent a diverse range of diagnoses, including patients with acute and chronic brain injury. Sufficient diagnostic information was not available in the de-identified dataset to account for the influence of baseline impaired consciousness on sedative requirements. Prospective assessment adjusting for impaired consciousness as a reason for reduced sedative requirements is necessary to determine whether baseline mental status is confounding the apparent association between rs1045642 genotype and fentanyl requirements in this cohort. Likewise, the hypothesized role of p-glycoprotein in relation to the BBB indicates a need to further study the influence of rs1045642 genotype in diseases that result in compromise to cerebral vasculature, such as cerebral vasculitis and TBI. The diagnostic heterogeneity of this modestly sized cohort prevented analysis for associations between specific disease and genotype or fentanyl exposure, as each diagnostic category contained a relatively heterogenous set of pathologies. The gastrointestinal category, for example, contained patients who underwent solid organ transplant, as well as patients with intussusception. Our study targeted patients expected to have a PICU LOS of at least 3 days, with 60% receiving fentanyl infusion. Patients receiving fentanyl infusions are generally sicker than patients who do not require a continuous sedative-analgesic medication during hospitalization. Therefore, it is not clear whether our findings are generalizable to PICU patients receiving only bolus fentanyl administration, infusions for short durations and/or with shorter expected PICU LOS. Similarly, the use of neuromuscular blockade in this cohort may be an indication of illness severity. Patients receiving neuromuscular blockade received more fentanyl, though it is unclear whether this finding is related to patient response or instead reflects extra vigilance on the part of clinicians to ensure adequate sedation in the absence of a reliable physical exam. Controlling for both diagnosis and illness severity will be essential in larger, prospective sedation-analgesia pharmacogenomic studies in PICU patients.
The current approach to sedation in the PICU is imprecise, can lead to major adverse events such as unplanned extubation and contributes to untoward outcomes such as prolonged mechanical ventilation (27,28). A clearer understanding of the molecular traits that determine response to sedative-analgesic medicines will provide the basis for individualized regimens for children in the PICU and could have implications for patients of all ages. In the emerging era of precision-medicine, genomic-based patient stratification of sedative-analgesic response could provide a novel framework for clinical trials designed with the aim of improving our ability to conscientiously administer opioids. Our findings, alongside existing evidence to-date, suggest that a larger, prospective assessment of pharmacogenomics-based approaches to sedation and analgesia in the PICU are warranted.
Supplementary Material
Acknowledgments
Statement of Financial Support
This work was supported by NIH grants NICHD T32 HD40686 (CMH), NINDS R01 NS069247 (RSBC, PEE, PMK), NCATS KL2 TR000146 (PEE), and CTSA UL1 TR001857 (Clinical and Translational Sciences Institute at the University of Pittsburgh); and the Children’s Hospital of Pittsburgh of UPMC Scientific Program.
We thank Eric Yablonsky for help with data sorting. We thank the nurses, residents, fellows, and pharmacists in the PICU for first-rate clinical care.
Footnotes
Disclosure: No disclosures by any author
References
- 1.Stein-Parbury J, McKinley S. Patients’ experiences of being in an intensive care unit: a select literature review. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2000;9(1):20–7. [PubMed] [Google Scholar]
- 2.Harris J, Ramelet A-S, van Dijk M, et al. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Med. 2016;42(6):972–86. doi: 10.1007/s00134-016-4344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vet NJ, Ista E, de Wildt SN, van Dijk M, Tibboel D, de Hoog M. Optimal sedation in pediatric intensive care patients: a systematic review. Intensive Care Med. 2013;39(9):1524–34. doi: 10.1007/s00134-013-2971-3. [DOI] [PubMed] [Google Scholar]
- 4.Curley MAQ, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313(4):379–89. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galinkin J, Koh JL Committee on Drugs, Section On Anesthesiology and Pain Medicine, American Academy of Pediatrics. Recognition and management of iatrogenically induced opioid dependence and withdrawal in children. Pediatrics. 2014;133(1):152–5. doi: 10.1542/peds.2013-3398. [DOI] [PubMed] [Google Scholar]
- 6.Traube C, Mauer EA, Gerber LM, et al. Cost Associated With Pediatric Delirium in the ICU. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamas A, López-Herce J. Monitoring sedation in the critically ill child. Anaesthesia. 2010;65(5):516–24. doi: 10.1111/j.1365-2044.2010.06263.x. [DOI] [PubMed] [Google Scholar]
- 8.Gong X-D, Wang J-Y, Liu F, et al. Gene polymorphisms of OPRM1 A118G and ABCB1 C3435T may influence opioid requirements in Chinese patients with cancer pain. Asian Pac J Cancer Prev APJCP. 2013;14(5):2937–43. doi: 10.7314/apjcp.2013.14.5.2937. [DOI] [PubMed] [Google Scholar]
- 9.Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34(1–2):47–54. doi: 10.1081/dmr-120001389. [DOI] [PubMed] [Google Scholar]
- 10.Kesimci E, Engin AB, Kanbak O, Karahalil B. Association between ABCB1 gene polymorphisms and fentanyl’s adverse effects in Turkish patients undergoing spinal anesthesia. Gene. 2012;493(2):273–7. doi: 10.1016/j.gene.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Levran O, O’Hara K, Peles E, et al. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum Mol Genet. 2008;17(14):2219–27. doi: 10.1093/hmg/ddn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H-J, Shinn HK, Ryu SH, Lee H-S, Park C-S, Kang J-H. Genetic polymorphisms in the ABCB1 gene and the effects of fentanyl in Koreans. Clin Pharmacol Ther. 2007;81(4):539–46. doi: 10.1038/sj.clpt.6100046. [DOI] [PubMed] [Google Scholar]
- 13.Sadhasivam S, Chidambaran V, Zhang X, et al. Opioid-induced respiratory depression: ABCB1 transporter pharmacogenetics. Pharmacogenomics J. 2015;15(2):119–26. doi: 10.1038/tpj.2014.56. [DOI] [PubMed] [Google Scholar]
- 14.1000 Genomes | A Deep Catalog of Human Genetic Variation [Internet] [cited 2016 Dec 7];Available from: http://www.internationalgenome.org/
- 15.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–7. [PubMed] [Google Scholar]
- 16.Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Tremper K, Naughton N. Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS) Br J Anaesth. 2002;88(2):241–5. doi: 10.1093/bja/88.2.241. [DOI] [PubMed] [Google Scholar]
- 17.Balding DJ. A tutorial on statistical methods for population association studies. Nat Rev Genet. 2006;7(10):781–91. doi: 10.1038/nrg1916. [DOI] [PubMed] [Google Scholar]
- 18.Narum SR. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conserv Genet. 2006;7(5):783–7. [Google Scholar]
- 19.Cousar JL, Conley YP, Willyerd FA, et al. Influence of ATP-binding cassette polymorphisms on neurological outcome after traumatic brain injury. Neurocrit Care. 2013;19(2):192–8. doi: 10.1007/s12028-013-9881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minardi C, SahillioVlu E, Astuto M, Colombo M, Ingelmo PM. Sedation and analgesia in pediatric intensive care. Curr Drug Targets. 2012;13(7):936–43. doi: 10.2174/138945012800675740. [DOI] [PubMed] [Google Scholar]
- 21.Sadeque AJ, Wandel C, He H, Shah S, Wood AJ. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther. 2000;68(3):231–7. doi: 10.1067/mcp.2000.109156. [DOI] [PubMed] [Google Scholar]
- 22.Hamabe W, Maeda T, Kiguchi N, Yamamoto C, Tokuyama S, Kishioka S. Negative relationship between morphine analgesia and P-glycoprotein expression levels in the brain. J Pharmacol Sci. 2007;105(4):353–60. doi: 10.1254/jphs.fp0071287. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand KJS, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125(5):e1208–1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics. 2012;13(15):1719–40. doi: 10.2217/pgs.12.152. [DOI] [PubMed] [Google Scholar]
- 26.Empey PE, Velez de Mendizabal N, Bell MJ, et al. Therapeutic hypothermia decreases phenytoin elimination in children with traumatic brain injury. Crit Care Med. 2013;41(10):2379–87. doi: 10.1097/CCM.0b013e318292316c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald RK, Davis AT, Hanson SJ National Association of Children’s Hospitals and Related Institution PICU Focus Group Investigators. Multicenter Analysis of the Factors Associated With Unplanned Extubation in the PICU. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2015;16(7):e217–223. doi: 10.1097/PCC.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 28.Yaghmai BF, Di Gennaro JL, Irby GA, Deeter KH, Zimmerman JJ. A Pediatric Sedation Protocol for Mechanically Ventilated Patients Requires Sustenance Beyond Implementation. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2016;17(8):721–6. doi: 10.1097/PCC.0000000000000846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.