Abstract
Neuroendocrine prostate cancer (NEPC) has increasingly become a clinical challenge. The mechanisms by which neuroendocrine (NE) cells arises from prostate adenocarcinoma cells are poorly understood. FOXA1 is a transcription factor of the forkhead family that is required for prostate epithelial differentiation. In this study, we demonstrated that FOXA1 loss drives neuroendocrine (NE) differentiation, demarcated by phenotypical changes and NEPC marker expressions. Mechanistically, this is mediated by FOXA1 binding to the promoter of IL-8, a chemokine previously shown elevated in NEPC, to directly inhibit its expression. Further, IL-8 up-regulation activates the MAPK/ERK pathway, leading to ERK phosphorylation and ENO2 expression. IL-8 knockdown or ERK inhibition, on the other hand, abolished FOXA1 loss-induced NE differentiation. Analysis of xenograft mouse models confirmed FOXA1 loss in NEPC tumors relative to its adenocarcinoma counterparts. Importantly, FOXA1 is down-regulated in human NEPC tumors compared to primary and castration-resistant prostate cancers, and its expression is negatively correlated with that of ENO2. These findings indicate that FOXA1 transcriptionally suppresses IL-8, the expression of which would otherwise stimulate MAPK/ERK pathway to promote NE differentiation of prostate cancer cells. Our data strongly suggest that FOXA1 loss may play a significant role in enabling prostate cancer progression to NEPC, while IL-8 and MAPK/ERK pathways may be promising targets for therapeutic intervention.
Keywords: neuroendocrine differentiation, NEPC, FOXA1, prostate cancer, IL-8
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed non-skin cancer in the American men. Localized and regional prostate cancers are well-managed with surgical and radiation treatments. Metastatic prostate cancer are commonly treated with androgen-deprivation therapy (ADT) and yet resistance develops quickly leading to castration-resistant prostate cancer (CRPC) 1. High-affinity AR antagonists such as abiraterone and enzalutamide have recently been developed to further inhibit CRPC. Albeit effective in the short-term, their use has been associated with increased incidences of neuroendocrine prostate cancer (NEPC) lately2, 3. This is consistent with previous reports that long-term androgen depletion causes neuroendocrine (NE) differentiation of prostate cancer cell lines 4, 5. NEPC tumors are often negative of androgen receptor (AR) but instead express neuroendocrine markers such as enolase 2 (ENO2), Chromogranin A (CHGA), and synaptophysin (SYP) 6. NEPC tumors are androgen-independent and there are limited treatment options available; platinum- and cisplatin-based therapies are often used, but prognosis remains dismal 7, 8. It was estimated that the median survival of NEPC patients is only 9.8 to 13.1 months, compared to 125 months for prostate adenocarcinoma 9.
The molecular basis by which NEPC arises from prostate adenocarcinoma is not fully understood. Interleukin 8 (IL-8), is one of the first few molecules implicated in prostate cancer progression to NEPC 5, 10. IL-8 is a CXC chemokine that binds to its G protein coupled receptors CXCR1 and CXCR2, all of which have been shown up-regulated in androgen-independent prostate cancer 11. Further, Immunohistochemistry experiments showed that IL-8 is expressed specifically by the neuroendocrine tumor cells in the human prostate cancer tissues, which also express the CXCR2 receptor 12, 13. IL-8 stimulates CXCR2 receptor through autocrine signaling to regulate the differentiation or function of neuroendocrine cells. Blockade of CXCR2 using a small molecule antagonist has been shown to decrease androgen-independent prostate cancer cell growth 14.
Besides IL-8, IL-6, intracellular cyclic AMP, and heparin-binding epidermal growth factor like growth factor (HB-EGF) have all been shown to induce neuron-like morphology of prostate cancer cells 15–18. Their changes in the tumor microenvironment were shown to induce NE differentiation through STAT3 and MAPK/ERK signal transduction pathways. For example, STAT3 expression and function were found required for IL-6-induced LNCaP cell neuroendocrine differentiation 19, whereas HB-EGF induces NE differentiation by activating MAPK/ERK pathway, independently of STAT3 phosphorylation. Further, androgen depletion was also shown to activate MAPK/ERK in androgen-sensitive LNCaP cells 20. An MEK inhibitor, PD98059, efficiently blocks neuroendocrine differentiation of androgen-depleted cells, while expression of constitutively active MEK drives NE-like differentiation of LNCaP cells in the presence of androgen.
FOXA1 is a transcription factor that is essential for the development and differential of epithelial cells including the pancreas, liver, breast, and prostate 21–25. FOXA1 knockout mice are embryonic lethal, but conditional knockout are viable and have been shown to inhibit prostate morphogenesis and epithelial cell differentiation 24, 26, 27. Furthermore, a recent study reported that prostate-specific deletion of FOXA1 in adult murine epithelium causes prostatic hyperplasia and alteration of differentiated phenotype 27. Moreover, we reported that loss of FOXA1 leads to AR reprogramming 28 and epithelial-to-mesenchymal transition (EMT) through direct regulation of SLUG expression 29.
Recent molecular characterization of NEPC tumors have begun to identify important regulators of NEPC, including DEK 30, SRRM4 31, AURKA and MYCN 32, as well as epigenetic modifiers such as EZH2 33. In the present study, we demonstrated that FOXA1 reduces MAPK/ERK activation and prevents neuroendocrine differentiation of prostate cancer cells. Mechanistically, this is mediated through FOXA1 binding to the promoter of the IL-8 gene to directly inhibit its expression, which is essential for MAPK/ERK inhibition. Our data thus establish FOXA1 as a major regulator of NEPC and that FOXA1 loss drives CRPC progression or trans-differentiation towards NEPC. Further, our findings support that IL-8/CXCR2-targeting agents and MAPK/ERK inhibitors may be useful in suppressing NEPC.
RESULTS
FOXA1 inhibits neuroendocrine differentiation of prostate cancer cells
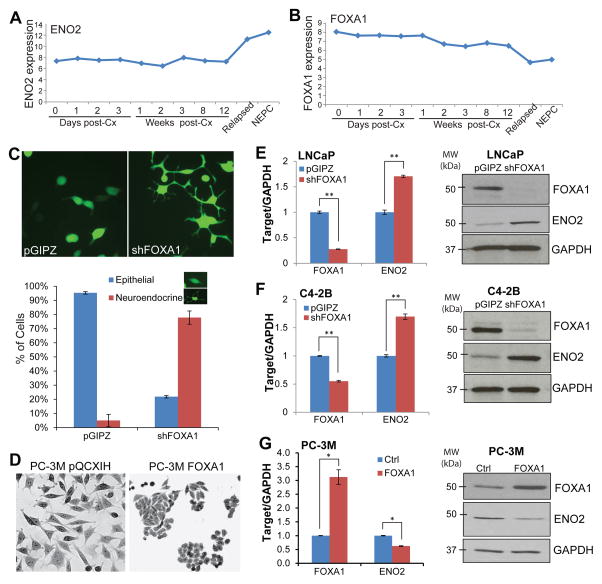
NEPC has increasingly become a major clinical challenge3. To determine whether FOXA1, a regulator of epithelial differentiation, is disrupted in NEPC, we analyzed the publically available dataset profiling gene expression of patient-derived xenograft modeling the progression from prostatic adenocarcinoma to NEPC 34. Our data showed that FOXA1 started to decrease at 1 week post-castration (post-Cx) and was remarkably down-regulated in relapsed and NEPC cells, wherein ENO2 was dramatically up-regulated (Figure 1A–B), suggesting a potential role of FOXA1 loss in NEPC progression. To test this, we performed FOXA1 depletion in LNCaP prostate adenocarcinoma cells over a time-course using shRNA-mediated knockdown and puromycin selection. After one week of puromycin selection, FOXA1-knockdown cells manifested clear morphological changes toward neuroendocrine (NE) phenotype with multiple neurite extensions, whereas the control cells remained the rounded shape of epithelial cells (Figure 1C). Nearly 96% of the control cells exhibited the rounded epithelial phenotype, while approximately 78% of the FOXA1-depleted cells displayed the NE phenotype, indicating a transition of LNCaP adenocarcinoma cells to NEPC. Stable overexpression of FOXA1, on the other hand, converted neuroendocrine-like PC-3M cells to the rounded shape forming typical epithelial cell clusters (Figure 1D). Moreover, analysis of gene expression showed that, upon stable FOXA1 knockdown, ENO2 expression was induced at both mRNA and protein levels in LNCaP (Figure 1E) and C4-2B (Figure 1F) cells compared to their respective control cells. Of note, AR levels were not significantly altered by FOXA1 depletion, precluding its potential regulation of ENO2 (Supplementary Figure 1). Conversely, we demonstrated that FOXA1 overexpression in PC-3M cells inhibited ENO2 mRNA and protein levels (Figure 1G). Taken together, our data strongly suggest that FOXA1 is a negative regulator of prostate cancer progression to NEPC.
Figure 1. FOXA1 knockdown induces neuroendocrine differentiation of prostate cancer cells.
A and B, Publically available microarray dataset GSE59986 34 was downloaded from the GEO database. The expression values of ENO2 and FOXA1 were retrieved and plotted over the time-course of post-castration (post-Cx), relapsed, and terminal NEPC tumors.
C, LNCaP cells were infected with pGIPZ or shFOXA1 lentivirus and selected for stable cells. Shown on the top is the morphology of the control and FOXA1-knockdown cells. The percentage of cells with epithelial and neuroendocrine-like phenotypes was quantified (bottom panel). Error bars indicate n=3, mean±SEM.
D, PC-3M cells were infected with pQCXIH or FOXA1-overexpressing retrovirus followed by two weeks of hygromycin selection. Cells were fixed, stained by crystal violet, and imaged.
E and F, FOXA1 knockdown induces ENO2 expression. LNCaP (E) and C4-2B (F) cells were infected with control or shFOXA1 lentivirus followed by puromycin selection, and then analyzed by qRT-PCR and western blotting. Error bars indicate n=3, mean±SEM, **p<0.001.
G, FOXA1 was stably overexpressed in PC-3M cells using FOXA1 retrovirus followed by two weeks of hygromycin selection. Error bars indicate n=3, mean±SEM, *p<0.05.
FOXA1 reduces ERK phosphorylation
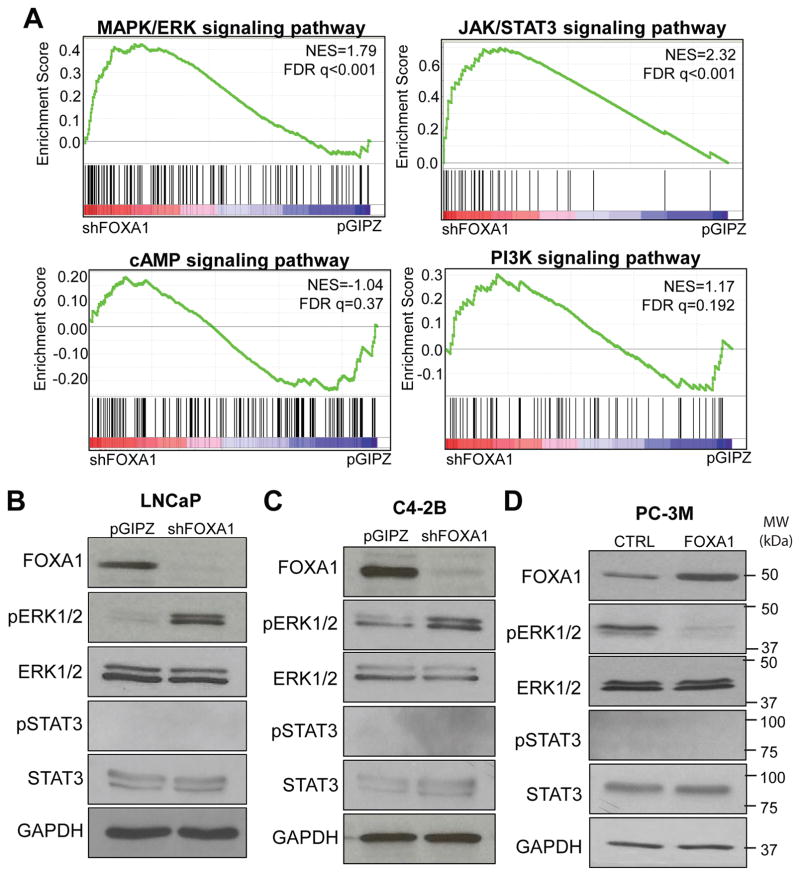
To identify potential mechanisms by which FOXA1 regulates NEPC, we examined whether FOXA1 regulates the MAPK/ERK, cAMP, JAK/STAT3, or PI3K/AKT signaling pathways as they have been previously associated with neuroendocrine differentiation 18, 19, 35. We retrieved corresponding pathway signature gene sets from the Molecular Signature Database and examined their rank ordered regulation in the expression microarray data comparing control and FOXA1-knockdown cells. Gene Set Enrichment Analysis (GSEA) revealed that MAPK/ERK (FDR q<0.001) and JAK/STAT3 (FDR q<0.001) pathway genes were enriched for up-regulation upon FOXA1 knockdown, while no significant enrichment was observed for other the cAMP and PI3K pathways (Figure 2A). To confirm this, we performed western blot analysis of the MAPK/ERK and JAK/STAT3 pathways in LNCaP cells with control or FOXA1 knockdown. Our data showed that FOXA1 depletion markedly induced pERK level, but pSTAT3 level remains undetectable (Figure 2B). Similar results were also observed in additional prostate cancer cell lines C4-2B following FOXA1 knockdown (Figure 2C). In contrast, FOXA1 stable overexpression in PC-3M cells significantly inhibited ERK1/2 phosphorylation, further supporting MAPK/ERK pathway as the candidate downstream mediator of FOXA1 (Figure 2D).
Figure 2. FOXA1 inhibits ERK phosphorylation.
A. MAPK/ERK and JAK/STAT3 signaling pathways are significantly enriched for up-regulation upon FOXA1 knockdown. MAPK/ERK (M10792), cAMP (M2720), JAK/STAT3 (M5897), PI3K (M5923) signaling pathway gene sets were obtained from Molecular Signatures Database and subjected to GSEA analysis using the gene expression dataset profiling LNCaP pGIPZ and shFOXA1 cells.
B and C, FOXA1 knockdown activates ERK but not STAT3. LNCaP (B) and C4-2B (C) control and stable FOXA1-knockdown cells were analyzed by western blotting.
D, FOXA1 overexpression reduces pERK but not pSTAT3. PC-3M control and stable FOXA1-overexpressing cells were analyzed by western blotting.
FOXA1 directly inhibits IL-8 gene expression
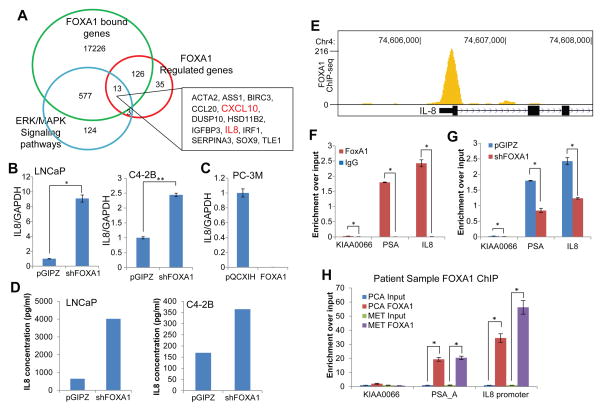
Next, we sought to examine how FOXA1 activates MAPK/ERK pathway. As FOXA1 is primarily known as a transcription factor, we wondered whether FOXA1 might regulate the transcription of some upstream regulators of the MAPK/ERK pathway. To identify direct FOXA1 targets that could induce ERK phosphorylation, we integrated multiple datasets to identify genes meeting the following selection criteria: 1) were differentially expressed by at least five-fold between LNCaP pGIPZ and shFOXA1 cells by microarray profiling; 2) harbored at least one FOXA1 binding site within the regulatory regions based on LNCaP FOXA1 ChIP-seq data, and 3) belong to the MAPK/ERK pathway. Through overlapping analysis, we obtained 13 genes, among which only 2 genes, CXCL10 and IL-8, were previously identified as upstream regulators of MAPK/ERK pathway 36–38 (Figure 3A). As most of prostate cell lines do not express the CXCL10 receptor CXCR3, we decided to focus on IL-8.
Figure 3. FOXA1 directly inhibit IL-8 gene transcription.
A, Venn diagram showing a core set of FOXA1-regulated MAPK/ERK pathway genes. FOXA1-bound genes were derived from LNCaP FOXA1 ChIP-seq data 28. Control and FOXA1-knockdown LNCaP cells were analyzed by microarray and genes with at least 5 folds differential expression were designated as FOXA1-regulated genes. The MAPK/ERK pathway gene sets including upstream regulators were obtained from InnateDB.
B, QRT-PCR analysis of IL-8 in LNCaP and C4-2B cells with stable FOXA1-knockdown relative to control cells. Error bars indicate n=3, mean ±SEM, *p<0.05 and **p<0.001.
C, QRT-PCR analysis of IL-8 in PC-3M cells with stable FOXA1 overexpression relative to control cells. Error bars indicate n=3, mean ±SEM, p<0.05.
D, ELISA analysis of IL-8 secreted protein was performed in LNCaP and C4-2B cells with stable FOXA1 knockdown relative to their respective control cells. One representative of three independent experiments is shown.
E, Genome browser view showing FOXA1 occupancy at the IL-8 promoter. FOXA1 ChIP-seq was performed in LNCaP cells.
F, ChIP-qPCR confirming FOXA1 binding at the IL-8 promoter in LNCaP cells. FOXA1 and IgG ChIP were performed in LNCaP cells. PSA enhancer is used as a positive control, whereas KIAA0066 a negative control gene that is not enriched by anti-FOXA1. Error bars indicate n=3, mean ±SEM, *p<0.05.
G, FOXA1 ChIP was performed in LNCaP cells with control or FOXA1 knockdown, followed by qPCR analysis.
H, FOXA1 occupies the IL-8 promoter in human prostate cancer tissues. FOXA1 ChIP was performed in one localized (PCA) and one metastatic (MET) prostate cancer tissues. Input and ChIP-enriched DNA were amplified using ligation-mediated PCR. Equal amount of amplicons were utilized to determine relative enrichment over input. Error bars indicate n=3, mean ±SEM, *p<0.05.
To validate the bioinformatics results, we examined IL-8 expression in LNCaP and C4-2B cells with stable FOXA1 knockdown and PC-3M cells with stable FOXA1 overexpression. Importantly, FOXA1 knockdown lead to a significant increase in IL-8 mRNA levels in both LNCaP and VCaP cells (Figure 3B), while FOXA1 overexpression in PC-3M cells remarkably abolished IL-8 expression (Figure 3C). Furthermore, as IL-8 is a secreted protein, we measured IL-8 secreted protein in cell culture media using ELISA and confirmed the increase of IL-8 at protein level in both LNCaP and C4-2B cells following FOXA1 stable knockdown (Figure 3D).
Next, we confirmed FOXA1 occupancy at the IL-8 promoter by examining LNCaP FOXA1 ChIP-seq, which showed that FOXA1 is strongly enriched at the IL-8 promoter (Figure 3E). To validate the ChIP-seq result, we performed ChIP-qPCR analysis using primers flanking the promoter of the IL-8 gene. Our results validated strong FOXA1 binding at the IL-8 promoter, the enrichment is equivalent or higher than FOXA1 occupancy at the positive control loci - the PSA enhancer (Figure 3F). As a negative control, the KIAA0066 gene was not enriched for FOXA1 binding. To further ensure the binding specificity, we performed FOXA1 ChIP in LNCaP pGIPZ control and shFOXA1 cells. ChIP-qPCR analysis demonstrated significantly decreased amount of FOXA1 occupancy at the IL-8 promoter following FOXA1 knockdown (Figure 3G). Furthermore, to determine if FOXA1 binding at IL-8 promoter holds true in primary prostate cancer tissues, we performed FOXA1 ChIP in localized and metastatic prostate cancer tissues. ChIP-enriched DNA was subjected to ligation-mediated PCR (LM-PCR) and potential enrichment of a specific genomic region was evaluated relative to the same amount of input DNA using qPCR. Our data showed that FOXA1 was highly enriched at the IL-8 promoter and PSA enhancer, but not at the negative control gene, in both primary and metastatic prostate cancers (Figure 3H). To further demonstrate that FOXA1 occupancy at the IL-8 promoter indeed regulates its transcriptional activity, we cloned the IL-8 promoter region into the pGL4.1[luc2] vector. Luciferase assay revealed that FOXA1 knockdown led to a marked increase of the transcriptional activity of the IL-8 promoter (Supplementary Figure 2). In good agreement of this, ChIP-qPCR showed significantly enhanced occupancy by active RNA Polymerase II (PolII p-Ser-5) and H3K4me3 at the IL-8 promoter upon FOXA1 knockdown (Supplementary Figure 3). Thus, we conclude that FOXA1 occupies the IL-8 promoter to directly inhibit its transcription. Next, we attempted to examine whether IL-8 is involved in FOXA1-mediated MAPK/ERK activation.
IL-8 mediates FOXA1 loss-induced MAPK/ERK activation and NE differentiation
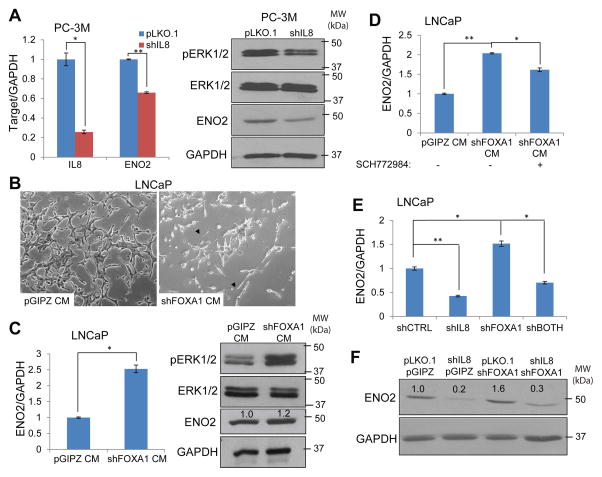
To determine whether IL-8 induces ERK phosphorylation and ENO2 expression, we performed IL-8 knockdown in neuroendocrine-like PC-3M cells. QRT-PCR analysis demonstrated significant decreased expression of NE marker ENO2 (Figure 4A). Moreover, western blot analysis confirmed ENO2 down-regulation at the protein level and also showed substantially decreased amount of pERK following IL-8 knockdown. Exogenous IL-8 stimulation on the other hand remarkably induced ENO2 expression (Supplementary Figure 4), supporting the role of IL-8 in promoting ERK phosphorylation and NE differentiation. To further elucidate that FOXA1 regulation of NEPC is due to secreted IL-8 protein, we harvested the conditioned media from LNCaP cells with control pGIPZ or FOXA1 knockdown, which were subsequently utilized to stimulate fresh LNCaP cells. Interestingly, LNCaP cells stimulated with conditioned medium from the FOXA1-depleted cells, which we have previously shown to express 8-fold more IL-8 protein (Figure 3D), exhibited cellular phenotype resembling neuroendocrine transdifferentiation (Figure 4B). Concordantly, qRT-PCR and western blot analysis demonstrated substantially increased amount of ENO2 expression and ERK phosphorylation in the cells stimulated with shFOXA1-conditioned media versus those treated with control media (Figure 4C). Most importantly, the effect is blocked, at least partially, by the MAPK/ERK inhibitor SCH77298439, suggesting that IL-8 induces NE differentiation through MAPK/ERK activation (Figure 4D). Finally, we attempted to examine whether this IL-8/pERK/ENO2 pathway is involved in mediating FOXA1 loss induced neuroendocrine differentiation. LNCaP cells were subjected to knockdown of IL-8 alone, FOXA1 alone, or both. QRT-PCR analysis confirmed that IL-8 knockdown inhibited ENO2 expression and, importantly, abolished FOXA1-loss induced ENO2 (Figure 4E). In good agreement with this, western blot showed that concurrent IL-8 knockdown dramatically reduced ENO2 protein level in FOXA1-depleted LNCaP cells. Therefore, IL-8 up-regulation is required for FOXA1 loss-induced neuroendocrine differentiation of prostate cancer cells.
Figure 4. IL-8 mediates FOXA1 loss-induced MAPK/ERK activation and neuroendocrine differentiation.
A. IL-8 induces ENO2 expression. PC-3M cells were infected with control or shIL-8 lentivirus followed by puromycin selection for one week before qRT-PCR and western blot analysis. Error bars indicate n=3, mean ±SEM, *p<0.05 and **p<0.001.
B and C, Conditioned media from FOXA1-knockdown cells induce neuroendocrine differentiation. Conditioned media were collected from LNCaP pGIPZ and FOXA1-knockdown stable cells and added to parental LNCaP cells for one week before imaging (B), and gene expression analysis using qRT-PCR and western blotting (C). Error bars indicate n=3, mean±SEM, *p<0.05.
D, MAPK/ERK inhibition abolishes FOXA1-loss-induced ENO2 expression. LNCaP cells were stimulated with conditioned media collected from either control or FOXA1-knockdown (shFOXA1) LNCaP cells with or without concurrent treatment of ERK inhibitor SCH772984. Error bars indicate n=3, mean±SEM, *p<0.05 and **p<0.001.
E and F, Depletion of IL-8 abolishes FOXA1-loss-induced ENO2. LNCaP cells were subjected to the knockdown of IL-8 and FOXA1 alone or in combination. ENO2 transcript and protein levels were examined by qRT-PCR (E) and western blotting (F). *p<0.05 and **p<0.001.
FOXA1 is down-regulated in NEPC and negatively correlated with ENO2 expression
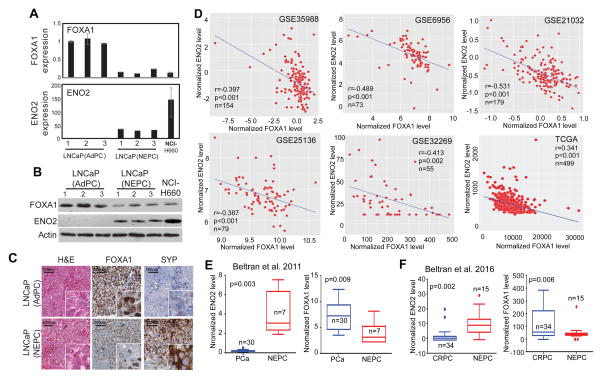
To further demonstrate that FOXA1 plays a role in prostate cancer progression to NEPC, we took advantage of a recently generated NEPC xenograft mouse model 40. Comparatively gene expression analysis revealed that FOXA1 is indeed down-regulated in LNCaP-derived NEPC xenografts relative to its adenocarcinoma counterparts, whereas ENO2 as expected is strongly up-regulated in NEPC xenografts (Figure 5A–B). Moreover, IHC staining of FOXA1 and neuroendocrine marker SYP in continuous xenograft tissue sections demonstrated an overall decrease of FOXA1 in NEPC tumors, especially in areas with strong SYP staining (Figure 5C).
Figure 5. FOXA1 is down-regulated in NEPC and negatively correlated with ENO2 expression.
A, and B, FOXA1 mRNA (A) and protein (B, C) levels decrease in LNCaP-derived NEPC xenografts. LNCaP(AdPC) were LNCaP xenografts (n=3) collected at the castration-resistant stage, when serum PSA concentrations recurred above the pre-castration levels. These tumors remain adenocarcinoma phenotype. LNCaP(NEPC) xenografts (n=3) were obtained from SRRM4-expressing LNCaP cells that were implanted in castrated nude mice. Phenotypical and histology analyses were reported previously 40. These tumors were castration resistant and had been maintained in castrated mice for three generations with low/no AR and PSA expression. NCI-H660 is an NEPC cell line used as a positive control.
C. FOXA1 protein is decreased in NEPC tumors and negative correlated with NE biomarker. Immunohistochemistry was performed on LNCaP(AdPC) and LNCaP(NEPC) xenografts with indicated antibodies and H&E staining. Representative images in the same field of the tumor were shown. Black arrows indicate areas of FOXA1 loss. Scale bars = 100μm
D, FOXA1 and ENO2 mRNA expression are negatively correlated. Six previously published prostate cancer gene expression datasets 41-45 were downloaded from the GEO database. The expression values of FOXA1 and ENO2 are retrieved and plotted.
E and F, FOXA1 is down-regulated in NEPC, wherein ENO2 is up-regulated. Previously published RNA-seq data of 37 PCa 32 (E) and 49 CRPC 33 (F) with or without neuroendocrine differentiation were obtained from cbioportal 57, 58. The expression values of ENO2 and FOXA1 were plotted comparing NEPC with PCa or CRPC.
Next, to test this in prostate cancer patients, we examined the expression levels of FOXA1 and ENO2 in a number of large prostate cancer gene expression profiling datasets 41–45 as well as the TCGA dataset. Importantly, we observed highly significant negative correlation between the expression of FOXA1 and NE markers in primary prostate cancer tissues (Figure 5D and Supplementary Figure 5). Further, to specifically examine this regulation in NEPC, we analyzed the Beltran et al. dataset that compared gene expression of 7 NEPC tissue with 30 prostate adenocarcinoma using RNA-seq 32. We found that FOXA1 mRNA level is indeed much lower in NEPC compared to primary PCa samples, whereas ENO2 expression was strongly up-regulated in NEPC (Figure 5E). Moreover, taking advantage of another RNA-seq dataset that has recently become available 33, we found that FOXA1 is also significantly down-regulated in NEPC relative to CRPC, while as positive control ENO2 expression was increased (Figure 5F). Altogether, our data showed that FOXA1 expression is lost in NEPC and is negatively correlated with the expression of neuroendocrine marker ENO2, strongly supporting the role of FOXA1 in preventing NEPC progression in clinical prostate cancer.
DISCUSSION
FOXA1 is a transcription factor that is indispensable for prostate development and epithelial cell differentiation 46. For a long time, FOXA1 was considered as a pioneer factor that recruits the AR to lineage-specific genomic loci to turn on prostatic gene expression 47. More recently, dual roles of FOXA1 have been reported in its regulation of AR including both pioneering and reprogramming effects 48, 49. In addition, in the context of castration-resistant prostate cancer, we showed that FOXA1 plays an inhibitory role in restricting the activity of residual AR in androgen-depleted cells, while FOXA1 loss unleashes an androgen-independent AR program 28. Moreover, we reported an androgen-independent function of AR wherein it directly inhibits SLUG gene transcription and thus hinders epithelial-to-mesenchymal transition 29. Along this line of research, in the present study we demonstrated that FOXA1 inhibits IL-8 transcription through direct occupancy at and regulation of its promoter. The concept of FOXA1 as a transcriptional repressor is new and the molecular mechanisms involved are yet to be defined. We predict that FOXA1, as a chromatin opening factor, might facilitate the recruitment of co-repressors to the regulatory elements of some specific genes that are to be maintained in a silenced mode in prostate cells, such as those involved in EMT and NE differentiation. Functionally, we showed that FOXA1 is able to prevent neuroendocrine differentiation of prostate cancer cells, being consistent with its central role as an epithelial factor.
As a prostatic transcription factor, FOXA1 is often expressed at high levels in the prostate along with the androgen receptor. We and other have shown earlier that FOXA1 is transiently up-regulated in localized prostate cancer but ultimately down-regulated in CRPC, prominently at mRNA levels 28, 48. In this study, we found that FOXA1 is further down-regulated in NEPC. This disease stage-dependent expression of FOXA1 is likely associated with its dual roles in promoting cell growth but inhibiting EMT and neuroendocrine differentiation, which are the dominating characteristics of primary and CRPC/NEPC tumors, respectively. Notably, while EMT is required for tumor dissemination, metastatic tumors that have successfully homed into distal organs will need to go through mesenchymal-to-epithelial transition in order to regain proliferation 50, 51. Likewise, NE cells are known to have low growth rate but NEPC or small cell carcinoma cells grow rapidly and are thus highly aggressive 52. During these reversal processes, it may be a necessity for FOXA1 re-expression. Consequently, we predict that FOXA1 levels may be tightly regulated through the disease transitions and might not always been down-regulated in all CRPC and NEPC tumors. The level of FOXA1 expression in these tumors may be dependent on or indicative of their respective growth and differentiation status. It would be interesting to determine in future studies whether/when FOXA1 expression is re-gained after tumor cells have successfully completed metastasis and/or NE differentiation.
High-throughput molecular characterization has revealed that NEPC patients harbor various genetic aberrations, such as MYCN and AURKA amplification, and epigenetic de-regulations 32, 33, 53. Upon identification of these genetic alterations, a Phase II clinical trial for AURKA inhibitor, MLN8237, has been initiated for NEPC and small cell carcinoma patients. In future studies, it would be interesting to test whether FOXA1 loss and function are linked with such genetic and epigenetic aberrations. We are the first to demonstrate that FOXA1 loss as an important upstream regulator of IL-8, one of the most extensively studied genes in NEPC 5. Further, we showed that this is mediated by IL-8-activated ERK phosphorylation, suggesting that IL-8 neutralizing antibodies, CXCR2 antagonists, and MEK/ERK inhibitors may hold great promise for the treatment of late-stage castration-resistant prostate cancer.
MATERIALS AND METHODS
Cell Lines
Prostate cancer cell lines LNCaP and PC-3M were obtained from American Type Culture Collection (ATCC) and C4-2B cells were a gift from Dr. Arul Chinnaiyan. All cell lines were authenticated and free of mycoplasma. Cells were cultured in RPMI1640 with 10% FBS. FOXA1 stable knockdown cells were created by infecting shFOXA1 lentivirus followed by one week of puromycin selection. FOXA1 stable overexpress cells were created by infecting FOXA1 overexpress retrovirus followed by two weeks of hygromycin selection.
Conditioned Media
LNCaP FOXA1 stable knockdown and control cells were made and media were incubated for one week prior to collection. Collected media were spin down to remove dead cells and 10mM HEPES and 1X glutamax were supplemented prior to use.
Plasmids and small interfering RNA
FOXA1 stable knockdown pGIPZ lentiviral shRNAmir construct (Clone ID#V2LHS_16780) was obtained from Open Biosystems. IL-8 stable knockdown construct was generated by inserting oligos (CGAACTTTAATTTCAGGAA) to the pLKO.1 backbone. FOXA1 overexpression construct was generated by cloning full-length FOXA1 into pQCXIH (Clontech). IL-8 promoter with FOXA1 binding region was amplified by PCR from HEK293T genomic DNA and cloned into pGL4.1[luc2] luciferase reporter vector (Promega).
Western blots, ChIP, ELISA and antibodies
Western blotting analyses were performed using standard protocols. Antibodies used were as follow: anti-FOXA1 (ab23738, Abcam), anti-NSE (M0873, DAKO), anti-pERK1/2 (4370S, cell signaling), anti-ERK1/2 (0192S, cell signaling), anti-AR (06-680, Millipore), and anti-GAPDH (ab9385, Abcam). ChIP was performed as previously described 54. Antibodies used include anti-FOXA1 (ab23738, Abcam), anti-RNA PolII p-Ser5 (04-1572, Millipore), and anti-H3K4me3 (04-745, Millipore). ELISA was performed using Human IL-8 ELISA kit II following manufacture’s protocol (550999, BD Bioscience).
Luciferase Assay
Luciferase assay was performed as previously described 55. In brief, pGL4.1[luc2]-IL-8 was transfected along with Renilla internal control into LNCaP shCtrl or shFOXA1 stable cells. Luciferase assays were performed 48hrs after the transfection.
Microscopy and morphology analysis
Fluorescent and crystal violet images were taken using Olympus CKX41. Cell morphologies were determined manually: GFP-positive Cells with two or more branches were considered neuroendocrine phenotype and cells with rounded shapes were considered epithelial.
Quantitative PCR assay
Quantitative PCR was conducted using Bullseye Evagreen qPCR 2X master mix (MIDSCI) using Applied Biosystems StepOne Plus Realtime PCR system. All primers were designed using Primer3 and synthesized by Integrated DNA Technology. All primers used in this study are listed in Supplementary Table1.
Immunohistochemistry
Paraffin embedded prostate cancer tissue blocks were from the Vancouver Prostate Centre Tissue Bank. Patient consent was reviewed and approved by the University of British Columbia Clinical Research Ethics Board (certificate no. H09-01628). Immunohistochemical staining was conducted as previously described 56 using the Ventana DiscoverXT Autostainer (Ventana Medical System) with enzyme labeled biotin streptavidin system and solvent-resistant DAB Map kit. Antibodies used in IHC include anti-FoxA1 (Abcam; ab23738), anti-SYP (Abcam; ab32127), anti-chromogranin A (Millipore; MAB5268), and anti-IL-8 (R&D systems).
Statistical Methods
All the experiments were performed at least in three biological replicates. All the figures shown are mean (±SEM) of technical replicates from 1 representative experiment. All statistical testing was done using two-sided t-test unless otherwise noted.
Bioinformatics analysis
Correlation plot was generated using R ggplot package. Geom_point function was used to create scatterplots and stat_smooth function was used to find the pattern by linear smooth methods. Correlation coefficient (r) and p values are calculated and shown on top left.
Supplementary Material
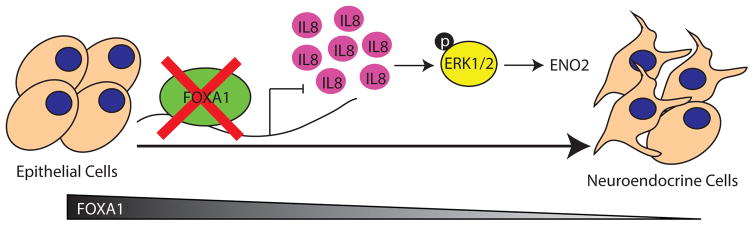
Figure 6. A model depicting FOXA1 regulation of neuroendocrine differentiation.
FOXA1 loss in prostate adenocarcinoma induces the expression of IL-8, which activates MAPK/ERK signaling, leading to neuroendocrine differentiation marked by high ENO2 expression.
Acknowledgments
This work was supported in part by the NIH R01CA172384 (to J.Y.), prostate cancer SPORE P50CA180995 (to J.Y.), the Institutional Ruth L. Kirschstein National Research Service Award from the National Institute of Diabetes and Digestive and Kidney Diseases T32 DK007169 (to J.K.).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–592. doi: 10.1016/j.eururo.2003.11.032. discussion 592. [DOI] [PubMed] [Google Scholar]
- 3.Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:e386–389. doi: 10.1200/JCO.2011.41.5166. [DOI] [PubMed] [Google Scholar]
- 4.Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocrine-related cancer. 2006;13:151–167. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 5.Santoni M, Conti A, Burattini L, Berardi R, Scarpelli M, Cheng L, et al. Neuroendocrine differentiation in prostate cancer: novel morphological insights and future therapeutic perspectives. Biochimica et biophysica acta. 2014;1846:630–637. doi: 10.1016/j.bbcan.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Sagnak L, Topaloglu H, Ozok U, Ersoy H. Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clinical genitourinary cancer. 2011;9:73–80. doi: 10.1016/j.clgc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Culine S, El Demery M, Lamy PJ, Iborra F, Avances C, Pinguet F. Docetaxel and cisplatin in patients with metastatic androgen independent prostate cancer and circulating neuroendocrine markers. J Urol. 2007;178:844–848. doi: 10.1016/j.juro.2007.05.044. discussion 848. [DOI] [PubMed] [Google Scholar]
- 8.Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–3630. doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parimi V, Goyal R, Poropatich K, Yang XJ. Neuroendocrine differentiation of prostate cancer: a review. American journal of clinical and experimental urology. 2014;2:273–285. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Sun Y, Wu C, Magyar CE, Li X, Cheng L, et al. Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway. Endocrine-related cancer. 2012;19:321–331. doi: 10.1530/ERC-11-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11:4117–4127. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Chen CJ, Wang JK, Hsia E, Li W, Squires J, et al. Neuroendocrine differentiation of prostate cancer. Asian J Androl. 2013;15:328–332. doi: 10.1038/aja.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Yao JL, Zhang L, Bourne PA, Quinn AM, di Sant’Agnese PA, et al. Differential expression of interleukin-8 and its receptors in the neuroendocrine and non-neuroendocrine compartments of prostate cancer. Am J Pathol. 2005;166:1807–1815. doi: 10.1016/S0002-9440(10)62490-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O’Sullivan JM, et al. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. The Journal of pharmacology and experimental therapeutics. 2008;327:746–759. doi: 10.1124/jpet.108.143826. [DOI] [PubMed] [Google Scholar]
- 15.Mori S, Murakami-Mori K, Bonavida B. Interleukin-6 induces G1 arrest through induction of p27(Kip1), a cyclin-dependent kinase inhibitor, and neuron-like morphology in LNCaP prostate tumor cells. Biochemical and biophysical research communications. 1999;257:609–614. doi: 10.1006/bbrc.1999.0515. [DOI] [PubMed] [Google Scholar]
- 16.Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, et al. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5330–5334. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox ME, Deeble PD, Bissonette EA, Parsons SJ. Activated 3′,5′-cyclic AMP-dependent protein kinase is sufficient to induce neuroendocrine-like differentiation of the LNCaP prostate tumor cell line. The Journal of biological chemistry. 2000;275:13812–13818. doi: 10.1074/jbc.275.18.13812. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Adam RM, Freeman MR. Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res. 2002;62:1549–1554. [PubMed] [Google Scholar]
- 19.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. The Prostate. 2000;42:186–195. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF. Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene. 2003;22:6704–6716. doi: 10.1038/sj.onc.1206764. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Washington MK, Crawford HC. Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal transition in pancreatic cancer. Cancer Res. 2010;70:2115–2125. doi: 10.1158/0008-5472.CAN-09-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besnard V, Wert SE, Kaestner KH, Whitsett JA. Stage-specific regulation of respiratory epithelial cell differentiation by Foxa1. American journal of physiology Lung cellular and molecular physiology. 2005;289:L750–759. doi: 10.1152/ajplung.00151.2005. [DOI] [PubMed] [Google Scholar]
- 23.Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, et al. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–2054. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes & development. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. The Prostate. 2005;62:339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- 26.Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, et al. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. The Journal of biological chemistry. 2005;280:13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- 27.DeGraff DJ, Grabowska MM, Case TC, Yu X, Herrick MK, Hayward WJ, et al. FOXA1 deletion in luminal epithelium causes prostatic hyperplasia and alteration of differentiated phenotype. Lab Invest. 2014;94:726–739. doi: 10.1038/labinvest.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin HJ, Zhao JC, Wu L, Kim J, Yu J. Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat Commun. 2014;5:3972. doi: 10.1038/ncomms4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin HJ, Zhao JC, Ogden I, Bergan RC, Yu J. Androgen receptor-independent function of FoxA1 in prostate cancer metastasis. Cancer Res. 2013;73:3725–3736. doi: 10.1158/0008-5472.CAN-12-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin D, Dong X, Wang K, Wyatt AW, Crea F, Xue H, et al. Identification of DEK as a potential therapeutic target for neuroendocrine prostate cancer. Oncotarget. 2015;6:1806–1820. doi: 10.18632/oncotarget.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Coleman IM, Brown LG, True LD, Kollath L, Lucas JM, et al. SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer. Clin Cancer Res. 2015;21:4698–4708. doi: 10.1158/1078-0432.CCR-15-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nature medicine. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akamatsu S, Wyatt AW, Lin D, Lysakowski S, Zhang F, Kim S, et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell reports. 2015;12:922–936. doi: 10.1016/j.celrep.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Orozco RM, Almaraz-Pro C, Rodriguez-Ubreva FJ, Cortes MA, Ropero S, Colomer R, et al. EGF prevents the neuroendocrine differentiation of LNCaP cells induced by serum deprivation: the modulator role of PI3K/Akt. Neoplasia. 2007;9:614–624. doi: 10.1593/neo.07337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wightman SC, Uppal A, Pitroda SP, Ganai S, Burnette B, Stack M, et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br J Cancer. 2015;113:327–335. doi: 10.1038/bjc.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 38.Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000;275:6868–6875. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- 39.Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer discovery. 2013;3:742–750. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Donmez N, Sahinalp C, Xie N, Wang Y, Xue H, et al. SRRM4 Drives Neuroendocrine Transdifferentiation of Prostate Adenocarcinoma Under Androgen Receptor Pathway Inhibition. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927–936. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 43.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Goodison S. Optimizing molecular signatures for predicting prostate cancer recurrence. The Prostate. 2009;69:1119–1127. doi: 10.1002/pros.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai C, Wang H, He HH, Chen S, He L, Ma F, et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. The Journal of clinical investigation. 2013;123:1109–1122. doi: 10.1172/JCI66666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao N, Zhang J, Rao MA, Case TC, Mirosevich J, Wang Y, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17:1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- 47.Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahu B, Laakso M, Ovaska K, Mirtti T, Lundin J, Rannikko A, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011;30:3962–3976. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 51.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature medicine. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Frontiers in oncology. 2014;4:60. doi: 10.3389/fonc.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosquera JM, Beltran H, Park K, MacDonald TY, Robinson BD, Tagawa ST, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15:1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Y, Liu L, Xie N, Xue H, Fazli L, Buttyan R, et al. Expression and function of the progesterone receptor in human prostate stroma provide novel insights to cell proliferation control. J Clin Endocrinol Metab. 2013;98:2887–2896. doi: 10.1210/jc.2012-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.