Abstract
Bone morphogenetic protein 2 (BMP2, HGNC:1069, GeneID: 650) is a classical morphogen; a molecule that acts at a distance and whose concentration influences cell proliferation, differentiation, and apoptosis. Key events requiring precise Bmp2 regulation include heart specification and morphogenesis and neural development. In mesenchymal cells, the concentration of BMP2 influences myogenesis, adipogenesis, chondrogenesis, and osteogenesis. Because the amount, timing, and location of BMP2 synthesis influence pattern formation and organogenesis, the mechanisms that regulate Bmp2 are crucial. A sequence within the 3′UTR of the Bmp2 mRNA termed the “ultra-conserved sequence” (UCS) has been largely unchanged since fishes and mammals diverged. Cre-lox mediated deletion of the UCS in a reporter transgene revealed that the UCS may repress Bmp2 in proepicardium, epicardium and epicardium-derived cells (EPDC) and in tissues with known epicardial contributions (coronary vessels and valves). The UCS also repressed the transgene in the aorta, outlet septum, posterior cardiac plexus, cardiac and extra-cardiac nerves and neural ganglia. We used homologous recombination and conditional deletion to generate three new alleles in which the Bmp2 3′UTR was altered as follows: a UCS flanked by loxP sites with or without a neomycin resistance targeting vector, or a deleted UCS. Deletion of the UCS was associated with elevated Bmp2 mRNA and BMP signaling levels, reduced fitness, and embryonic malformations.
Keywords: neural, birth defects, genetics, transcription, mutagenesis, mammal
INTRODUCTION
BMP2 is a critical signal whose concentration influences essential morphogenetic mechanisms in multiple structures. As expected for a morphogen, precise regulation of BMP2 levels and downstream signaling is crucial. BMP2-deficiencies lead to embryo lethality as early as E7.5 to E9 with defects in both extraembryonic and embryonic tissues (Ma et al., 2005; Singh et al., 2008; Zhang and Bradley, 1996). The absence of BMP2 disrupts key morphological processes such as enclosure of the fetus in extraembryonic tissues and the positioning of ventral structures such as the gut endoderm and heart (Gavrilov and Lacy, 2013), heart specification and cardiogenesis (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Zhang and Bradley, 1996), primordial germ cell generation (Ying and Zhao, 2001), and craniofacial development (Graf et al., 2016). BMP signal gradients control the dorsal-ventral patterning and morphogenesis of the neural tube with BMP2 playing irreplaceable roles in neural plate morphogenesis (Ybot-Gonzalez et al., 2007) and cranial neural tube closure and neural crest cell migration (Castranio and Mishina, 2009; Correia et al., 2007; Kanzler et al., 2000). In the adult, BMP2 is required to maintain bone function and quality post-weaning (Tsuji et al., 2006; Yang et al., 2013) and for the uterine decidual response during embryo implantation (Lee et al., 2007).
Sub-normal levels of BMP2 also negatively impact embryogenesis. Later studies of mice heterozygous for global ablation of Bmp2 (Zhang and Bradley, 1996) identified cephalic neural tube defects in heterozygotes (Uchimura et al., 2009). Detailed analyses of compound mutant mice heterozygous for a Bmp2 null allele with a Bmp4 null or hypomorphic allele identified embryonic and post-natal lethality associated with multiple organ defects (Goldman et al., 2009; Uchimura et al., 2009). In the limb, a threshold level of BMP signaling resulting from the combined activities of BMP2, 4, and 7 is required for normal osteogenesis (Bandyopadhyay et al., 2006). Consistent with the absolute requirement for BMP2 in decidualization, a Bmp2 hypomorphic allele created by appending a Neo cassette to the Bmp2 3′UTR caused embryonic defects (Singh et al., 2008). Dams with the hypomorphic allele exacerbated the embryonic phenotype. These studies showed that other BMP ligands such as BMP4 and BMP7 that are synthesized in overlapping or adjacent patterns fail to fully compensate for insufficient levels of maternal and embryonic BMP2.
Although multiple strategies have demonstrated the detrimental impact of elevated BMP signaling (Estrada et al., 2011; Galvin et al., 2000; Komatsu et al., 2013; Singh et al., 2008; Tylzanowski et al., 2006), few studies have directly assessed the impact of elevated BMP2 levels. The apparent failure of embryos synthesizing elevated BMP2 in hair follicles provides circumstantial evidence that elevated BMP2 can be embryo-lethal (Blessing et al., 1993). In contrast, mice that over expressed Bmp2 in vascular smooth muscle cells survived with no reported developmental abnormalities. These mice had enhanced atherosclerotic intimal calcification in the ApoE null/high cholesterol model (Nakagawa et al., 2010). Our analyses of new regulatory alleles that stimulate BMP2 synthesis begin to fill this gap in our understanding of how excess BMP2 impacts development.
Post-transcriptional gene regulation mechanisms involving the 3′untranslated region (UTR) often control the rapid and precise synthesis of potent signaling proteins like BMP2. Bmp2 gene expression is greatly influenced by post-transcriptional mechanisms (reviewed in (Rogers et al., 2015)). The entire Bmp2 3′UTR is extensively conserved between chicken and mammals (Fritz et al., 2004; Hu et al., 2006; Tian et al., 2005). In addition, a unique ultra-conserved sequence (UCS) of several hundred nucleotides in the 3′UTR is 73% identical between mammals and fishes with some motifs present in the AmphiBMP2/4 gene in Amphioxus, a chordate cousin whose family branched 650 million years ago (Abrams et al., 2004; Fritz et al., 2006; Fritz et al., 2004). This remarkable evolutionary conservation strongly supports ancient regulatory mechanisms necessary for controlling BMP2 synthesis during critical periods such as embryogenesis.
The UCS from both mice and humans can repress diverse gene constructs in developing and adult cells in vitro and in vivo (Devaney et al., 2009; Fotinos et al., 2016; Fotinos et al., 2014; Jiang et al., 2010; Kruithof et al., 2011a; Kruithof et al., 2011b). It is important to emphasize that the UCS does not repress in ALL cell types. Indeed, the UCS activates transcription in retinoic acid treated F9 embryonal cells and transformed lung cells (Abrams et al., 2004; Fritz et al., 2004; Jiang et al., 2010). Furthermore, post-transcriptional control only works after a gene is transcribed. In embryos and many non-transformed tissues, the Bmp2 gene appears to be “active”; i.e., transcribed in specific tissues, but a post-transcriptional block limits BMP2 synthesis in a subset of those tissues. In these cells, reduced levels of post-transcriptional repression may lead to ectopic, precocious, and/or elevated levels of this key instructive developmental signal.
The purpose of this study was to use gene targeting in mice to assess the effects of deleting the UCS in the 3′ UTR of the BMP2 mRNA on viability, morphology, and development. Three new Bmp2 alleles were generated: a conditional allele with the UCS flanked by loxP sites (Bmp2flUCS), a conditional allele with the UCS and a Pgk-neo targeting vector flanked by loxP sites (Bmp2UCSNeo), and an allele with the UCS deleted (Bmp2ΔUCS). Our findings confirm that the UCS limits Bmp2 mRNA abundance and BMP signaling in vivo and that disturbing Bmp2 3′UTR-mediated events negatively impact embryonic development.
RESULTS
Modified Bmp2 3′UTRs reduce survival
Given the unchanged nature of the Bmp2UCS over evolutionary time, we hypothesized that its deletion would decrease viability in mammals. To test this hypothesis, we used a new floxed UCS Bmp2 allele in which the repressive UCS within the 3′UTR is flanked with loxP sites at +22 and +714 relative to the stop codon (Bmp2flUCS, MMRRC# 042043, Fig. 1). Mice with one or two copies of the floxed allele (Bmp2flUCS/+ or Bmp2flUCS/flUCS) were viable and fertile. Mice bearing the floxed UCS allele were bred to mice bearing a constitutively expressed Cre gene under the transcriptional control of a human cytomegalovirus promoter (Jackson Labs #B6.C-Tg(CMV-cre)1Cgn/J). The CMV-Cre gene is constitutively expressed from early embryogenesis on and reliably mediates the global recombination of a floxed gene in all tissues. The resulting pups had one copy of the recombined Bmp2 allele (Bmp2ΔUCS/+) without the UCS in all tissues.
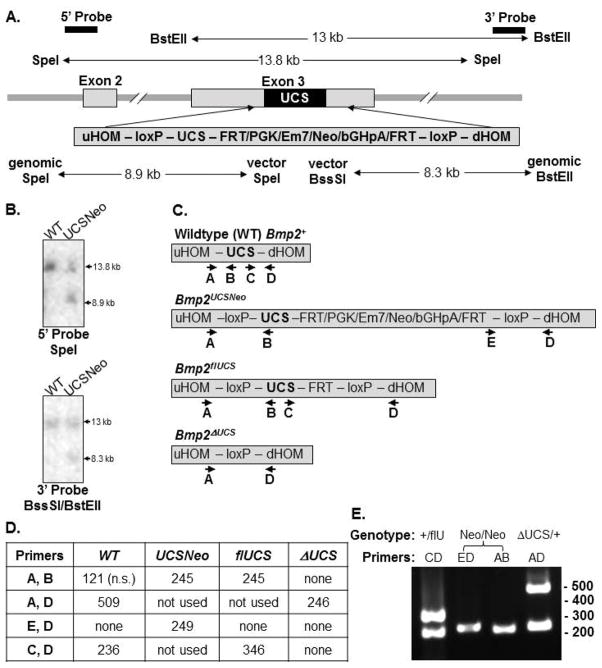
Fig. 1. Targeting construct, alleles, and genotyping.
A. A PvuII to an AccI fragment bearing the natural Bmp2 UCS in exon 3 (+100 to +446 relative to the stop codon) was replaced by a 124 bp loxP site followed by the Bmp2 UCS and 1.9 kb Pgk-neo cassette flanked by FRT sites and a downstream loxP site. The positions of 5′ and 3′ probes and restriction enzyme sites used for Southern analyses are indicated with the expected wildtype fragment sizes shown above and the targeted fragment sizes shown below the construct diagram. B. Genomic DNA from WT embryonic stem cells (lane 1) and correctly targeted cells (lane 2) were digested with the indicated enzymes and probes. C. A diagram of each allele and the positions of primers used for PCR genotyping. D. The expected size in bp of the amplicons generated by each primer pair by each allele. n.s. = not shown in E. E. Sample genotyping results.
To test the impact of the complete absence of the UCS, we bred mice with 1 copy each of the recombined allele (Bmp2ΔUCS/+) and CMV-Cre to mice with 2 floxed alleles (Bmp2flUCS/flUCS). Among the pups derived from this mating, 50% were expected to inherit the CMV-Cre gene. The floxed UCS allele in these pups would sustain a deletion of the UCS. The expected proportion of each genotype were 25% each Bmp2flUCS/+ and Bmp2ΔUCS/flUCS without CMV-Cre and 25% each of Bmp2ΔUCS/+ or Bmp2ΔUCS/ΔUCS with CMV-Cre. We tabulated the number of pups with each genotype at weaning. Table 1 shows that mice with all potential genotypes: one copy of the floxed allele (Bmp2fl/+), 1 copy of the recombined allele mice (Bmp2ΔUCS/+), compound heterozygotes bearing 1 copy each of the floxed and the recombined alleles (Bmp2ΔUCS/fl), or homozygotes for the recombined Bmp2 gene (Bmp2ΔUCS/ΔUCS) were viable. However, Bmp2ΔUCS homozygotes were significantly underrepresented (Chi squared equals 25.5 with 3 degrees of freedom; 2 tailed P value < 0.0001). A few pups were found dead between birth and weaning, but the frequency did not differ from that typically observed in our colony, nor was any particular genotype over-represented.
Table 1. Reduced fitness in mice lacking the Bmp2 UCS.
Mice with 1 copy each of the recombined allele (Bmp2ΔUCS/+) and CMV-Cre were bred to mice with 2 floxed alleles (Bmp2flUCS/flUCS).
| Expected genotypes: | 25% Bmp2+/Fl | 25% CMV-Cre; Bmp2+/ΔUCS | 25%Bmp2ΔUCS/Fl | 25% CMV-Cre; Bmp2ΔUCS /ΔUCS | |
|---|---|---|---|---|---|
| Paternal genotype | Maternal genotype | Number of pups (%): | |||
| CMV-Cre; Bmp2ΔUCS/+ | Bmp2flUCS/flUCS | 35 (43%) | 19 (23%) | 20 (25%) | 7 (9%) |
| Bmp2flUCS/flUCS | CMV-Cre; Bmp2ΔUCS/+ | 29 (29%) | 34% (34%) | 26 (26%) | 11 (11%) |
| All | 64 (35%) | 53 (29%) | 46 (25%) | 18 (10%) | |
BMP signaling and BMP2 in particular are required for normal uterine function during pregnancy (Clementi et al., 2013; Lee et al., 2007; Nagashima et al., 2013). Therefore, we assessed the impact of parental homozygosity for the floxed alleles (Bmp2flUCS/flUCS) or parental heterozygosity for the recombined allele (Bmp2ΔUCS/+) on viability. The genotype of the parents did not alter the underrepresentation of pups homozygous for the UCS deletion (Table 1). The genotypes of pups derived from mothers or fathers with either allele combination were both significantly skewed from Mendelian expectations (maternal Bmp2flUCS/flUCS: Chi squared equals 19.5 with 3 degrees of freedom; 2 tailed P value < 0.0001; paternal Bmp2flUCS/flUCS; Chi squared equals 11.8 with 3 degrees of freedom; 2 tailed P value < 0.0002). These results do not support a large maternal influence of either having two floxed alleles or one allele lacking the UCS.
The floxed Bmp2 allele was generated via an allele bearing a 1.9 kb Pgk-neo cassette (see Methods). Mice with one or two copies of the Neo-containing allele inserted into the Bmp2 3′UTR (Bmp2UCSNeo/+ or Bmp2UCSNeo/Neo, MMRRC #42279, Fig. 1C) also were viable and fertile. However, a previous study showed that compound heterozygotes bearing a deletion of the Bmp2 coding region and an allele that generates the same Neo cassette appended to the end of the Bmp2 transcript impacted embryonic survival (Singh et al., 2008). Therefore, we quantified the impact of the Pgk-neo cassette on mice lacking the UCS. We bred mice with 1 copy each of the recombined allele (Bmp2ΔUCS/+) and CMV-Cre to mice with 2 Neo-bearing alleles (Bmp2UCSNeo/UCSNeo). Table 2 shows that mice with one copy of the Bmp2UCSNeo allele or the recombined Bmp2ΔUCS allele formed a majority of the surviving pups. As observed in the progeny of parents bearing the floxed allele, pups with two recombined alleles (Bmp2ΔUCS/ΔUCS) were significantly underrepresented. Furthermore, compound heterozygotes bearing 1 copy each of the Bmp2UCSNeo allele and the recombined Bmp2ΔUCS allele were significantly underrepresented. Again, the resulting genotypes were significantly skewed from Mendelian expectations (Chi squared equals 102.5 with 3 degrees of freedom; 2 tailed P value < 0.0001). As with the floxed alleles, we failed to observe an obvious impact of parental genotype with the distribution of pup genotypes being equally skewed from expectations (maternal Bmp2UCSNeo/UCSNeo; Chi squared equals 31.4 with 3 degrees of freedom; 2 tailed P value < 0.0001; paternal Bmp2UCSNeo/UCSNeo; Chi squared equals 30.5 with 3 degrees of freedom; 2 tailed P value < 0.0001). These observations suggest that both the insertion of the 1.9 kb of targeting cassette in the middle of the 3′UTR and the deletion of the UCS severely impact tissues sensitive to changes in Bmp2 regulation.
Table 2. Reduced fitness in compound heterozygotes with a Neo insertion and a deleted Bmp2 UCS.
Mice with 1 copy each of the recombined allele (Bmp2ΔUCS/+) and CMV-Cre were bred to mice with 2 Neo alleles (Bmp2UCSNeo/UCSNeo).
| Expected genotypes: | 25% Bmp2+/UCSNeo | 25% CMV-Cre; Bmp2+/ΔUCS | 25%Bmp2ΔUCS/UCSNeo | 25% CMV-Cre; Bmp2ΔUCS /ΔUCS | |
|---|---|---|---|---|---|
| Paternal genotype | Maternal genotype | Number of pups (%): | |||
| CMV-Cre; Bmp2ΔUCS/+ | Bmp2Neo/Neo | 43 (27%) | 68 (42%) | 31 (19%) | 20 (12%) |
| Bmp2Neo/Neo | CMV-Cre; Bmp2ΔUCS/+ | 44 (23%) | 79 (42%) | 30 (16%) | 19 (19%) |
| All | 87 (25%) | 147 (42%) | 61 (17%) | 56 (16%) | |
Previously, we had never observed developmental anomalies in many litters of embryos bearing the ubiquitously expressed CMV-Cre gene (Kruithof et al., 2011a; Kruithof et al., 2011b). However, tissue-selective expression of Cre-recombinase has been reported to cause developmental anomalies (Schmidt-Supprian and Rajewsky, 2007; Wang et al., 2015). Therefore, we assessed the impact of the UCS deletion in mice lacking Cre-recombinase. Parents with 1 copy each of the recombined allele (Bmp2ΔUCS/+) alone were bred. The expected proportions were 25% wild type, 50% heterozygous, and 25% homozygous UCS null pups. Table 3 shows that mice homozygous for the recombined Bmp2 gene (Bmp2ΔUCS/ΔUCS) were significantly underrepresented (Chi squared equals 6.663 with 2 degrees of freedom, two-tailed P value < 0.036). The deletion appeared to more severely impact female pups.
Table 3. Cre recombinase does not account for reduced fitness in mice lacking the Bmp2 UCS.
Parents with 1 copy each of the recombined allele (Bmp2ΔUCS/+) were bred.
| Expected genotypes: | 25%Bmp2+/+ | 50% Bmp2+/ΔUCS | 25% Bmp2ΔUCS/ΔUCS |
|---|---|---|---|
| Pup Sex | Number of pups (%): | ||
| Male | 44 (26%) | 89 (52%) | 37 (22%) |
| Female | 41 (23%) | 107 (60%) | 31 (17%) |
| All | 89 (25%) | 202 (56%) | 71 (20%) |
UCS deletion increased Bmp2 mRNA abundance and BMP signaling
The UCS repressed diverse genes bearing different promoters and 3′UTRs in many cultured cell types and mouse tissues (Devaney et al., 2009; Fotinos et al., 2016; Fotinos et al., 2014; Jiang et al., 2010; Kruithof et al., 2011a; Kruithof et al., 2011b). Therefore, we hypothesized that Cre-mediated deletion of the UCS will induce elevated or ectopic BMP2 synthesis. We used reverse transcription and real time PCR to determine the effect of UCS deletion on Bmp2 mRNA expression in embryos obtained from dams bred as described in Tables 1 – 3. Because we were unable to authenticate antibodies directed at BMP2 specifically, we assessed BMP signaling using western blotting and authenticated antibodies directed at phosphorylated, BMP-activated SMAD proteins. Bmp2 mRNA levels and the phosphorylation of SMAD1/5/9(8) (pSMAD1/5/9, HGNC:6774) both were significantly elevated in E10.5 embryos lacking the UCS relative to embryos with 1 wild type and one floxed allele (Fig. 2A, B). Furthermore, embryos heterozygous for the UCS deletion (Bmp2ΔUCS/+ or Bmp2ΔUCS/FL) displayed intermediate levels of Bmp2 mRNA abundance and BMP signaling.
Fig. 2. Bmp2 mRNA expression and BMP signaling in embryos lacking the Bmp2 UCS.

A. Bmp2 mRNA expression in E10.5 embryos normalized to actin determined using reverse transcription and real time PCR (Average ± SEM, n = 6–13). B. BMP signaling as assessed by normalizing phosphorylated SMAD1/5/9(8) (pSMAD) levels to actin levels in E10.5 embryos (Average ± SEM, n = 8–17). C. Western blot panels showing pSMAD1/5/9(8), total SMAD1/5/9(8) (tSMAD), and actin levels in representative pairs of E10.5 embryos.
Among many litters of embryos, we observed congenitally deformed embryos and embryos undergoing resorption that likely account for the reduced proportion of specific genotypes at birth. We tabulated the frequency and nature of defects at E10.5. Each of the 3 breeding strategies generated embryonic phenotypes that were highly variable as described below, with no parental combination obviously promoting any particular defect. Thirty out 65 photographed and inspected embryos that were heterozygous for the UCS deletion had observable defects (46%). Twenty-six out of 43 homozygous embryos had observable defects (60%). In addition, at E10.5, we observed uterine swellings that contained apparently reabsorbing embryos that could not be cleanly removed and genotyped. Defects ranged from severely deformed, runted, fragile embryos to distinct neural tube defects (NTDs) and ventral body wall defects to subtler defects such as edema and superficial hemorrhage. Samples of each are shown in Fig. 3. NTDs were most commonly observed, although embryos with NTDs often had other defects such as delays ascertained by limb bud development. Fig. 3 shows representative embryos with open and closed neural tube and ventral body wall defects (E9.5, E10.5, 11.5) and holoproencephaly (inadequate midline division of forebrain), anophthalmia (absence of one or both eyes), and micrognathia (undersized jaw; E13.5). The presence of edema in some embryos is consistent with reduced cardiac function (Fig. 3B, lower left panel).
Fig. 3. Malformations and BMP2 levels in embryos lacking the Bmp2 UCS.
Representative embryonic floxed or recombined heterozygotes (Bmp2fl/+ or Bmp2ΔUCS/+), compound heterozygotes (Bmp2fl/ΔUCS), and recombined homozygotes (Bmp2ΔUCS/ΔUCS) lacking the UCS. A. E9.5, B. E10.5, C. E11.5, D. E13.5. Embryos within each panel are littermates. Both normal appearing (N) and defective (D) embryos with the same genotypes were observed. E. E10.5 embryos with elevated BMP signaling were more likely to be defective. The filled or open circles indicate the pSMAD1/5/9(8) levels of embryos that were visibly defective or visibly normal, respectively.
Homozygous deletion of the UCS was not uniformly embryo-lethal as apparently normal embryos were observed (Fig. 3B) and healthy pups were born (Tables 1–3). Defective embryos heterozygous for the deletion and one wild type or floxed allele also were observed (Fig. 3A, C). UCS-mediated post-transcriptional repression is just one of several mechanisms that repress Bmp2 gene expression in particular (Rogers et al., 2015) and BMP signaling in general (Katagiri and Watabe, 2016). Other regulators may partly compensate for deletion of the repressive UCS. In the mixed strain 129 and C57Bl/6background used here, genetic variation may influence the efficacy of alternative repression mechanisms. Indeed, different inbred strains were associated with variation in the frequency and severity of defects observed in the survival of Bmp2 null embryos and in Bmp2 chimeras of Bmp2 null ES and WT cells (Castranio and Mishina, 2009; Goldman et al., 2009; Uchimura et al., 2009). We observed that the level of SMAD1/5/9(8) phosphorylation in embryos with visible anomalies or growth retardation was nearly three times that of embryos with apparently normal morphologies (Fig. 3E, n = 37 defective vs. 23 normal E10.5 embryos, p< 2 × 10−6). Elevated BMP signaling was observed in all defective embryos including those with both the Bmp2UCSNeo and the Bmp2ΔUCS alleles.
DISCUSSION
The new alleles described here query the impact of UCS - mediated regulation in the context of normal transcriptional regulation. In contrast, we previously assessed UCS function using a lacZ reporter gene bearing 3.9 kb of Bmp2 non-coding sequence that included the distal promoter and the intact 3′UTR. The intact reporter was expressed in several tissues that synthesize BMP2: pericardial mesoderm, interdigital cells, eye, cartilage primordium, and trigeminal nerves (Kruithof et al., 2011b). Deletion of the UCS both intensified and expanded the domains of βgal activity into cells adjacent to regions that expressed the intact transgene. Additional ectopic expression was observed developing neural and cardiovascular tissues. Assuming the strictly post-transcriptional role for the UCS supported by in vitro findings (Fotinos et al., 2016; Fotinos et al., 2014; Jiang et al., 2010; Kruithof et al., 2011a), reporter gene transcription must have occurred in the cells with the expanded βgal activity.
A shortcoming of the previous studies was that the relatively small reporter gene did not completely recapitulate endogenous BMP2 transcription patterns in all tissues. For example, the reporter gene was not expressed in the roof plate of the telencephalon or the dorsal surface ectoderm, where BMP2 plays a key role in cephalic neural tube closure (Castranio and Mishina, 2009; Furuta et al., 1997). Similarly, reporter gene activity was undetectable in the craniofacial structures where BMP2 influences cranial neural crest-derived bone development (Bonilla-Claudio et al., 2012). These limitations were likely due to the absence of distant Bmp2 regulatory regions and those in the proximal promoter and introns (Rogers et al., 2015). In contrast, the new Bmp2 alleles bear 3′UTR modifications of the endogenous gene. All regulatory elements outside of the loxP-flanked UCS are intact and should drive transcription in all appropriate tissues.
Both in vivo and in vitro observations support a widely spread repressive role for the UCS (Fotinos et al., 2016; Fotinos et al., 2014; Jiang et al., 2010; Kruithof et al., 2011a; Kruithof et al., 2011b; Rogers et al., 2015). Consistent with these studies, Bmp2 mRNA abundance and BMP signaling was inversely proportional to the number of UCS copies in mid-gestation embryos (Fig. 2A, B). Numerous studies have shown that the 3′UTR of mRNAs regulates gene expression at a post transcriptional level either by regulating mRNA stability and thus RNA abundance or translation efficiency or both. The observation that UCS deletion elevates Bmp2 RNA abundance is consistent with a role in controlling Bmp2 mRNA stability. Ongoing studies are investigating the trans-regulatory factors that may control Bmp2 expression via the UCS.
The positive correlation between the Bmp2ΔUCS allele and deformed embryos is consistent with our hypothesis that the UCS restrains BMP2 synthesis. We initially hypothesized that the Bmp2UCSNeo allele might have produced lower BMP2 levels based upon the hypomorphic effect of an allele with the same Pgk-neo cassette appended to the transcript (Singh et al., 2008). However, our results are not consistent with reduced BMP2 levels in embryos with the Bmp2UCSNeo allele. Indeed, the range of phosphorylated SMAD1/5/9(8) levels in embryos with one copy of the Neo-containing allele (Bmp2UCSNeo/+) or compound heterozygotes with 1 copy each of the Neo and the recombined allele (Bmp2UCSNeo/ΔUCS) were comparable to embryos with other genotypes (Fig. 3E).
Two points are relevant to this observation. First, the structure of a transcript with a 1.9 kb Neo cassette inserted within the 3′UTR (our allele) may differ greatly from a transcript bearing the same cassette appended to the end as in Singh et al. Our understanding of how RNA structure per se impacts post-transcriptional processes is limited. Therefore, the impact of each alteration must be defined empirically. Second, Fig. 3E shows average pSMAD1/5/9(8) levels in embryos with the Bmp2ΔUCS/UCSNeo genotype were not significantly different from those with the Bmp2ΔUCS/ΔUCS genotype. Elevated pSMAD levels in the overtly defective embryos account for the upward spread of pSMAD level in both genotypes. Our working hypothesis is that both the Neo insertion and the deletion of the UCS disrupt the precision of post-transcriptional Bmp2 regulatory processes leading to a greater range of BMP2 levels. Redundant layers of gene expression and signaling control can compensate for some added variation, but only partly.
Our analysis of morphological defects found in the embryos showed neural tube and ventral body wall defects to be among the most common defects observed (Fig. 3). Such ventral and dorsal patterning defects also are prevalent in in mice with reduced BMP2 levels (Castranio and Mishina, 2009; Correia et al., 2007; Gavrilov and Lacy, 2013; Graf et al., 2016; Kanzler et al., 2000; Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Ybot-Gonzalez et al., 2007; Ying and Zhao, 2001; Zhang and Bradley, 1996). Thus, as expected for a true morphogen, too little or too much of this ligand disrupts morphogenesis.
Despite strong evolutionary pressure against sequence change in the UCS (Abrams et al., 2004; Fritz et al., 2004), some individuals survive the absence of the UCS and develop to adulthood without obvious anomalies. The simplest explanation is that excess BMP2 levels activate other repressors that dampen BMP signaling. Indeed, BMP signaling directly induces the extracellular antagonist NOGGIN, the intracellular repressor SMAD6, and the miR-17 family of miRNAs which repress BMPRII (Gazzerro and Minetti, 2007; Sun et al., 2013). Negative feedback from excess BMP signaling would buffer the developing embryo from elevated BMP2 levels. Our observation that BMP signaling levels were significantly elevated in embryos with overt anomalies relative to those that were visibly normal (Fig. 2C) is consistent with the hypothesis that the UCS is just one of many redundant restraints on the activity of this potent growth factor. Just as different inbred strains vary in susceptibility to reduced BMP2 levels (Castranio and Mishina, 2009; Goldman et al., 2009; Uchimura et al., 2009), genetic variation in other BMP signaling repressors may compensate for deletion of the UCS.
The incomplete penetrance of these regulatory alleles is consistent with a reduction in the developmental robustness or “canalization” of these embryos. The UCS in the 3′UTR is a natural target of miRNA-mediated regulation that we have begun to explore (Fotinos et al., 2016; Fotinos et al., 2014). Interestingly, miRNAs have been proposed as part of a buffering network that ensures normal development in the face of genetic and environmental variation (Posadas and Carthew, 2014). Critical growth factors whose transcripts have conserved 3′UTRs like Bmp2 are prime candidates for such a stabilizing mechanism.
The UCS was a repressive regulatory element in a majority of tested cell types including all mesenchymal cells and non-transformed lung cells (Fotinos et al., 2016; Fotinos et al., 2014; Jiang et al., 2010; Kruithof et al., 2011a; Kruithof et al., 2011b; Rogers et al., 2015). However, the UCS is an activator in retinoid-treated F9 embryonal cells, in transformed bronchial epithelial cells, and in human lung adenocarcinoma cells (Abrams et al., 2004; Fritz et al., 2004; Jiang et al., 2010). All of these cells strongly transcribe Bmp2–driven reporter constructs and synthesize high levels of BMP2. Thus it is formally possible that deletion of the UCS also reduces BMP2 synthesis in some embryonic tissues. We did not observe reduced reporter gene expression in embryos bearing the short transgene (Kruithof et al., 2011a; Kruithof et al., 2011b). However, deletion of the UCS caused exuberant beta-galactosidase synthesis that would mask small regions where reduced reporter gene expression may have occurred. Furthermore, the small lacZ reporter lacked distantly located enhancers that would activate transcription (Rogers et al., 2015).
The potential of the UCS to act as a switch that may activate or repress depending on cell type is highly relevant in the context of embryogenesis where Bmp2 expression is highly dynamic. A recent study supports the idea that midline positioning of the dorsal mesentery depends on the relative, rather than the actual, amount of BMP signaling on the left and right sides of the developing embryo (Arraf et al., 2016; Gavrilov and Lacy, 2016). By potentially up-regulating BMP2 synthesis in some tissues, but down-regulating in other tissues, the UCS deletion would disrupt the relative levels of BMP2 in developing tissues and profoundly influence tissue morphogenesis. An expanding body of evidence supports the critical need to maintain BMP2 levels within a narrow developmentally tolerated range. Regulatory mutations as in these new alleles are essential reagents for elucidating the role of gene expression in establishing an acceptable level of BMP2. Despite the experimental challenges, a full assessment of the mechanisms that control the quantity and pattern of BMP2 and other important development factors is required to fully understand animal development.
METHODS
Generation of mice with Bmp2 alleles with modified 3′UTRs
All animals were handled in accordance with the Guidelines for Care and Use of Experimental Animals and approved by the NJ Medical School Institutional Animal Care and Use Committee (IACUC protocol #15069). A Bmp2 fragment was isolated from strain 129 and used to construct a 15.9 kb conditional gene targeting vector (Fig. 1A). The following fragments were subcloned into a bluescript SK vector in order: 1. The upstream homology domain (uHOM) beginning at a SalI site 7313 bp upstream of the translational stop codon to 76 bp past the stop codon followed by a 124 bp loxP site which replaced a mouse specific PvuII site. 2. The Bmp2 UCS from +100 to +446 relative to the stop codon ending at an AccI site. 3. A 1.9 kb Pgk-neo cassette flanked by FRT sites and bearing a downstream loxP site replaced the AccI site. The neo gene conferred kanamycin resistance in E. coli and G418 resistance in mammalian cells. 4. The downstream homology domain beginning at the AccI site at +446 and extending 6197 bp downstream.
The positions of the probes used for Southern analysis are shown in Fig. 1A. The sizes of the restriction fragments detected by these probes in the wildtype allele are shown above the locus. The fragment sizes detected by these probes in the targeted allele are shown below the construct.
Linearized targeting vector was electroporated into AB2.2 ES cells (Lexicon Genetics, Woodlands, TX). G418-resistant clones were screened by Southern blot using the indicated external probes flanking the Pgk-neo insertion (Fig. 1B). Germline targeted mice were generated at the Transgenic/Knock-out core at UT Southwestern Medical Center (UTSW) in Dallas, TX) by injection into C57Bl/6 blastocysts and subsequently screened for agouti coat color. The resulted chimeras were bred to C57BL/6 females and F1 agouti offspring were genotyped by PCR as described below. Subsequently, mice heterozygous for floxedUCS allele with a Pgk-neo cassette (Bmp2UCSNeo/+, MMRRC #42279) were bred with mice bearing a FLP recombinase gene driven by the constitutive ROSA promoter (JAX #007844; 129S4/SvJae-Gt(ROSA)26Sortm2(FLP*)Sor/J) to remove the Pgk-neo cassette. This yielded mice with a loxP-flanked UCS (Bmp2flUCS/+, MMRRC# 042043). A ubiquitously expressed CMV-Cre gene under the transcriptional control of the cytomegalovirus promoter (JAX #006054; B6.C-Tg(CMV-cre)1Cgn/J) was then introduced into the Bmp2flUCS/+ mice to delete the UCS and to generate a recombined null UCS allele (Bmp2ΔUCS/+). The wildtype and 3 new alleles are shown in Fig. 1C.
These mice will be made available to the research community from the authors or the Mutant Mouse Resource & Research Centers (https://www.mmrrc.org/).
DNA isolation
Genomic DNA was isolated from tails from mouse pups at weaning and from either tails or amnions of embryos as follows. Tails or amnion membranes were lysed with DNA lysis buffer (100 mMTris, pH 8.5; 5 mM EDTA; 200 mM NaCl; 0.2% SDS; 0.2 mg/ml proteinase K added fresh) with rotation at 50 – 55°C for 12–16 hours. After solubilizing, debris was spun down for 10 minutes in a micro-centrifuge at full speed (16,000 × g). One volume of isopropanol was added to supernatants and mixed by inversion. DNA was centrifuged for 5 minutes at full speed, then washed with 70% ethanol.
Genotyping
Genomic DNA was genotyped by semi-quantitative PCR as follows: an initial denaturation at 97°C for 5 min; followed by 28 cycles of denaturation at 94°C for 30 secs; annealing at 55°C for 45 sec; and extension at 72°C for 1 min; ending with a final cycle of 94°C for 1 min, 55°C for 1 min, and 72°C for 5 min. Approximate PCR primer locations are shown in Fig. 1D. Primers A (5′GTC GTT AGC ACA GCA AGA ATA AA 3′) and B (5′AAA GTG TTC ATT GGG AAA TAT TAA AGT 3′) amplified a 121 bp fragment from the wildtype allele or a 245 bp fragment from the Bmp2flUCS or Bmp2UCSNeo alleles. Primers C (5′TCC AGC ACA TGA AGT ATA ATG GT 3′) and D (5′GCA AGA AAC AGA CAG CAA GTG 3′) amplified a 236 bp fragment from the wildtype allele or a 346 bp fragment from the Bmp2flUCS allele. Primers E (5′ GGG AGG ATT GGG AAG ACA ATA G3′) and D amplified a 138 bp fragment from the Bmp2UCSNeo allele. Primers A and D amplified a 509 bp fragment from the wildtype allele or a 246 bp fragment from the Bmp2ΔUCS allele. Fig. 1E shows a sample PCR.
Embryo fixation and histology preparation
Pregnant mice were euthanized and sacrificed according to IACUC regulations at embryonic stages E9.5, 10.5, and 13.5. Whole embryos were dissected in phosphate buffer solution (PBS) and separated from the amnion which was used for genotyping. Embryos were photographed and compared with littermates before fixation in chilled 3.7% paraformaldehyde-PBS solution or snap freezing for RNA and protein analyses.
RNA Isolation and Quantitative Real Time PCR (qPCR)
Total RNA was isolated from whole embryos using the Ribozol RNA isolation protocol (VWR Life Science, Radnor, PA, #N580) following manufacturer’s instructions. RNA was quantified with a NanoDrop spectrophotometer (Agilent Technologies). RNA was reverse transcribed using the QuantiTect® Reverse Transcription kit (Qiagen Inc., Germantown, MD, # 205313) according to the manufacturer’s instructions. Quantitative PCR was performed using the QuantiTect® SYBR® Green PCR kit (Qiagen Inc., Germantown, MD, #204145) and a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, #1855196). The mRNA expression was normalized to actin as the reference gene. Relative Bmp2 mRNA expression was calculated using CFX96 Manager software (Bio-Rad Laboratories, Hercules, CA, # 1845000). Intron-spanning primers were used to eliminate amplicons generated from any contaminating genomic DNA. The primer sequences used were Bmp2 - Forward (TAGATCTGTACCGCAGGCA) and Reverse (GTTCCTCCACGGCTTCTTC) and Actin - Forward (CGCCACCAGTTCGCCATGGA) and reverse (TACAGCCCGGGGAGCATCGT).
Western blotting
Lysates from whole embryos were prepared in RIPA buffer (20 mM Tris pH 8.0, 137 mM NaCl, 10% (v/v) Glycerol, 1% (v/v) NP-40, 0.1% (w/v) SDS, 0.5% Sodium deoxycholate, 2 mM EDTA pH 8.0, 1 mM PMSF, 1 mM aprotinin, and 20 μM leupeptin). Equal amounts of protein determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA, # 5000006) was separated by 12% SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membrane (45uM, Bio-Rad Laboratories, Hercules, CA, #1620115) at 90V for 90 minutes in a wet transfer apparatus (Bio-Rad Laboratories, Hercules, CA). The blots were blocked with 5% milk in Tris-buffered saline (10 mM Tris, 150 mM NaCl, pH 7.5) with 1% (v/v) Tween-20 (TBS-T) for 1 hour at room temperature and then incubated overnight at 4°C with the specific primary antibody in TBS-T with 3% (w/v) BSA. BMP signaling was measured using a polyclonal phospho-SMAD 1/5/9(8) antibody (Cell Signaling Technology, Danvers, MA, #9511) or a monoclonal phospho-SMAD 1/5/9 antibody (Cell Signaling Technology, Danvers, MA, #13820) at a dilution of 1:1000. Polyclonal total SMAD (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, #sc-6031-R) and Actin antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, #sc-1616-R) were used as loading controls at a dilution of 1:1,000 and 1:500 respectively. The pSMAD antibodies were authenticated using MC3T3-E1 (clone 4) and C3H 10T½ cells transfected with a Bmp2 expression vector or a control vector expressing luciferase. Specific proteins were detected using Clarity Western ECL Substrate (Bio-Rad Laboratories, Hercules, CA, # 1705061) and imaged using FluoroChem M (Protein Simple, San Jose, California). Densitometric analysis of the blots was done using AlphaView SA 3.0 software (Protein Simple, San Jose, California).
Acknowledgments
Grant support: Rutgers NJMS Dean’s Biomedical Research Support Program; National Heart, Lung, and Blood Institute 1R01HL114751
References
- Abrams KL, Xu J, Nativelle-Serpentini C, Dabirshahsahebi S, Rogers MB. An evolutionary and molecular analysis of Bmp2 expression. J Biol Chem. 2004;279:15916–15928. doi: 10.1074/jbc.M313531200. [DOI] [PubMed] [Google Scholar]
- Arraf AA, Yelin R, Reshef I, Kispert A, Schultheiss TM. Establishment of the Visceral Embryonic Midline Is a Dynamic Process that Requires Bilaterally Symmetric BMP Signaling. Dev Cell. 2016;37:571–580. doi: 10.1016/j.devcel.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing M, Nanney LB, King LE, Jones CM, Hogan BL. Transgenic mice as a model to study the role of TGF-beta-related molecules in hair follicles. Genes Dev. 1993;7:204–215. doi: 10.1101/gad.7.2.204. [DOI] [PubMed] [Google Scholar]
- Bonilla-Claudio M, Wang J, Bai Y, Klysik E, Selever J, Martin JF. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development. 2012;139:709–719. doi: 10.1242/dev.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castranio T, Mishina Y. Bmp2 is required for cephalic neural tube closure in the mouse. Dev Dyn. 2009;238:110–122. doi: 10.1002/dvdy.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi C, Tripurani SK, Large MJ, Edson MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP, Pangas SA, DeMayo FJ, Matzuk MM. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet. 2013;9:e1003863. doi: 10.1371/journal.pgen.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007;236:2493–2501. doi: 10.1002/dvdy.21256. [DOI] [PubMed] [Google Scholar]
- Devaney JM, Tosi LL, Fritz DT, Gordish-Dressman HA, Jiang S, Orkunoglu-Suer FE, Gordon AH, Harmon BT, Thompson PD, Clarkson PM, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Brandoli C, Hoffman EP, Rogers MB. Differences in fat and muscle mass associated with a functional human polymorphism in a post-transcriptional BMP2 gene regulatory element. J Cell Biochem. 2009;107:1073–1082. doi: 10.1002/jcb.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada KD, Retting KN, Chin AM, Lyons KM. Smad6 is essential to limit BMP signaling during cartilage development. J Bone Miner Res. 2011;26:2498–2510. doi: 10.1002/jbmr.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotinos A, Fritz DT, Lisica S, Liu Y, Rogers MB. Competing Repressive Factors Control Bone Morphogenetic Protein 2 (BMP2) in Mesenchymal Cells. J Cell Biochem. 2016;117:439–447. doi: 10.1002/jcb.25290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotinos A, Nagarajan N, Martins AS, Fritz DT, Garsetti D, Lee AT, Hong CC, Rogers MB. Bone morphogenetic protein-focused strategies to induce cytotoxicity in lung cancer cells. Anticancer Res. 2014;34:2095–2104. [PMC free article] [PubMed] [Google Scholar]
- Fritz DT, Jiang S, Xu J, Rogers MB. A polymorphism in a conserved posttranscriptional regulatory motif alters bone morphogenetic protein 2 (BMP2) RNA:protein interactions. Mol Endocrinol. 2006;20:1574–1586. doi: 10.1210/me.2005-0469. [DOI] [PubMed] [Google Scholar]
- Fritz DT, Liu D, Xu J, Jiang S, Rogers MB. Conservation of Bmp2 post-transcriptional regulatory mechanisms. J Biol Chem. 2004;279:48950–48958. doi: 10.1074/jbc.M409620200. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Gavrilov S, Lacy E. Genetic dissection of ventral folding morphogenesis in mouse: embryonic visceral endoderm-supplied BMP2 positions head and heart. Curr Opin Genet Dev. 2013;23:461–469. doi: 10.1016/j.gde.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov S, Lacy E. Symmetric BMPs on the Developmental Road. Dev Cell. 2016;37:488–490. doi: 10.1016/j.devcel.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Minetti C. Potential drug targets within bone morphogenetic protein signaling pathways. Curr Opin Pharmacol. 2007;7:325–333. doi: 10.1016/j.coph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Goldman DC, Donley N, Christian JL. Genetic interaction between Bmp2 and Bmp4 reveals shared functions during multiple aspects of mouse organogenesis. Mech Dev. 2009;126:117–127. doi: 10.1016/j.mod.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf D, Malik Z, Hayano S, Mishina Y. Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine Growth Factor Rev. 2016;27:129–139. doi: 10.1016/j.cytogfr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Fritz DT, Tian B, Rogers MB. Using Emerging Genome Data to Identify Conserved Bone Morphogenetic Protein (Bmp)2 Gene Expression Mechanisms. ACM First International Workshop on Text Mining in Bioinformatics (TMBIO2006) Proceedings; Arlington, VA, USA. New York, NY: ACM Press; 2006. [Google Scholar]
- Jiang S, Fritz DT, Rogers MB. A conserved post-transcriptional BMP2 switch in lung cells. J Cell Biochem. 2010;110:509–521. doi: 10.1002/jcb.22567. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Watabe T. Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol. 2016:8. doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Yu PB, Kamiya N, Pan H, Fukuda T, Scott GJ, Ray MK, Yamamura K, Mishina Y. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res. 2013;28:1422–1433. doi: 10.1002/jbmr.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof BP, Fritz DT, Liu Y, Garsetti DE, Frank DB, Pregizer SK, Gaussin V, Mortlock DP, Rogers MB. An autonomous BMP2 regulatory element in mesenchymal cells. J Cell Biochem. 2011a;112:666–674. doi: 10.1002/jcb.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof BP, Xu J, Fritz DT, Cabral CS, Gaussin V, Rogers MB. An in vivo map of bone morphogenetic protein 2 post-transcriptional repression in the heart. Genesis. 2011b;49:841–850. doi: 10.1002/dvg.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Nagashima T, Li Q, Clementi C, Lydon JP, DeMayo FJ, Matzuk MM. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest. 2013;123:2539–2550. doi: 10.1172/JCI65710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Ikeda K, Akakabe Y, Koide M, Uraoka M, Yutaka KT, Kurimoto-Nakano R, Takahashi T, Matoba S, Yamada H, Okigaki M, Matsubara H. Paracrine osteogenic signals via bone morphogenetic protein-2 accelerate the atherosclerotic intimal calcification in vivo. Arterioscler Thromb Vasc Biol. 2010;30:1908–1915. doi: 10.1161/ATVBAHA.110.206185. [DOI] [PubMed] [Google Scholar]
- Posadas DM, Carthew RW. MicroRNAs and their roles in developmental canalization. Curr Opin Genet Dev. 2014;27:1–6. doi: 10.1016/j.gde.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MB, Shah TA, Shaikh NN. Turning Bone Morphogenetic Protein 2 (BMP2) on and off in Mesenchymal Cells. J Cell Biochem. 2015;116:2127–2138. doi: 10.1002/jcb.25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Singh AP, Castranio T, Scott G, Guo D, Harris MA, Ray M, Harris SE, Mishina Y. Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex Dev. 2008;2:134–141. doi: 10.1159/000143431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Mao S, Li H, Zen K, Zhang CY, Li L. Role of miR-17 family in the negative feedback loop of bone morphogenetic protein signaling in neuron. PLoS One. 2013;8:e83067. doi: 10.1371/journal.pone.0083067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Hu J, Zhang H, Lutz CS. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005;33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Tylzanowski P, Mebis L, Luyten FP. The Noggin null mouse phenotype is strain dependent and haploinsufficiency leads to skeletal defects. Dev Dyn. 2006;235:1599–1607. doi: 10.1002/dvdy.20782. [DOI] [PubMed] [Google Scholar]
- Uchimura T, Komatsu Y, Tanaka M, McCann KL, Mishina Y. Bmp2 and Bmp4 genetically interact to support multiple aspects of mouse development including functional heart development. Genesis. 2009;47:374–384. doi: 10.1002/dvg.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mishina Y, Liu F. Osterix-Cre transgene causes craniofacial bone development defect. Calcif Tissue Int. 2015;96:129–137. doi: 10.1007/s00223-014-9945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Guo D, Harris MA, Cui Y, Gluhak-Heinrich J, Wu J, Chen XD, Skinner C, Nyman JS, Edwards JR, Mundy GR, Lichtler A, Kream BE, Rowe DW, Kalajzic I, David V, Quarles DL, Villareal D, Scott G, Ray M, Liu S, Martin JF, Mishina Y, Harris SE. Bmp2 in osteoblasts of periosteum and trabecular bone links bone formation to vascularization and mesenchymal stem cells. J Cell Sci. 2013;126:4085–4098. doi: 10.1242/jcs.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232:484–492. doi: 10.1006/dbio.2001.0173. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Devl. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]