Abstract
Interferon Regulatory Factor 6 (IRF6) is a critical regulator of differentiation, proliferation and migration of keratinocytes. Mutations in IRF6 cause two autosomal dominant disorders characterized by cleft lip with or without cleft palate. In addition, DNA variation in IRF6 confers significant risk for non-syndromic cleft lip and palate. IRF6 is also implicated in adult onset development and disease processes, including mammary gland development and squamous cell carcinoma. Mice homozygous for a null allele of Irf6 die shortly after birth due to severe skin, limb, and craniofacial defects, thus impeding the study of gene function after birth. To circumvent this, a conditional allele of Irf6 was generated. To validate the functionality of the conditional allele, we used three “deleter” Cre strains: Gdf9-Cre, CAG-Cre, and Ella-Cre. When Cre expression was driven by the Gdf9-Cre or CAG-Cre transgenes, 100% recombination was observed as indicated by DNA genotyping and phenotyping. In contrast, use of the Ella-Cre transgenic line resulted in incomplete recombination, despite expression at the one-cell stage. In sum, we generated a novel tool to delete Irf6 in a tissue specific fashion, allowing for study of gene function past perinatal stages. However, recombination efficiency of this allele was dictated by the Cre-driver used.
Keywords: IRF6, conditional allele, cleft lip and palate, Van der Woude Syndrome, epithelium, Cre/lox
Introduction
Interferon Regulatory Factor 6 (IRF6) is a member of the Interferon Regulatory Factor family of transcription factors. This nine-member family contains a highly conserved DNA binding domain and a less conserved protein association domain. With the exception of IRF6, other members of the IRF family have been implicated in immune response (Tamura et al., 2008). Alternatively, mutations in IRF6 cause two autosomal dominant orofacial clefting disorders, Van der Woude syndrome (VWS) and Popliteal Pterygium syndrome (PPS) (Kondo et al., 2002). In addition, a DNA variant in IRF6, present in 30% of the population worldwide, confers significant risk for non-syndromic cleft lip and palate, one of the most common congenital defects (Rahimov et al., 2008; Zucchero et al., 2004).
Irf6 regulates the development of multiples tissues during embryogenesis. Specifically, Irf6 is expressed in embryonic skin and other ectodermally-derived tissues (Ingraham et al., 2006; Kondo et al., 2002; Richardson et al., 2006). More recent reports also indicated Irf6 expression and function in mesoderm derived tissues, including the tongue (Goudy et al., 2013). Knockout murine models using conventional gene targeting strategies were generated to study Irf6 function during embryogenesis. Mice homozygous for a null allele of Irf6 (Irf6gt/gt) displayed severe skin, limb, and craniofacial abnormalities (Ingraham et al., 2006; Richardson et al., 2006). Skin from Irf6gt/gt embryos at embryonic day 17.5 (E17.5) had increased proliferation of the epidermis. In addition, these embryos failed to develop a stratified epidermis, lacking both the granular and cornified layers. Thus, Irf6 is an essential regulator of proliferation and differentiation in keratinocytes (Ingraham et al., 2006; Richardson et al., 2006).
Other studies indicate a role for Irf6 in adult development and disease. In the mouse, Irf6 is expressed throughout development of the mammary gland, reaching maximal expression during lactation (Bailey et al., 2009). Interestingly, IRF6 expression is reduced or absent in breast carcinomas (Bailey et al., 2005). Re-introduction of Irf6, along with its binding partner Maspin, into breast cancer cells resulted in cell cycle arrest (Bailey and Hendrix, 2008; Bailey et al., 2005). In addition, down-regulation of IRF6 was associated with squamous cell carcinoma (Botti et al., 2011; Restivo et al., 2011; Stransky et al., 2011). In squamous cell carcinoma biopsies, down-regulation of IRF6 was strongly associated with hypermethylation of CpG islands near the IRF6 promoter (Botti et al., 2011). Children with VWS have increased risk of wound complications following surgical repair of orofacial clefts, suggesting a role for IRF6 in wound healing (Jones et al., 2010). Studies of wound closure in Irf6gt/gt embryos indicate a role for Irf6 in the regulation of cell migration (Biggs et al., 2012). In sum, Irf6 has been implicated in both congenital and adult disease processes of significant clinical impact. However, studies of Irf6 function post embryogenesis are precluded by current mouse models because the loss of Irf6 in the mouse results in perinatal lethality. The aim of this work was to develop a conditional allele of Irf6 to allow investigation of gene function post-embryogenesis in a spatial and temporal fashion.
Results and Discussion
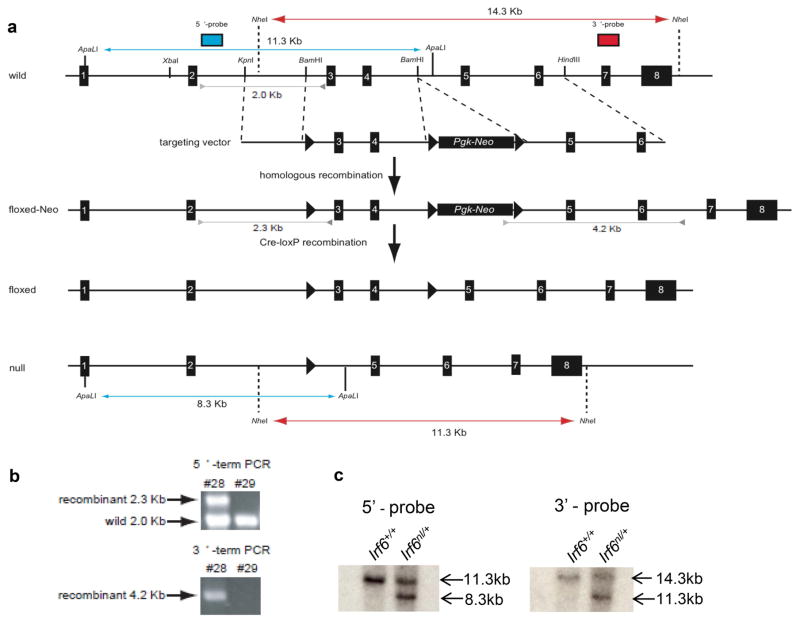
To generate a conditional allele of Irf6 (Irf6fl), we utilized a three LoxP site strategy (Fig 1a). Two LoxP sites flanked a Pgk-Neo selectable marker cassette in intron four, and a third LoxP site was located in intron two (Irf6neo). Recombination between LoxP sites flanking the selectable marker produced a floxed allele with exons three and four being flanked by LoxP sites. Recombination between the remaining LoxP sites would delete exons three and four (Irf6nl). This allele is predicted to be a null allele, since exon three encodes a critical region of the DNA Binding Domain, and excision of these exons results in frameshift and the introduction of a premature stop codon.
Figure 1. Construction of a conditional allele of Irf6.
a) A targeting vector was generated to contain three LoxP sites (triangles). The targeting vector contained a LoxP flanked PGK-neo cassette inserted 3′ of exon four. A third LoxP site was incorporated 5′ of exon three. b) Cells carrying the recombinant allele were detected via PCR. Regions amplified are indicated by green tracks. c) Southern blot analysis of ES cells. A 5′ probe (indicated by blue tracks) and 3′ probe (indicated by red tracks) were used. Irf6+ - wildtype allele, Irf6nl – null allele.
Embryonic stem cells containing the recombinant clones were acquired by G418 selection followed by PCR-based screening (Fig 1b). Excision of the floxed Pgk-Neo cassette in ES cells (SV129) was conducted by transfection with a Cre-expression vector (de Greef et al., 2016). PCR-based genotyping identified clones with the wild type, Irf6neo, Irf6fl and the Irf6nl alleles. Southern blot analysis confirmed correct targeting and subsequent recombination to generate the Irf6nl allele (Fig 1c). Chimeric mice were generated by injection of ES cells containing the Irf6fl allele into C57Bl/6J blastocysts. Mice homozygous for the Irf6 conditional allele (Irf6fl/fl) were viable and born at the expected Mendelian ratio (data not shown). Also, they showed no developmental or reproductive defects when compared to Irf6+/+ or Irf6fl/+ littermates, suggesting that this allele does not affect normal gene function.
To test the functionality of the Irf6fl allele, we employed the Gdf9-Cre transgenic mouse line (tgGdf9-Cre/+). The Gdf9 promoter is sufficient to drive expression of Cre recombinase in the oocyte beginning at postnatal day three (Lan et al., 2004). Thus, recombination at the Irf6 locus could only occur in females carrying the Cre transgene and the conditional Irf6 allele (tgGdf9-Cre/+; Irf6fl/+). In tgGdf9-Cre/+; Irf6fl/+ females, we expected a recombination event in the oocyte to produce Irf6+ or Irf6nl gametes. To test this, tgGdf9-Cre/+; Irf6fl/+ females were mated to Irf6gt/fl males. If recombination of the conditional allele was complete, we expect 25% of embryos to phenocopy embryos deficient for Irf6 (Ingraham et al., 2006). From these matings, six litters were collected at weaning (N = 43 pups). We observed a loss of two expected genotypes, tgGdf9-Cre/+; Irf6gt/nl and tg+/+; Irf6gt/nl (χ2-test; p=0.002; Table 1). These data were consistent with the perinatal lethality observed in Irf6gt/gt. Additionally, we note the presence of Irf6fl/nl and Irf6fl/+ animals and the absence of Irf6nl/nl or Irf6nl/+ animals, suggesting that the conditional allele from the sire did not recombine.
Table 1.
Gdf9-Cre mediated recombination of Irf6fl
 tgGdf9-Cre/+ ; Irf6fl/+ X tgGdf9-Cre/+ ; Irf6fl/+ X
 tg+/+; Irf6gt/fl tg+/+; Irf6gt/fl
| ||
|---|---|---|
| Genotype | Expected | Observed |
| tgGdf9-Cre/+; Irf6gt/nl | 5.125 | 0 |
| tgGdf9-Cre/+; Irf6fl/nl | 5.125 | 7 |
| tgGdf9-Cre/+; Irf6gt/+ | 5.125 | 3 |
| tgGdf9-Cre/+; Irf6fl/+ | 5.125 | 6 |
| tg+/+; Irf6gt/nl | 5.125 | 0 |
| tg+/+; Irf6fl/nl | 5.125 | 8 |
| tg+/+; Irf6gt/+ | 5.125 | 5 |
| tg+/+; Irf6fl/+ | 5.125 | 12 |
| CNG | 2 (not included) | |
| TOTAL | 41 | 41 |
Recombination of the Irf6fl allele in the female results in production of the Irf6nl allele. Data represent offspring from six litters after weaning. There is a significant underrepresentation of mice with Irf6gt/nl (Χ2 test: p = 0.0018). CNG – Could Not Genotype.
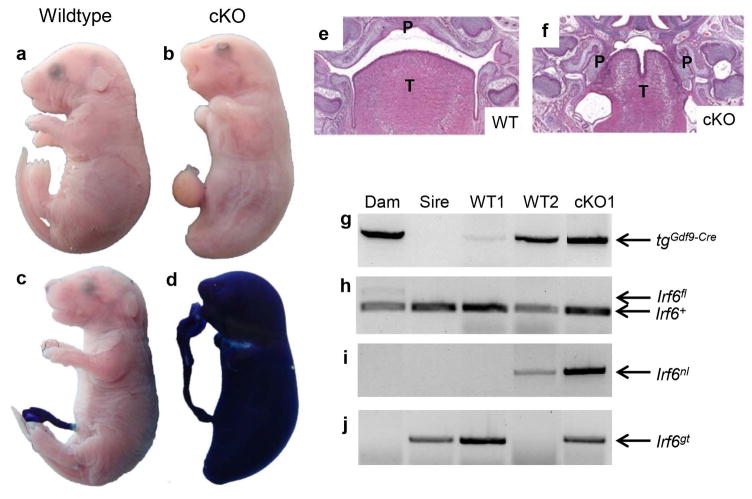
To confirm if the observed underrepresentation of genotypes was due to peri-natal lethality of mutant pups, we performed timed matings between tgGdf9-Cre/+;Irf6fl/+ females and Irf6gt/+ males. Pregnant females were sacrificed at E17.5 and three litters were collected (N = 34 embryos). We observed nine embryos that phenocopied Irf6gt/gt embryos (Fig 2a,b) and were referred to as conditional knockouts (cKO). In addition to severe skin, limb, and craniofacial abnormalities, cKO embryos had impaired barrier function (Fig 2c,d) and severe oral adhesions (Fig 2e,f). These embryos lacked the Irf6fl allele and were positive for the Irf6nl and Irf6gt alleles (Fig 2e). Notably, the mutant phenotype was independent of the presence of the Gdf9-Cre transgene, indicating that the Gdf9-Cre mediated recombination of the Irf6fl allele occurred prior to completion of meiosis I. Furthermore, these data demonstrate that the Irf6fl allele is capable of efficient recombination, resulting in a null allele.
Figure 2. Conditional knockout embryos (cKO) phenocopy Irf6gt/gt embryos.
a) Wildtype and b) cKO embryos generated from Gdf9-Cre mediated recombination of the Irf6fl allele. cKO embryos (d; genotype - Irf6gt/nl with or without the Gdf9-Cre transgene) display severe skin, limb, and craniofacial abnormalities and impaired barrier function, as indicated by a dye exclusion assay, when compared to wildtype littermates (c). Cross sections of wildtype (e) and cKO (f) heads indicate severe oral adhesions in cKO embryos (P – palate; T – tongue). PCR-based genotyping for the Gdf9-Cre transgene (g), Irf6+ and Irf6fl (h), Irf6nl (i), and Irf6gt (j). Complete recombination of the Irf6fl is observed irrespective of whether embryos were positive for the Gdf9-Cre transgene. cKO embryos were also positive for the Irf6gt allele. No embryos were positive for the Irf6fl allele.
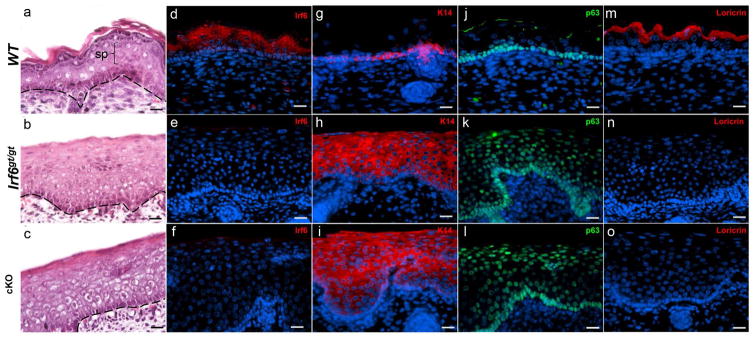
Histological analysis of embryonic skin from wildtype, Irf6gt/gt and cKO embryos showed an expanded epidermis (Fig 3a–c). To confirm the molecular profile, wildtype, Irf6gt/gt, and cKO embryos were immunostained at E17.5. In wildtype embryos, Irf6 expression was seen throughout the spinous and basal layers of the epidermis. As expected, this expression was lost in Irf6gt/gt and cKO embryos (Fig 3d–f). In wildtype skin, Keratin14 (K14) was restricted to the basal layer. K14 expression was expanded throughout the epidermis in Irf6gt/gt and cKO embryos (Fig 3g–i). A similar pattern of expression was observed for p63, another marker of the basal layer and a pro-proliferation gene (Fig 3k–l). Lastly, expression of loricrin, a marker of terminally differentiated keratinocytes, was completely lost in cKO embryos (Fig. 3m–o). These results are consistent with those observed in Irf6-deficient skin (Ingraham et al., 2006).
Figure 3. Molecular profile of embryonic skin.
H&E staining of a) Wildtype (WT), b) Irf6gt/gt and c) cKO skin. Black dashed line indicates the basal layer of the epidermis. The spinous layer (sp) is indicated in a. Immunofluorescent staining for Irf6 (d–f), K14 (g–i), p63 (j–l), and Loricrin (m–o). Images acquired using brightfield fluorescent microscope. Scale bars represent 100μm.
To further confirm the functionality of the Irf6fl allele, we utilized two additional Cre transgenic lines. First, we used CAG-Cre, where Cre expression is driven by the cytomegalovirus immediate early enhancer-chicken beta-actin promoter in mature oocytes. In this line, Cre mRNA or protein was stored in the oocyte and can facilitate recombination following fertilization (Sakai and Miyazaki, 1997). CAG-Cre transgene positive (tgCAG-Cre/+; Irf6+/+) animals were mated to Irf6fl/fl animals (N=30 offspring). Genotypic analysis indicated that ten animals were tgCAG-Cre/+. These animals showed complete recombination of the Irf6fl allele, as evidenced by the presence of only the Irf6nl allele (supplementary Table 1).
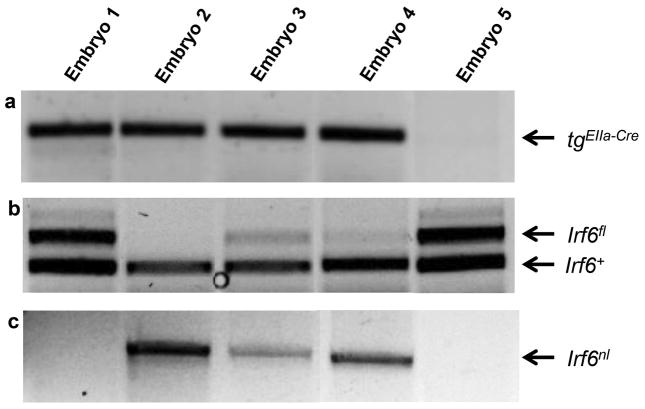
We also used the classic deleter strain, EIIa-Cre, which targets expression of the recombinase to the stages of embryogenesis preceding implantation. We mated tgElla-Cre/+; Irf6gt/+ animals with Irf6gt/fl animals (N=124 embryos). This allowed us to produce wildtype or mutant embryos carrying two genotypes: 1) Irf6gt/gt or 2) tgElla-Cre/+; Irf6gt/nl. Sixty-five embryos were tgElla-Cre/+. Of these embryos, nineteen were also positive for the Irf6fl allele but showed no evidence of recombination. We observed eight tgElla-Cre/+ embryos that showed incomplete recombination, as indicated by the presence of both the Irf6fl and Irf6nl alleles (Fig 4). These embryos were grossly normal. Nine tgElla-Cre/+ embryos showed complete recombination in tail tissues, as indicated by presence of the Irf6nl only (Table 2). Strikingly, only one embryo phenocopied Irf6gt/gt embryos (tgElla-Cre/+; Irf6gt/nl), even though three embryos had the correct genotype to generate the mutant phenotype in the event of recombination (tgElla-Cre/+; Irf6gt/fl). This finding was in accordance with published results where complete recombination using the EIIa-cre allele was only observed in 50% of animals (Lakso et al., 1996).
Figure 4. PCR-based genotyping of EIIa-Cre transgenic embryos.
(a), Irf6+ and Irf6fl (b) and Irf6nl (c) from E17.5 embryos. When using the EIIa-Cre transgene was used, we observed embryos with no recombination (Embryo 1), complete recombination (Embryo 2), or incomplete recombination (Embryos 3 and 4) of the Irf6fl allele. The Irf6nl was not detected in the absence of the EIIa-Cre transgene (Embryo 5).
Table 2.
EIIa-Cre mediated recombination of Irf6fl
| tg EIIa-Cre/+ ; Irf6gt/+ x tg+/+ ; Irf6gt/fl | |||
|---|---|---|---|
| Ella-Cre genotype | total | Irf6 genotype | total |
| tg+/+ | 46 | Irf6fl | 18 |
| Irf6fl and Irf6nl | 0 | ||
| Irf6nl | 0 | ||
| tgEIIa/+ | 66 | Irf6fl | 19 |
| Irf6fl and Irf6nl | 8 | ||
| Irf6nl | 9 | ||
Data represent offspring from 15 litters collected at E17.5. Mice lacking the Ella-Cre transgene showed no recombination of the Irf6fl allele. Mice carrying the Ella-Cre transgene showed mosaic recombination of Irf6fl. Some offspring showed no recombination (Irf6fl only), presence of both the Irf6fl and Irf6nl alleles, or complete recombination (Irf6nl only).
We created a conditional allele for Irf6. While the allele is capable of recombination, we observed variable efficiency that appeared to be dependent on the cell type. While this could simply reflect variable expression or activity of individual Cre transgenes or recombinase, respectively (McLellan et al., 2017) (Bao et al., 2013), we hypothesize that a cis effect at the Irf6 locus contributes to the variable efficiency. This hypothesis is based on the following: 1) In the current study, we observed no or incomplete recombination with the EIIa-Cre “deleter strain, and with two other tissue specific Cre transgenes, including K14-Cre-ER (Vasioukhin et al., 1999) and Tgfb3-Cre (Yang et al., 2008) (data not shown). And 2), A previous study used this Irf6fl allele to examine the role of Irf6 in dental epithelium, using the Pitx2-Cre transgene (Chu et al., 2016). While the Pitx2-Cre driver was previously shown to be competent to knockout other genes in oral epithelium that were required for palatogenesis (Xiong et al., 2009) (He et al., 2011), and Irf6 function in oral epithelium is required for palatogenesis (Ingraham et al., 2006; Richardson et al., 2009), no pathology was observed during palatogenesis (Chu et al., 2016). Thus, the failure of multiple Cre drivers to recombine the Irf6fl allele is consistent with the hypothesis that a cis effect at the Irf6 locus is inhibiting Cre recombination.
While, we do not know the mechanism underlying the tissue dependence of Cre recombination at the Irf6 locus, one possible explanation is variation in chromatin structure at the Irf6 locus. In support of this hypothesis, a recent study showed that hypermethylation of the IRF6 promoter region was associated with down-regulation of expression in squamous cell carcinoma (Botti et al., 2011; Rotondo et al., 2016; Stransky et al., 2011). DNA methylation is associated with nucleosome compaction, rendering the DNA inaccessible to transcription factors required to facilitate gene expression. As a result, in tissue types where Irf6 is methylated, we hypothesize that the DNA was inaccessible to Cre recombinase. Long and Rossi (2009) showed that methylation of promoter elements upstream of reporter genes activated by Cre expression (Z/AP and Z/EG strains) inhibited Cre-mediated recombination (Long and Rossi, 2009). Further studies to determine the methylation state of Irf6 in different cell types must be done to confirm this hypothesis.
Finally, we note that Ella-Cre was not an effective transgene for assessing the functionality of newly derived conditional alleles. Instead, we propose the usage of oocyte specific Cre-drivers, such as Gdf9-Cre, or other more efficient one-cell stage drivers, such as CAG-Cre, to validate the functionality of conditional alleles. However, it is important to note that the mating strategy employed for these studies may have promoted the observed recombination inefficiency. It would be beneficial to address recombination with tgElla-Cre/+; Irf6fl/+ females.
Methods
Generation of a conditional allele for Irf6
Mouse BAC clone (RPCI22-516G1) was digested with restriction enzymes. A 1.8 kb KpnI/BamHI fragment for the 5′-arm and a 3.9 kb of BamHI/HindIII fragment for the 3′-arm were cloned into pBluescript II SK(-) (Agilent Technologies). Three kilobases (kb) of the BamHI fragment, containing exons three and four, was cloned into the ploxP3-Neo-pA vector (kind gift from Professor Takeshi Yagi, Osaka University). 5.8 kb of the XbaI fragment which contains floxed exons and Pgk-Neo cassette was subcloned into the BamHI site between 5′- and 3′-arms (Fig. 1a). The resulting targeting construct was digested with NotI and electroporated into mouse R1 ES cells. After G418 selection, ES cells were screened by PCR. Primer set of 5′-GAGAAATAGGGCCTTCACGGTG-3′ (sense) and 5′-TGTGCCCTCTGATGCTGGAACAG-3′ (antisense) for 5′-side, 5′-TCGCCTTCTTGACGAGTTCTTCTG-3′ (sense, in Pgk-Neo cassette) and 5′-GCTCAACTCCCTTTGTGACTGTCC-3′ (antisense) for 3′ side were used (Fig 1b). Recombinant ES clones were used for establishment of Irf6 hypomorphic mouse (Irf6neo). To establish floxed exons (fl) and null (nl) strains, the Pgk-Neo cassette and the floxed exons, respectively, were removed in the recombinant ES cells by transfection with a Cre expression vector (de Greef et al., 2016). Resultant clones were then screened by Southern hybridization. Genomic DNA was digested with ApaL1 or Nhe1 and hybridized to a 5′ or 3′ probe, respectively (Fig 1c). The 5′ probe corresponded to a region in intron two. The 3′ probe corresponded to a region within exon and intron seven. Hybridization with the 5′ probe following restriction enzyme digest resulted in two products, 11.3kb (wildtype allele) and 8.3kb (null). Hybridization with the 3′ probe also produced two fragments, 14.3kb (wildtype) and 11.3kb (null). The 5′ hybridization probe was amplified with primer set 5′-AGTTGTGACTGACTGTAGGATCAGG-3′ (forward) and 5′- ACCAAAACTTCACCAGGAGTATAGGA-3′ (reverse). The 3′ hybridization probe was amplified with primer set of 5′- AGAGTAAAGAATGGTTGTCAGTGGAG-3′ (forward) and 5′- GACACCAGTATTCAAGAGGATTGAG-3′ (reverse) (Fig 1c). To generate a conditional mouse line for Irf6, embryonic stem cells carrying the Irf6fl allele were injected into C57Bl/6J blastocyst and inserted into pseudo-pregnant dams. Chimeric males were mated to C57Bl/6J females and germline transmission was determined by Polymerase Chain Reaction (PCR)-based genotyping of progeny. The Irf6fl/fl mice reported here are available to the scientific community.
Mouse and embryo genotyping
To attain genomic DNA, tail tissue was digested in lysis buffer (10 mM Tris-HCl, 150 mM NaCl, 10 mM EDTA, 0.1% SDS) with Proteinase-K (20mg/ml, Roche) at 55°C, overnight, followed by ethanol precipitation of DNA. PCR-based genotyping was used to identify alleles (see below). All PCR was conducted using JumpStart REDTaq Ready Mix (Sigma-Aldrich, St. Louis, MO) and separated and visualized by electrophoresis on 1.5% agarose gels.
Detection of the Irf6neo, Irf6fl, and Irf6nl alleles
Primer set of 5′-GCAGAGTGGAGCACACTTCA-3′ and 5′-AAGCATGTCTATTTGGGGGTT-3′ was used to determine the absence of the 5′ LoxP site in the Irf6+ (221 bp) but its presence (570 bp) in the Irf6fl and Irf6neo alleles. Primer set of 5′-TGGCAAAATCTATTTCGAGTGG-3′ and 5′-CACACTGACCTCAATGTCCAA-3′ was used to determine the absence of the 3′ LoxP site in the Irf6+ (222 bp) but its presence (379 bp) in the Irf6fl alleles. This primer set also distinguished the Irf6fl and Irf6neo alleles, as these primers do not amplify a product from the Irf6neo allele under these conditions because the expected product is too large. Primer set of 5′-GCAGAGTGGAGCACACTTCA-3′ and 5′-CACACTGACCTCAATGTCCAA-3′ was used to detect the Irf6nl allele (499 bp). PCR was performed as follows: 1) 95°C for two minutes, 2) 95°C for 15 seconds, 3) 55°C for 15 seconds, 4) 72°C for 45 seconds, 5) repeat steps 2–4 35 times, 6) 72°C for five minutes.
Detection of the Irf6gt allele
Primer set of 5′-GACCAGACCGTGCAGGGGCTGTGG-3′ and 5′-GAGAGGCTAGGGTGGAAGGGATTC-3′ identifies the Irf6gt allele (283bp). PCR conditions were: 1) 95°C for three minutes, 2) 95°C for 38 seconds, 3) 60°C for 50 seconds, 4) 72°C for 15 seconds, 5) repeat steps 2–4 40 times, 6) 72°C for five minutes. PCR Master Mix was supplemented with 5M Betaine monohydrate to aid in amplification of GC-rich regions.
Detection of Cre transgenes
The Gdf9-Cre transgene was detected as described by Lan et al (2004) using primer set of 5′-TCTGATGAAGTCAGGAAGAACC-3′ and 5′-GAGATGTCCTTCACTCTGATTC-3′. PCR conditions were: 1) 95°C for five minutes, 2) 95°C for one minute, 3) 58°C for two minutes, 4) 72°C for one minute, 5) repeat steps 2–4 35 times, 6) 72°C for five minutes. Master mix was supplemented with 5 Molar (5M) Betaine monohydrate to aid in amplification of GC-rich regions.
The EIIa-Cre transgene was detected using primer set of 5′-GCGGTCTGGCAGTAAAAACTATC-3′ and 5′-GTGAAACAGCATTGCTGTCACTT-3′. PCR conditions were: 1) 94°C for three minutes, 2) 94°C for 30econds, 3) 51.7°C for one minute, 4) 72°C for one minute, 5) repeat steps 2–4 35 times, 6) 72°C for five minutes.
The CAG-Cre transgene was detected using primer set of 5′-CCTACAGCTCCTGGGCAACGTGC-3′ and 5′-CTAATCGCCATCTTCCAGCAGG-3′. PCR conditions were: 1) 94°C for three minutes, 2) 94°C for 30 seconds, 3) 60°C for 30 seconds, 4) 72°C for two minutes, 5) repeat steps 2–4 30 times, 6) 72°C for five minutes.
Mating Strategies
Gdf9-Cre
A male hemizygous for the Gdf9-iCre transgene (tgGdf9-Cre/+) was purchased from Jackson Labs (www.jax.org) and mated to Irf6fl/fl females to generate females carrying both the Cre transgene and one copy of the Irf6fl allele (tgGdf9-Cre/+; Irf6fl/+). These females were mated to compound heterozygous males for Irf6 (Irf6gt/fl). Tail snips were collected from pups upon weaning and genotyped. A chi-squared test was used to determine deviations from expected Mendelian ratios.
TgGdf9-Cre/+; Irf6fl/+ females were then placed into timed matings with males heterozygous for a null allele of Irf6 (Irf6gt/+). The presence of a copulation plug was denoted as embryonic day 0.5 (E0.5) and embryos were collected on E17.5.
EIIa-Cre
A homozygous EIIa-Cre male (tgElla-Cre/Ella-Cre) was purchased from Jackson Labs (www.jax.org) and mated to Irf6gt/+ females to produce double heterozygous tgElla-Cre/+;Irf6gt/+ mice. Timed matings were set up between tgElla-Cre/+;Irf6gt/+ animals and Irf6gt/fl animals.
CAG-Cre
Validation of the Irf6fl allele using CAG-Cre was conducted multiple ways. 1) Females homozygous (tgCAG-Cre/CAG-Cre) or heterozygous (tgCAG-Cre/+) for the CAG-Cre transgene were mated to an Irf6fl/fl males. 2) TgCAG-Cre/+ males were mated to Irf6fl/fl females. Litters were born and tail snips were collected upon weaning and subjected to PCR based genotyping. A chi-squared test was used to verify Mendelian ratios.
Embryo Collection and processing
On E17.5, pregnant females were sacrificed using isoflurane induced comatosis followed by cervical dislocation. Embryos were decapitated. Heads and bodies were fixed overnight in 10% neutral buffered formalin at 4°C. Following fixation, embryos were processed and embedded in paraffin by the Histopathology Laboratory at Michigan State University using standard protocols. All animals were used in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Michigan State University Institutional Animal Use and Care Committee.
Histological analysis
Whole heads from embryos at E17.5 were sectioned sagitally at 7μm. Sections were then stained with hematoxylin and eosin (H&E). Briefly, for depariffinization and rehydration, sections were passed through three changes of xylene followed by washes through reducing grades of ethanol. Slides were then incubated in Gill’s Hematoxylin No. III (Sigma-Aldrich, St. Louis, MO) for 1.5 minutes, washed briefly in tap water, and incubated in a 1% Eosin solution (Eosin Y-VWR, West Chester, PA) for 1.5 minutes. To dehydrate, slides were passed through increasing grades of ethanol. Lastly, slides were mounted using Permount mounting media (VWR, Radnor, PA). Stained sections were imaged using a Nikon 90i upright microscope.
Dye Exclusion Assay
The dye exclusion assay was carried out as described by Ingraham, et al (2006). Briefly, whole embryos were collected at E17.5 and fixed in 100% methanol for five minutes. Following fixation, embryos were rinsed briefly in 1X PBS and stained in 0.1% toluidine blue for one minute. Staining was followed by washing with 1X PBS.
Immunofluorescence
For immunofluorescent detection of markers of the epidermis, sections were deparaffinized and rehydrated in reducing concentrations of ethanol. Sections were subjected to antigen retrieval by boiling in 10mM Sodium citrate (pH6.0) for 30 minutes. Tissues sections were permeabilized using 0.5% Triton X-100 followed by blocking in blocking solution (10% normal goat serum, 0.1% Bovine Serum Albumin in 1X Phosphate Buffered Saline (PBS)) for one hour at room temperature. Sections were then incubated in anti-mouse F′ (ab) fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) for five minutes to reduce non-specific binding of anti-mouse secondary. Sections were incubated with 1:100 rabbit anti-Irf6 (Sigma-Aldrich; St. Louis, MO), 1:250 rabbit anti-Keratin 14 (Covance), 1:150 mouse anti-p63 (Santa Cruz), and 1:250 rabbit anti-loricrin (Covance) overnight at 4°C, followed by incubation with either goat anti-mouse AlexaFluor 488 or goat anti-rabbit AlexaFluor 555 (Life Technologies, Grand Island, NY). Nuclei were counterstained for 10 minutes in a 1:10,000 dilution of 4′ 6-diaminidino-2-phenylindole (DAPI; Life Technologies, Grand Island, NY). Slides were then mounted in ProLong GOLD Antifade reagent (Life Technologies, Grand Island, NY). Images were taken using a Nikon Eclipse 90i fluorescent microscope.
Supplementary Material
Acknowledgments
Funding for this work was provided by National Institute of Health grants DE13513 (B.C.S.) and F31DE022696 (Y.A.K.). The authors thank the Michigan State University Histopathology Core and animal care facility for their services. The authors also thank Jeannie I. Klavanian, Krysta Wierzbicki, Kendra Siegersma, and Raeuf Roushangar for their technical support.
References
- Bailey CM, Hendrix MJ. IRF6 in development and disease: a mediator of quiescence and differentiation. Cell Cycle. 2008;7:1925–1930. doi: 10.4161/cc.7.13.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CM, Khalkhali-Ellis Z, Kondo S, Margaryan NV, Seftor RE, Wheaton WW, Amir S, Pins MR, Schutte BC, Hendrix MJ. Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership. J Biol Chem. 2005;280:34210–34217. doi: 10.1074/jbc.M503523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CM, Margaryan NV, Abbott DE, Schutte BC, Yang B, Khalkhali-Ellis Z, Hendrix MJ. Temporal and spatial expression patterns for the tumor suppressor Maspin and its binding partner interferon regulatory factor 6 during breast development. Dev Growth Differ. 2009;51:473–481. doi: 10.1111/j.1440-169X.2009.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Ma HY, Schuster A, Lin YM, Yan W. Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1(lox/lox) and Stra8-iCre; Mov10l1(lox/Delta) mice. Genesis. 2013;51:481–490. doi: 10.1002/dvg.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs LC, Rhea L, Schutte BC, Dunnwald M. Interferon regulatory factor 6 is necessary, but not sufficient, for keratinocyte differentiation. J Invest Dermatol. 2012;132:50–58. doi: 10.1038/jid.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti E, Spallone G, Moretti F, Marinari B, Pinetti V, Galanti S, De Meo PD, De Nicola F, Ganci F, Castrignano T, Pesole G, Chimenti S, Guerrini L, Fanciulli M, Blandino G, Karin M, Costanzo A. Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proc Natl Acad Sci U S A. 2011;108:13710–13715. doi: 10.1073/pnas.1110931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu EY, Tamasas B, Fong H, Foster BL, LaCourse MR, Tran AB, Martin JF, Schutte BC, Somerman MJ, Cox TC. Full Spectrum of Postnatal Tooth Phenotypes in a Novel Irf6 Cleft Lip Model. J Dent Res. 2016;95:1265–1273. doi: 10.1177/0022034516656787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greef JC, Hamlyn R, Jensen BS, O’Campo Landa R, Levy JR, Kobuke K, Campbell KP. Collagen VI deficiency reduces muscle pathology, but does not improve muscle function, in the gamma-sarcoglycan-null mouse. Hum Mol Genet. 2016;25:1357–1369. doi: 10.1093/hmg/ddw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudy S, Angel P, Jacobs B, Hill C, Mainini V, Smith AL, Kousa YA, Caprioli R, Prince LS, Baldwin S, Schutte BC. Cell-autonomous and non-cell-autonomous roles for IRF6 during development of the tongue. PLoS One. 2013;8:e56270. doi: 10.1371/journal.pone.0056270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Li L, Liu C, Yamagami T, Taketo MM, Zhou C, Chen Y. Epithelial Wnt/beta-catenin signaling regulates palatal shelf fusion through regulation of Tgfbeta3 expression. Dev Biol. 2011;350:511–519. doi: 10.1016/j.ydbio.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, Murray JC, Schutte BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Canady JW, Brookes JT, Wehby GL, L’Heureux J, Schutte BC, Murray JC, Dunnwald M. Wound complications after cleft repair in children with Van der Woude syndrome. J Craniofac Surg. 2010;21:1350–1353. doi: 10.1097/SCS.0b013e3181ec6aad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, Watanabe Y, Howard E, de Lima RL, Daack-Hirsch S, Sander A, McDonald-McGinn DM, Zackai EH, Lammer EJ, Aylsworth AS, Ardinger HH, Lidral AC, Pober BR, Moreno L, Arcos-Burgos M, Valencia C, Houdayer C, Bahuau M, Moretti-Ferreira D, Richieri-Costa A, Dixon MJ, Murray JC. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- Long MA, Rossi FM. Silencing inhibits Cre-mediated recombination of the Z/AP and Z/EG reporters in adult cells. PLoS One. 2009;4:e5435. doi: 10.1371/journal.pone.0005435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan MA, Rosenthal NA, Pinto AR. Cre-loxP-Mediated Recombination: General Principles and Experimental Considerations. Curr Protoc Mouse Biol. 2017;7:1–12. doi: 10.1002/cpmo.22. [DOI] [PubMed] [Google Scholar]
- Rahimov F, Marazita ML, Visel A, Cooper ME, Hitchler MJ, Rubini M, Domann FE, Govil M, Christensen K, Bille C, Melbye M, Jugessur A, Lie RT, Wilcox AJ, Fitzpatrick DR, Green ED, Mossey PA, Little J, Steegers-Theunissen RP, Pennacchio LA, Schutte BC, Murray JC. Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet. 2008;40:1341–1347. doi: 10.1038/ng.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo G, Nguyen BC, Dziunycz P, Ristorcelli E, Ryan RJ, Özuysal Ö, Di Piazza M, Radtke F, Dixon MJ, Hofbauer GF, Lefort K, Dotto GP. IRF6 is a mediator of Notch pro-differentiation and tumour suppressive function in keratinocytes. EMBO J. 2011;30:4571–4585. doi: 10.1038/emboj.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Jiang R, Dixon MJ. Integration of IRF6 and Jagged2 signalling is essential for controlling palatal adhesion and fusion competence. Hum Mol Genet. 2009;18:2632–2642. doi: 10.1093/hmg/ddp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–1334. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- Rotondo JC, Borghi A, Selvatici R, Magri E, Bianchini E, Montinari E, Corazza M, Virgili A, Tognon M, Martini F. Hypermethylation-Induced Inactivation of the IRF6 Gene as a Possible Early Event in Progression of Vulvar Squamous Cell Carcinoma Associated With Lichen Sclerosus. JAMA Dermatol. 2016;152:928–933. doi: 10.1001/jamadermatol.2016.1336. [DOI] [PubMed] [Google Scholar]
- Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortes ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, He F, Morikawa Y, Yu X, Zhang Z, Lan Y, Jiang R, Cserjesi P, Chen Y. Hand2 is required in the epithelium for palatogenesis in mice. Dev Biol. 2009;330:131–141. doi: 10.1016/j.ydbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LT, Li WY, Kaartinen V. Tissue-specific expression of Cre recombinase from the Tgfb3 locus. Genesis. 2008;46:112–118. doi: 10.1002/dvg.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, Natsume N, Yoshiura K, Vieira AR, Orioli IM, Castilla EE, Moreno L, Arcos-Burgos M, Lidral AC, Field LL, Liu YE, Ray A, Goldstein TH, Schultz RE, Shi M, Johnson MK, Kondo S, Schutte BC, Marazita ML, Murray JC. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004;351:769–780. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.