Abstract
Purpose
To develop a saturation-recovery myocardial T1-mapping method for the simultaneous multi-slice acquisition of three slices.
Methods
Saturation Pulse-Prepared Heart-Rate Independent Inversion Recovery (SAPPHIRE) T1-mapping was implemented with simultaneous multi-slice imaging using FLASH readouts for faster coverage of the myocardium. Controlled aliasing in parallel imaging (CAIPI) was used to achieve minimal noise amplification in three slices. Multi-band reconstruction was performed using three linear reconstruction methods: Slice- and in-plane GRAPPA; CG-SENSE; and Tikhonov-regularized CG-SENSE. Accuracy, spatial variability and inter-slice leakage were compared with single-band T1-mapping in a phantom and in six healthy subjects.
Results
Multi-band phantom T1-times showed good agreement with single-band T1-mapping for all three reconstruction methods (Normalized root-mean-square error<1.0%). Increase in spatial variability compared with single-band imaging was lowest for GRAPPA (1.29-fold), with higher penalties for Tikhonov-regularized CG-SENSE (1.47-fold) and CG-SENSE (1.52-fold). In-vivo multi-band T1-times showed no significant difference compared with single-band (T1-Time±inter-segmental variability: Single-Band: 1580±119ms, GRAPPA: 1572±145ms, CG-SENSE: 1579±159ms, Tikhonov: 1586±150ms; ANOVA: p=0.86). Inter-slice leakage was smallest for GRAPPA (5.4%), and higher for CG-SENSE (6.2%) and Tikhonov-regularized CG-SENSE (7.9%).
Conclusion
Multi-band accelerated myocardial T1-mapping demonstrated the potential for single-breath hold T1-quantification in 16 AHA segments over 3 slices. 1.2 to 1.4-fold higher in-vivo spatial variability was observed, where GRAPPA-based reconstruction showed the highest homogeneity and the least inter-slice leakage.
Keywords: Myocardial T1 mapping, Simultaneous Multi-Slice Imaging, Multi-band, saturation recovery, SAPPHIRE
Introduction
Quantitative imaging of the heart using Magnetic Resonance Imaging (MRI) has recently emerged to a major focus area within the cardiac MRI (CMR) community. The quantification of various relaxation parameters (T1 (1,2), T1ρ (3,4), T2 (5–7), T2* (8,9)) has revealed clinical sensitivity to a wide range of ischemic and non-ischemic cardiomyopathies (10–12). In particular, the spatially resolved assessment of the longitudinal relaxation time T1 (referred to as T1 mapping) shows promising potential to enhance quality of CMR for prognosis and diagnosis of cardiomyopathies (13).
Parameter maps of the myocardium are commonly obtained from a series of single-shot images with different contrast-weightings, all of which are acquired during a single breath-hold. Three slice coverage in short axis orientation is recommended for evaluation, as it captures the heterogeneity across left ventricle better than single slice acquisitions (14). Conventional myocardial T1 mapping methods acquire only a single-slice per breath-hold, necessitating rest periods between subsequent breath-holds, which lead to patient discomfort and long scan times. Furthermore, repeated breath-holds may compromise image registration (15–18).
Free-breathing T1 mapping methods have been proposed to improve patient comfort and enable increased spatial resolution or coverage. In these techniques, respiratory motion compensation may be performed via prospective triggering (19) or gating based on diaphragmatic image navigators (20–22), retrospective self-gating (23,24), or prospective slice-tracking (25). However, respiratory gating and triggering lead to increased scan times, while tracking potentially induces blurring in the presence of heavy breathing. Consequently, a fast single breath-hold acquisition is the preferred approach.
Image acceleration techniques, such as parallel imaging, are frequently used in breath-held myocardial T1 mapping, in order to provide sufficient spatial resolution in the single-shot acquisitions during the brief diastolic quiescence (14). Compressed sensing has also been used to improve spatial resolution in T1 mapping in a single-breath hold (26,27). However, these approaches, as commonly used, do not affect the coverage or the total acquisition time, thus not changing the breath-hold duration.
Simultaneous multi-slice (SMS) or multi-band (MB) imaging is an alternative acceleration technique for acquiring multiple slices simultaneously (28), where the only SNR reduction compared to single-slice imaging is due to coil geometry (29). In MB imaging, the simultaneous excitation of multiple slices is achieved by playing an excitation pulse, which is obtained as the sum of pulses at different resonance frequencies, corresponding to different slice locations (30). To decrease the noise-amplification from un-aliasing, better encoding has been proposed with Controlled aliasing in volumetric parallel imaging (CAIPIRINHA) (29). Here, cyclic-phase shifts, or equivalent a sheared under-sampling pattern, is used to induce a shifted object position benefitting 3D and 2D SMS un-aliasing (31).
MB imaging has become a popular tool in neurological applications (32), however, its use in cardiac applications has been limited, due to unfavorable coil geometries used in body imaging. MB imaging has been used in myocardial perfusion imaging with a 2-fold MB and 2.5-fold in-plane acceleration (33), as well as 3 to 5-fold MB and no in-plane acceleration (34). Cardiac cine imaging with 2-fold MB and 3-fold in-plane acceleration at 3T (35), and with 2 to 3-fold MB and 2 to 4-fold in-plane acceleration at 7T (36) have also been investigated. However, its effect on myocardial MR parameter quantification and precision has not been explored.
In this study, we sought to evaluate the potential of MB imaging to accelerate myocardial T1 mapping and to enable 16-segment quantification in a single breath-hold. A Saturation Pulse Prepared Heart-rate Independent Inversion REcovery (SAPPHIRE) sequence, with CAIPIRINHA-MB accelerated FLASH imaging was proposed for the simultaneous acquisition of three slices. T1 time accuracy and precision were compared to conventional single-band (SB) SAPPHIRE imaging in phantom scans. In-vivo results are presented for native T1 mapping in healthy subjects.
Methods
Sequence
Figure 1a depicts the schematic of the proposed pulse-sequence. Combined sinc-excitation pulses (bandwidth-time-product, BWT=2.0, pulse-duration=1.0ms) at three frequencies were employed for MB excitation in the FLASH imaging readout of a SAPPHIRE (37) sequence, using hybrid saturation/inversion preparation for T1 sensitization. 15 images with different inversion times were acquired during a single-breath hold. All inversion times are confined to a single heart-beat, resulting in an acquisition over 15 heart-beats. The inversion times are linearly distributed between the minimal inversion time (185 ms in this study) and the maximum inversion time, determined by the start of the diastolic phase. For phantom imaging, the maximum inversion time was selected as 760 ms, corresponding to a heart-rate of 60 bpm. Pulse phases of the three base excitation pulses were cycled with a phase increment of 2π/3 from slice to slice, to achieve a field-of-view (FOV) shift of 1/3 in the images between adjacent slices. Additionally a constant slice-specific phase-shift was added to each individual pulse phase, previously optimized to minimize peak B1+ amplitude to reduce SAR burden of the sequence (38,39).
Figure 1.
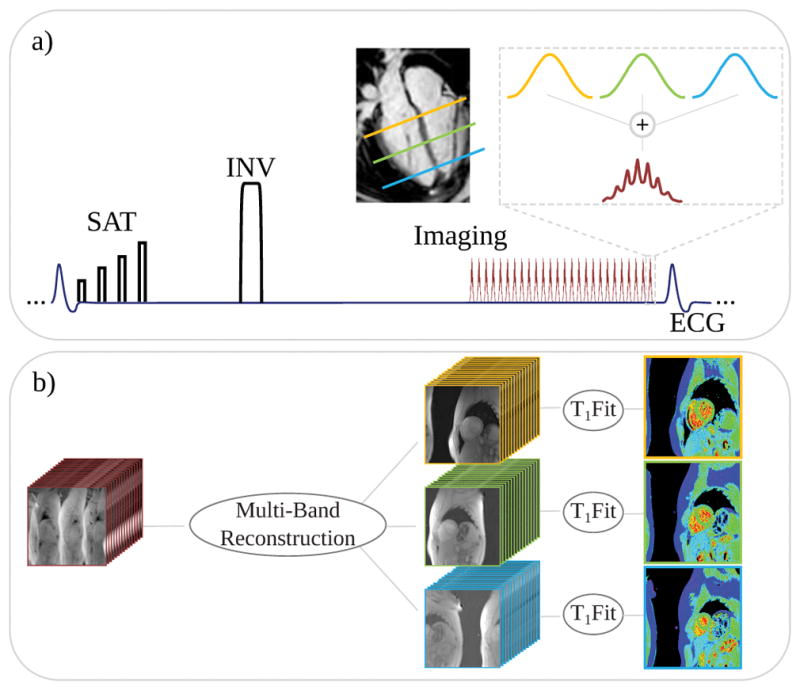
(a) Schematic description of the multi-band T1 mapping sequence and (b) the reconstruction pipeline. A SAPPHIRE sequence with combined saturation/inversion-recovery preparation is combined with FLASH imaging readout. The MB excitation pulses are obtained as the sum of three sinc single-band excitations at different frequency bands. Image reconstruction is performed to unalias the multi-band slices and the in-plane undersampling, with subsequent phase-sensitive fitting of the saturation/inversion-recovery curve to obtain quantitative T1 maps.
All MB and SB T1 mapping were performed at 3 Tesla field-strength with a single-shot ECG-triggered FLASH sequence with the following imaging parameters: Uniform in-plane undersampling=2, FOV=320×320mm2, spatial resolution=2.0×2.1mm2, slice thickness=10mm, slice gap=10mm, partial-Fourier=6/8, #phase-encode lines=69, TR/TE/FA=4.0/2.0ms/10°, bandwidth=505Hz/Px, linear k-space ordering, inversion-pulse: 2.56ms tan/tanh adiabatic full passage (40), saturation-pulse: 4-compartment WET (41). 24 reference lines were acquired in the k-space center for each image.
To enable the reconstructions for slice unaliasing, a 1-second reference scan was used to acquire low-resolution images of 3 slices, during free-breathing and without ECG-gating (FOV=320×320mm2, spatial resolution=2×5mm2, slice thickness=10mm, TR/TE/FA=3.6/1.8ms/10°, bandwidth=500Hz/Px). For comparison, an additional 3 heart-beat reference scan with the same parameters was also acquired, with end-diastolic ECG-triggering and breath-holding.
Reconstruction
The acquired raw data with MB-aliasing and in-plane acceleration were exported from the scanner, and the T1-weighted images were reconstructed offline in Matlab (The Mathworks, Nattick, MA, USA) using three different linear reconstruction approaches: i) Multi-slice unaliasing performed using slice-GRAPPA (42), followed by in-plane GRAPPA (43), whose kernels were calibrated from the low resolution reference scan, with (5,5) and (5,4) kernel sizes, respectively. The final images were generated using a coil-sensitivity weighted combination of the individual coil images. ii) A CG-SENSE (44) reconstruction for slice and in-plane unaliasing. CG-SENSE was used instead of SENSE to utilize the signal from the fully-sampled MB-encoded k-space center. Coil-sensitivity maps for each band and each coil were generated from the reference scan. iii) The reconstruction in ii) with additional Tikhonov regularization (45). A separate sub-study, detailed in the Supporting Information S1 and Supporting Figure S1, was performed to empirically optimize the Tikhonov regularization parameter as 0.05.
For each of the three methods, following the MB slice and in-plane unaliasing, phase-sensitive fitting, as proposed for inversion recovery T1 mapping (15) was performed on the final T1-weighted images to obtain T1 maps (Fig. 1b).
Phantom Imaging
All imaging was performed at a 3T Siemens Magnetom Prisma (Siemens Healthcare, Erlangen, Germany) system using a 30-channel receiver coil-array.
Phantom imaging for T1 quantification accuracy and precision, was performed using SB and MB SAPPHIRE in a cylindrical phantom containing multiple spherical compartments of Gadolinium or sucrose doped agarose-gel, with T1 and T2 times in the in-vivo range (T1: 200–2500ms, T2: 50–250ms, (46)). All scans were performed with 10 repetitions to allow assessment of noise-dependent variation.
In-vivo Imaging
The study was approved by the institutional review board, and written informed consent was acquired before each examination. Imaging was performed in six healthy subjects (3 male; 36±16 years) with no contraindications to MRI. Native T1 maps were acquired using conventional SB and proposed MB SAPPHIRE in these short-axis slices. Conventional SB SAPPHIRE was performed in three breath-holds for coverage of the three slices. MB SAPPHIRE images of matching slices were acquired in a single breath-hold. Free-breathing and breath-hold calibration scans were acquired prior to the multi-band acquisition.
Data Analysis
Phantom accuracy was defined as the deviation of the average T1 time within manually drawn region-of-interests (ROIs), averaged over all repetitions. Spatial variability was assessed as the standard deviation across the ROI in the homogenous phantom vials, averaged over all repetitions.
Leakage analysis was performed using the respective MB reconstruction on spatially shifted SB acquisitions (31). The 3 slices from the SB acquisitions, corresponding to no saturation preparation, were shifted to match the FOV shifts of the MB acquisition. Then, each of these shifted slices were run through the three separate reconstruction algorithms, using the kernels/coil sensitivity maps as generated from the respective reference scans. Ideally, this leads to only the original input slice being reconstructed, with no signal content in the other two slices. Thus, the leakage was defined as the resulting residual signal in the two non-input slices. This was repeated for the other two shifted SB slices. Using the linearity of the reconstructions, the total leakage in each slice was generated by addition of the leakages from the three shifted SB slices as inputs.
In-vivo T1 and leakage maps were evaluated in manually drawn ROIs, delineating the endo- and epi-cardial contours, while carefully avoiding areas of partial-voluming. Quantitative comparison of the T1 times and T1 time spatial variability was performed according to the American Heart Association (AHA) 16 segment model (47). In-vivo T1 time spatial variability was defined as the inter-segment variation.
Statistical differences in the T1 times, and inter-slice leakage were assessed using one way ANOVA with subsequent paired Student’s T-Tests. Kruskal-Wallis group analysis and Wilcoxon signed rank tests were used to statistically compare spatial variability of T1 time. P-values < 0.05 were considered to be significant for group tests, and Bonferroni correction was applied for pair-wise tests with significance levels of 0.0083 for T1 time and spatial variability comparison (6 tests) and 0.017 for Leakage analysis (3 tests).
Results
Phantom Imaging
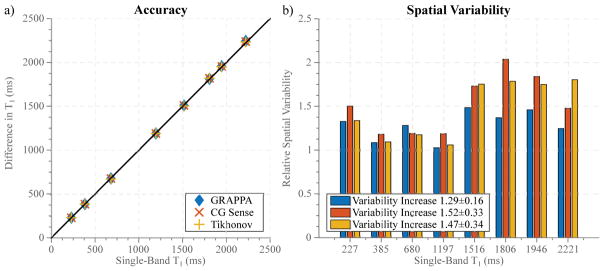
Figure 2 depicts the results of phantom imaging, showing good agreement between SB T1 mapping and all MB reconstruction methods, with minor differences between the three reconstruction techniques (Normalized root-mean-square error (NRMSE): GRAPPA: 0.62%, CG-SENSE: 0.66%, Tikhonov: 0.59%). Spatial variability in the homogeneous T1 phantom shows the highest increase for the non-regularized CG-SENSE reconstruction and only minor changes for GRAPPA (Variability relative to single-band T1 mapping: GRAPPA: 1.29±0.16, CG-SENSE: 1.52±0.33, Tikhonov: 1.47±0.34).
Figure 2.
Phantom results depicting the accuracy (a) and spatial-variability (b) of the proposed technique in comparison with conventional SB imaging for three different linear reconstruction methods. The MB T1 times show good agreement with the SB acquisition. 1.3 to 1.5-fold increase of noise variability is shown with the different MB reconstruction techniques compared to single-band imaging.
In-vivo Imaging
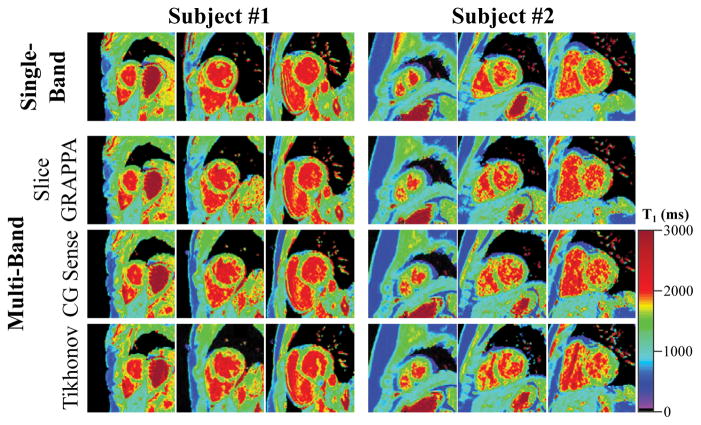
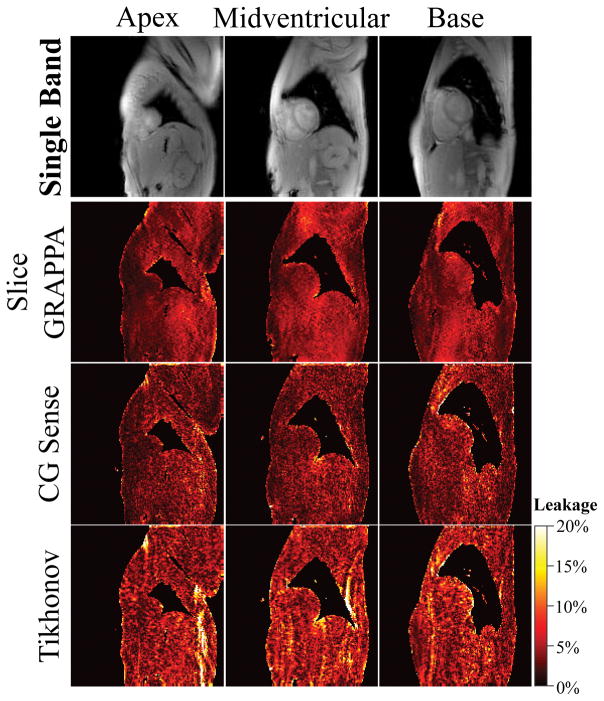
Figure 3 shows representative T1 maps acquired in two subjects with single- and multi-band SAPPHIRE, using the free-breathing calibration scan. All volunteer scans are shown in Supporting Figure S2. Conventional SB acquisition produces visually homogeneous T1 maps (T1 time ± spatial variability, Subject #1: 1572±95ms, Subject #2: 1546±126ms). For the MB T1 scans, reconstruction with slice GRAPPA resulted in the least amount of spatial variability (Subject #1: 1567±111ms, Subject #2: 1563±139ms), providing images that are visually comparable to SB imaging. Tikhonov-regularized CG-SENSE also provided images with comparable quality, although with slightly increased spatial variability (Subject #1: 1567±112ms, Subject #2: 1583±155ms). CG-SENSE showed the strongest increase in spatial variability (Subject #1: 1563±118ms, Subject #2: 1593±170ms). By visual inspection CG-SENSE based reconstructions showed higher leakage, especially in the basal slice, while less leakage was observed for slice GRAPPA. The same trend is observed in the leakage maps of the three reconstruction techniques (Figure 4): Leakage using GRAPPA reconstruction appears visually homogeneous and noise-like. Increased structure but reduced noise-like variation can be observed using CG-SENSE, while the highest leakage is observed with regularized CG-SENSE.
Figure 3.
Representative T1 maps from two subjects, comparing a multi-band acquisition with various linear reconstructions to conventional single-band T1 mapping. Increased heterogeneity of the T1 times is observed using conventional CG-SENSE, compared with single-band T1 mapping. GRAPPA and Tikhonov-regularized MB imaging achieves image quality that is visually comparable to single-band imaging, although Tikhonov-regularized CG-SENSE displayed increased inter-slice leakage, as apparent in subject #1.
Figure 4.
Leakage maps comparing three multi-band reconstructions. The top row, presents the corresponding single-band images with no magnetization preparation, as used to generate the leakage maps. GRAPPA shows mild and noise-like leakage across the FOV. Slightly decreased leakage, albeit with increased intensity hot-spots is depicted for CG-SENSE. Tikhonov-regularized CG-SENSE displays the highest inter-slice leakage of the three methods, though the intensity hot-spots predominantly lie outside the myocardium.
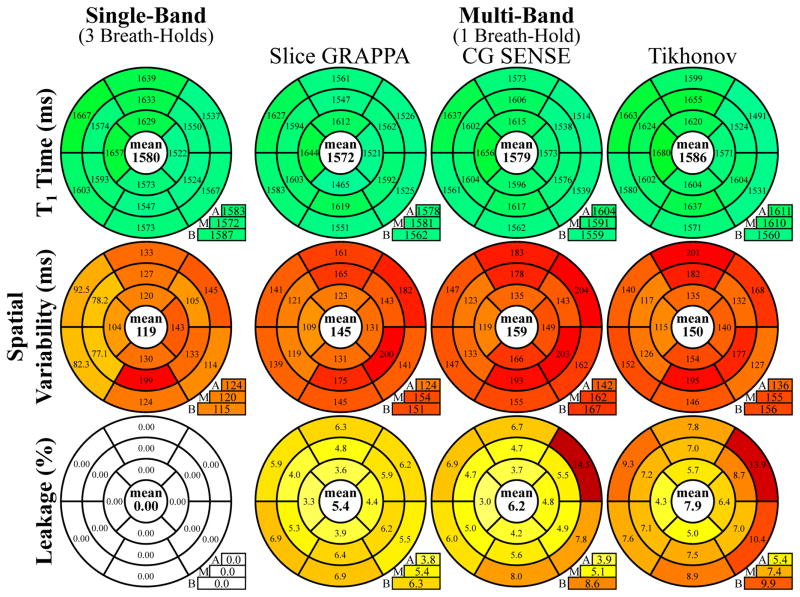
Bullseye representations of the quantitative evaluation of myocardial T1 times, in-vivo spatial variability and inter-slice leakage for MB and SB T1 mapping across all subjects in the 16 AHA segments are depicted in Figure 5. For MB acquisitions, all reconstruction techniques result in T1 values comparable to the SB reference (ANOVA p=0.86). As in phantom scans, the increase in spatial variability is the least for GRAPPA, followed by Tikhonov-regularized CG-SENSE, and CG-SENSE (Kruskal-Wallis p=0.07, pair-wise p≤0.031, except for GRAPPA vs. Tikhonov–regularized CG-SENSE: p=0.44 and CG-SENSE vs. Tikhonov-regularized CG-SENSE p = 0.094). However, the Tikhonov-regularized reconstruction also shows the highest inter-slice leakage, in particular in segments #2, #5 and #6 of the basal slice. GRAPPA showed the most uniform leakage performance across segments with the smallest mean (Kruskal-Wallis p=0.22; GRAPPA vs. Tikhonov p=0.25, GRAPPA vs. CG-SENSE p=0.031, CG-SENSE vs. Tikhonov-regularized CG-SENSE p=0.031).
Figure 5.
Bullseye representation of myocardial T1 times, T1 time spatial variability and inter-slice leakage, as quantitatively analyzed according to the AHA 16-segment model. All three reconstruction methods show T1 times comparable to single-band imaging, although with increased intra-segment variability. Slice GRAPPA shows the smallest increase in spatial variability compared to SB T1 mapping and the smallest inter-slice leakage.
The same trend can be observed in blood T1 times. No significant difference was found between SB and the three MB reconstructions (ANOVA: p=0.98, SB 2043±80ms; GRAPPA: 2034±71; CG-SENSE: 2042±94ms; Tikhonov-regularized CG-SENSE: 2026±93ms). Blood T1 time spatial variability was 1.3 to 1.6-fold higher using MB compared with SB T1 mapping, but differences were not found to be significant (Kruskal-Wallis: p=0.102, SB 130±13ms; GRAPPA: 170±31; CG-SENSE: 206±82; Tikhonov-regularized CG-SENSE: 172±51ms).
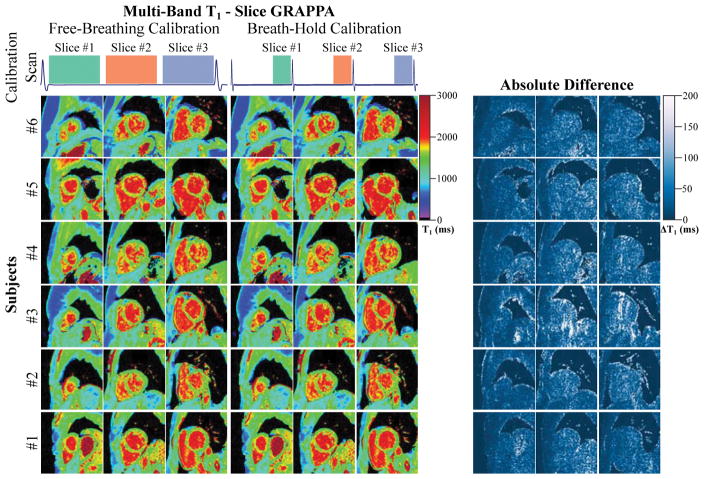
In our study, no visual difference was observed when performing the MB reconstructions using the free-breathing vs. breath-held calibration scans. T1 maps reconstructed with GRAPPA using the different types of calibration data are depicted in Figure 6. There were no significant differences among the T1 values (p>0.57) or the spatial variability (p>0.59) for the two types of calibration data. GRAPPA displayed the highest amount of resilience to changes in the calibration data (relative difference: T1: 0.015±0.269%, Variability: 0.223±2.28%). CG-SENSE reconstructions showed a slight but non-significant trend of increased variability with the free-breathing calibration-data (relative difference CG-SENSE: T1: −0.472±1.03%, Variability: −7.82±11.9%, relative difference Tikhonov-regularized CG-SENSE: T1: −0.517±0.994%, Variability: −3.62 ±7.19%).
Figure 6.
Multi-band T1 maps reconstructed with slice GRAPPA using free-breathing or breath-hold calibration data. The acquisition scheme for the calibration data is depicted in the top row. Images of six healthy subjects are presented below, along with the corresponding difference maps. Multi-band T1 maps with both calibration data are of similar quality and differences are hardly noticeable by visual assessment. Difference maps reveal minor noise like changes when using a differently acquired calibration data set.
Discussion
In this study, we evaluated a multi-band imaging sequence for accelerated myocardial T1 mapping that enables 16-segment quantification in a single breath-hold. We evaluated three linear reconstruction algorithms for unaliasing the MB data, and their effect on T1 estimation and spatial variability. Phantom and in-vivo experiments show that all three methods show comparable accuracy to conventional single-band imaging, albeit at 1.2–1.4-fold loss in spatial variability.
Multi-band imaging suffers from decreased SNR due to unfavorable coil geometry in cardiac applications. Our results show that a combination of slice and in-plane GRAPPA showed the least noise amplification, with the least amount of inter-slice leakage and a uniform leakage profile across all the myocardial segments. Among the SENSE-based reconstructions, Tikhonov regularization reduced the effects of noise amplification. However, it also increased the inter-slice leakage, albeit most of the increased leakage being evident outside the heart, with the exception of some basal segments. Nonlinear reconstruction techniques with appropriate regularization can also be utilized for further removal of artifacts due to noise and leakage. However, these were not explored in the current study, in order to provide a uniform comparison of spatial variability and leakage for the linear reconstruction techniques.
The position of the heart is known to show major variations between separate breath-holds even in healthy volunteers. Hence, T1 map acquisition of three short-axis slices in separate breath-holds provides potentially non-equidistant coverage with bias towards basal or apical T1 times. As all slices are acquired simultaneously in MB T1 mapping, equidistant and uniform coverage of the left-ventricle is ensured in a short-axis stack scan with the proposed technique.
In this study, MB reconstructions, in particular slice GRAPPA, were observed to be resilient to inter-scan motion between the calibration and the measurement data. This result is encouraging for the applicability of SMS imaging to cardiac applications, where potential mismatches in cardiac or respiratory phases might be unavoidable. The use of calibration scans without cardiac or respiratory gating is advantageous, as additional scan time requirements are minimized. Further studies are needed to verify this trend in other cardiac MR applications.
Myocardial T1 mapping is most commonly performed using bSSFP imaging readouts, which are less disruptive to the longitudinal magnetization recovery curve (48). Recently, the use of FLASH imaging has been explored for inversion-recovery based T1 mapping (49,50). Saturation-recovery T1 mapping methods are known to allow for accurate T1 quantification with FLASH imaging readout (51). Accordingly T1 times assessed with FLASH SAPPHIRE in healthy subjects are in good agreement with a recent study of steady-state free precession based saturation recovery T1 mapping at 3T (41). FLASH imaging has been proven to be beneficial at 3T due to its resilience against off-resonance artifacts, which might be a major disruptive factor to bSSFP image quality at high field strengths. However, in T1 mapping, this comes at the cost of reduced noise-resilience and increased end-diastolic imaging times. Due to the linear k-space ordering, and due to the fact that border zones are commonly excluded when evaluating myocardial T1 maps, the increase in acquisition window duration caused by the long FLASH TR, has previously been reported to be not an issue at 1.5T (52). Nonetheless, the TR of the FLASH sequence can be further shortened by optimizing spoiling strategies or increasing imaging bandwidth as a trade-off against T1 mapping precision. For multi-band imaging, FLASH has the additional advantage that MB phase cycling can be encoded in the RF phase of each band, rather than using encoding gradients as for EPI or SSFP imaging, which might introduce additional signal loss due to in-band dephasing (53).
In order to mitigate the reduced baseline SNR of FLASH imaging, T1 maps were acquired over 15 heart-beats, in contrast to previous bSSFP-based SAPPHIRE protocols, which use 9 to 11 images (37,41,54,55). The longer breath-hold duration was not disruptive in the study cohort. However, in critically ill patients or patients with respiratory restrictions, reduced sequence duration can be achieved at a trade-off against a slight loss in precision.
While multi-band T1 mapping was demonstrated with a SAPPHIRE saturation-recovery sequence design, this acceleration technique can straightforwardly be applied to other saturation recovery techniques, such as saturation-recovery single shot acquisition (SASHA) (56). In this study, we chose SAPPHIRE instead of SASHA, due to recent results showing that SAPPHIRE is more artifact resilient and more precise (41,54). The technique may also be applied to myocardial T1 mapping where the T1 recovery curve spans several heart-beats, such as the Modified look-locker inversion-recovery technique (MOLLI) and its variants (48,57,58). However, progressive saturation of the blood in several slices simultaneously, might lead to diminished signal from the blood pool over time, especially at high multi-band factors. Furthermore, the FLASH imaging readouts as used in the proposed multi-band acquisition substantially compromise the accuracy in commonly used inversion-recovery T1 mapping sequences. Although tailored reconstruction schemes have been proposed to mitigate this effect (59), MB inversion-recovery T1 mapping is beyond the scope of this work, but warrants further investigation.
All SAPPHIRE T1 maps in the current study were reconstructed using curve fitting to phase-sensitive data. This approach has previously been proposed for inversion-recovery T1 mapping (15), resulting in reduced in-vivo variability as it eliminates the necessity to restore the signal polarity along the inversion-recovery curve. Furthermore, a Gaussian noise characteristic is maintained, potentially increasing T1 mapping accuracy, when least-squares fitting is used with low baseline SNR or when the T1-weighted myocardial signal falls close to the zero crossing. These advantages of phase-sensitive T1 mapping can also be harvested in hybrid SAPPHIRE T1 mapping, as the dynamic range spans across both the positive and negative longitudinal magnetizations.
In this study, in vivo imaging was performed during breath-holding. However, in some patients, end-expiration breath-holding cannot be maintained even for short durations. In these cases, respiratory drift may corrupt the T1 map quality and precision. Dedicated image registration algorithms have been proposed, to closely re-align the T1-weighted baseline images, despite the substantial contrast variations (16,17). Although, no significant motion-induced artifacts have been observed in our healthy cohort study, such registration techniques can be applied to MB T1 mapping. Additionally, due to the simultaneous acquisition of multiple slices, similar motion can be expected and simultaneous registration of all three slices may be performed. This may help to reduce the dimensionality of the registration problem and improve the re-alignment of the baseline images in post-processing. Potential synergies of image registration and MB cardiac imaging will be explored in future studies.
This study has several limitations. Only a limited number of healthy subjects was included in this proof-of-concept study. Clinical evaluation of single breath-hold whole-heart T1 mapping in larger cohorts exhibiting specific pathologies is warranted. Since the excitation bands in MB imaging have to be parallel, T1 maps have only been evaluated in short-axis, to allow for the 16-segment analysis. Only a single MB acceleration factor of 3 was studied in this work to allow for T1 mapping in a single breath-hold, in accordance with the coverage requirements of the SCMR T1 mapping task-force consensus statement (14).
Conclusion
The proposed technique enables acquisition of native myocardial T1 maps with improved spatial coverage, allowing for the quantification of the 16 AHA-segments over 3 slices in a single breath-hold. More than 3-fold savings in acquisition time is achieved in young healthy volunteers, at an increased T1 spatial variability of 1.2 to 1.4-fold using a linear slice GRAPPA reconstruction.
Supplementary Material
Figure S1: Spatial variability in the MB T1 maps reconstructed with Tikhonov-regularized CG-SENSE using different values for the regularization parameter. Example images of the mid-ventricular slice are provided for one volunteer at four different parameter values. Minimum spatial variability is observed at 0.05. Lower regularization parameters cause noise-induced spatial inhomogeneity, while higher values lead to residual leakage artifacts (white arrows).
Figure S2: Single- and multi-band T1 maps acquired in six healthy subjects. Multi-band data was reconstructed using slice GRAPPA. Visually comparable T1 map quality with largely homogeneous myocardial T1 and clear delineation towards the blood-pools can be observed with both T1 mapping sequences. Slightly increased spatial inhomogeneity is observed in the multi-band T1 maps.
Acknowledgments
Funding:
NIH; Grant numbers: R00HL111410, P41EB015894
The authors acknowledge grant support from the NIH, grant numbers: R00HL111410, P41EB015894.
Footnotes
Competing Interests
SW and MA are inventors of a pending U.S. and European patent entitled “Methods for scar imaging in patients with arrhythmia”, which described the SAPPHIRE imaging sequence.
References
- 1.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. doi: 10.1186/1532-429X-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burt JR, Zimmerman SL, Kamel IR, Halushka M, Bluemke DA. Myocardial T1 mapping: techniques and potential applications. Radiographics. 2014;34(2):377–395. doi: 10.1148/rg.342125121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oorschot JW, Visser F, Eikendal AL, Vonken EJ, Luijten PR, Chamuleau SA, Leiner T, Zwanenburg JJ. Single Breath-Hold T1rho-Mapping of the Heart for Endogenous Assessment of Myocardial Fibrosis. Invest Radiol. 2016;51(8):505–512. doi: 10.1097/RLI.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 4.Muthupillai R, Flamm SD, Wilson JM, Pettigrew RI, Dixon WT. Acute myocardial infarction: tissue characterization with T1rho-weighted MR imaging--initial experience. Radiology. 2004;232(2):606–610. doi: 10.1148/radiol.2322030334. [DOI] [PubMed] [Google Scholar]
- 5.He T, Gatehouse PD, Anderson LJ, Tanner M, Keegan J, Pennell DJ, Firmin DN. Development of a novel optimized breathhold technique for myocardial T2 measurement in thalassemia. J Magn Reson Imaging. 2006;24(3):580–585. [Google Scholar]
- 6.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang TY, Liu YJ, Stemmer A, Poncelet BP. T2 measurement of the human myocardium using a T2-prepared transient-state TrueFISP sequence. Magn Reson Med. 2007;57(5):960–966. doi: 10.1002/mrm.21208. [DOI] [PubMed] [Google Scholar]
- 8.Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, Pennell DJ. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18(1):33–39. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 9.Sandino CM, Kellman P, Arai AE, Hansen MS, Xue H. Myocardial T2* mapping: influence of noise on accuracy and precision. J Cardiovasc Magn Reson. 2015;17(1):7. doi: 10.1186/s12968-015-0115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6(7):806–822. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamlin SA, Henry TS, Little BP, Lerakis S, Stillman AE. Mapping the future of cardiac MR imaging: case-based review of T1 and T2 mapping techniques. Radiographics. 2014;34(6):1594–1611. doi: 10.1148/rg.346140030. [DOI] [PubMed] [Google Scholar]
- 12.Han Y, Liimatainen T, Gorman RC, Witschey WR. Assessing Myocardial Disease Using T1rho MRI. Curr Cardiovasc Imaging Rep. 2014;7(2):9248. doi: 10.1007/s12410-013-9248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schelbert EB, Messroghli DR. State of the Art: Clinical Applications of Cardiac T1 Mapping. Radiology. 2016;278(3):658–676. doi: 10.1148/radiol.2016141802. [DOI] [PubMed] [Google Scholar]
- 14.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB Society for Cardiovascular Magnetic Resonance I, Cardiovascular Magnetic Resonance Working Group of the European Society of C. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue H, Greiser A, Zuehlsdorff S, Jolly MP, Guehring J, Arai AE, Kellman P. Phase-sensitive inversion recovery for myocardial T1 mapping with motion correction and parametric fitting. Magn Reson Med. 2013;69(5):1408–1420. doi: 10.1002/mrm.24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67(6):1644–1655. doi: 10.1002/mrm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roujol S, Foppa M, Weingartner S, Manning WJ, Nezafat R. Adaptive registration of varying contrast-weighted images for improved tissue characterization (ARCTIC): application to T1 mapping. Magn Reson Med. 2015;73(4):1469–1482. doi: 10.1002/mrm.25270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roujol S, Basha TA, Weingartner S, Akcakaya M, Berg S, Manning WJ, Nezafat R. Impact of motion correction on reproducibility and spatial variability of quantitative myocardial T2 mapping. J Cardiovasc Magn Reson. 2015;17:46. doi: 10.1186/s12968-015-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coniglio A, Di Renzi P, Vilches Freixas G, Della Longa G, Santarelli A, Capparella R, Nardiello B, Loiudice C, Bianchi S, D’Arienzo M, Begnozzi L. Multiple 3D inversion recovery imaging for volume T1 mapping of the heart. Magn Reson Med. 2013;69(1):163–170. doi: 10.1002/mrm.24248. [DOI] [PubMed] [Google Scholar]
- 20.Weingartner S, Akcakaya M, Roujol S, Basha T, Stehning C, Kissinger KV, Goddu B, Berg S, Manning WJ, Nezafat R. Free-breathing post-contrast three-dimensional T1 mapping: Volumetric assessment of myocardial T1 values. Magn Reson Med. 2015;73(1):214–222. doi: 10.1002/mrm.25124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weingartner S, Akcakaya M, Roujol S, Basha T, Tschabrunn C, Berg S, Anter E, Nezafat R. Free-breathing combined three-dimensional phase sensitive late gadolinium enhancement and T1 mapping for myocardial tissue characterization. Magn Reson Med. 2015;74(4):1032–1041. doi: 10.1002/mrm.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta BB, Chen X, Bilchick KC, Salerno M, Epstein FH. Accelerated and navigator-gated look-locker imaging for cardiac T1 estimation (ANGIE): Development and application to T1 mapping of the right ventricle. Magn Reson Med. 2015;73(1):150–160. doi: 10.1002/mrm.25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow K, Yang Y, Shaw P, Kramer CM, Salerno M. Robust free-breathing SASHA T1 mapping with high-contrast image registration. J Cardiovasc Magn Reson. 2016;18(1):47. doi: 10.1186/s12968-016-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai JM, Huang TY, Tseng YS, Lin YR. Free-breathing MOLLI: application to myocardial T(1) mapping. Med Phys. 2012;39(12):7291–7302. doi: 10.1118/1.4764915. [DOI] [PubMed] [Google Scholar]
- 25.Weingartner S, Roujol S, Akcakaya M, Basha TA, Nezafat R. Free-breathing multislice native myocardial T1 mapping using the slice-interleaved T1 (STONE) sequence. Magn Reson Med. 2014 doi: 10.1002/mrm.25387. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Joseph AA, Kalentev O, Merboldt KD, Voit D, Roeloffs VB, van Zalk M, Frahm J. High-resolution myocardial T1 mapping using single-shot inversion recovery fast low-angle shot MRI with radial undersampling and iterative reconstruction. Br J Radiol. 2016;89(1068):20160255. doi: 10.1259/bjr.20160255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Mehta BB, Salerno M, Epstein FH. High Resolution Myocardial T1 Mapping using MOLLI with Parallel Imaging and Compressed Sensing. Proc Intl Soc Mag Reson Med; Salt Lake City, Utah, USA. 2013. p. 1407. [Google Scholar]
- 28.Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001;13(2):313–317. doi: 10.1002/1522-2586(200102)13:2<313::aid-jmri1045>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53(3):684–691. doi: 10.1002/mrm.20401. [DOI] [PubMed] [Google Scholar]
- 30.Muller S. Multifrequency selective rf pulses for multislice MR imaging. Magn Reson Med. 1988;6(3):364–371. doi: 10.1002/mrm.1910060315. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Ugurbil K. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 2013;83:991–1001. doi: 10.1016/j.neuroimage.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ugurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, Van de Moortele PF, Strupp J, Sapiro G, De Martino F, Wang D, Harel N, Garwood M, Chen L, Feinberg DA, Smith SM, Miller KL, Sotiropoulos SN, Jbabdi S, Andersson JL, Behrens TE, Glasser MF, Van Essen DC, Yacoub E Consortium WU-MH. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage. 2013;80:80–104. doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stab D, Wech T, Breuer FA, Weng AM, Ritter CO, Hahn D, Kostler H. High resolution myocardial first-pass perfusion imaging with extended anatomic coverage. J Magn Reson Imaging. 2014;39(6):1575–1587. doi: 10.1002/jmri.24303. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Adluru G, Chen L, Kholmovski EG, Bangerter NK, DiBella EV. Radial simultaneous multi-slice CAIPI for ungated myocardial perfusion. Magn Reson Imaging. 2016;34(9):1329–1336. doi: 10.1016/j.mri.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Stab D, Ritter CO, Breuer FA, Weng AM, Hahn D, Kostler H. CAIPIRINHA accelerated SSFP imaging. Magn Reson Med. 2011;65(1):157–164. doi: 10.1002/mrm.22600. [DOI] [PubMed] [Google Scholar]
- 36.Schmitter S, Moeller S, Wu X, Auerbach EJ, Metzger GJ, Van de Moortele PF, Ugurbil K. Simultaneous multislice imaging in dynamic cardiac MRI at 7T using parallel transmission. Magn Reson Med. 2016 doi: 10.1002/mrm.26180. [DOI] [PubMed] [Google Scholar]
- 37.Weingartner S, Akcakaya M, Basha T, Kissinger KV, Goddu B, Berg S, Manning WJ, Nezafat R. Combined saturation/inversion recovery sequences for improved evaluation of scar and diffuse fibrosis in patients with arrhythmia or heart rate variability. Magn Reson Med. 2014;71(3):1024–1034. doi: 10.1002/mrm.24761. [DOI] [PubMed] [Google Scholar]
- 38.Wong E. Optimized phase schedules for minimizing peak RF power in simultaneous multi-slice RF excitation pulses. Proc Intl Soc Mag Reson Med; Melbourne, Australia. p. 2209. [Google Scholar]
- 39.Auerbach EJ, Xu J, Yacoub E, Moeller S, Ugurbil K. Multiband accelerated spin-echo echo planar imaging with reduced peak RF power using time-shifted RF pulses. Magn Reson Med. 2013;69(5):1261–1267. doi: 10.1002/mrm.24719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kellman P, Herzka DA, Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magn Reson Med. 2014;71(4):1428–1434. doi: 10.1002/mrm.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weingartner S, Messner NM, Budjan J, Lossnitzer D, Mattler U, Papavassiliu T, Zollner FG, Schad LR. Myocardial T1-mapping at 3T using saturation-recovery: reference values, precision and comparison with MOLLI. J Cardiovasc Magn Reson. 2016;18(1):84. doi: 10.1186/s12968-016-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 44.Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46(4):638–651. doi: 10.1002/mrm.1241. [DOI] [PubMed] [Google Scholar]
- 45.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 46.Lakkadi N, Rosen M, Bolan PJ. Design of a Phantom for Multiparametric Quantitative MR Imaging and Spectroscopy. Proc Intl Soc Mag Reson Med; Toronto, Canada. 2015. p. 4285. [Google Scholar]
- 47.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS American Heart Association Writing Group on Myocardial S, Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 48.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 49.Shao J, Rapacchi S, Nguyen KL, Hu P. Myocardial T1 mapping at 3.0 tesla using an inversion recovery spoiled gradient echo readout and bloch equation simulation with slice profile correction (BLESSPC) T1 estimation algorithm. J Magn Reson Imaging. 2016;43(2):414–425. doi: 10.1002/jmri.24999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gensler D, Morchel P, Fidler F, Ritter O, Quick HH, Ladd ME, Bauer WR, Ertl G, Jakob PM, Nordbeck P. Myocardial T1: quantification by using an ECG-triggered radial single-shot inversion-recovery MR imaging sequence. Radiology. 2015;274(3):879–887. doi: 10.1148/radiol.14131295. [DOI] [PubMed] [Google Scholar]
- 51.Higgins DM, Ridgway JP, Radjenovic A, Sivananthan UM, Smith MA. T1 measurement using a short acquisition period for quantitative cardiac applications. Med Phys. 2005;32(6):1738–1746. doi: 10.1118/1.1921668. [DOI] [PubMed] [Google Scholar]
- 52.Jang J, Bellm S, Roujol S, Basha TA, Nezafat M, Kato S, Weingartner S, Nezafat R. Comparison of spoiled gradient echo and steady-state free-precession imaging for native myocardial T1 mapping using the slice-interleaved T1 mapping (STONE) sequence. NMR Biomed. 2016;29(10):1486–1496. doi: 10.1002/nbm.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barth M, Breuer F, Koopmans PJ, Norris DG, Poser BA. Simultaneous multislice (SMS) imaging techniques. Magn Reson Med. 2016;75(1):63–81. doi: 10.1002/mrm.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roujol S, Weingartner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272(3):683–689. doi: 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weingartner S, Messner NM, Zollner FG, Akcakaya M, Schad LR. Black-blood native T1 mapping: Blood signal suppression for reduced partial voluming in the myocardium. Magn Reson Med. 2016 doi: 10.1002/mrm.26378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T(1) mapping. Magn Reson Med. 2014;71(6):2082–2095. doi: 10.1002/mrm.24878. [DOI] [PubMed] [Google Scholar]
- 57.Piechnik SK, Ferreira VM, Dall’Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shao J, Rashid S, Renella P, Nguyen KL, Hu P. Myocardial T1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magn Reson Med. 2017;77(4):1495–1504. doi: 10.1002/mrm.26223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Spatial variability in the MB T1 maps reconstructed with Tikhonov-regularized CG-SENSE using different values for the regularization parameter. Example images of the mid-ventricular slice are provided for one volunteer at four different parameter values. Minimum spatial variability is observed at 0.05. Lower regularization parameters cause noise-induced spatial inhomogeneity, while higher values lead to residual leakage artifacts (white arrows).
Figure S2: Single- and multi-band T1 maps acquired in six healthy subjects. Multi-band data was reconstructed using slice GRAPPA. Visually comparable T1 map quality with largely homogeneous myocardial T1 and clear delineation towards the blood-pools can be observed with both T1 mapping sequences. Slightly increased spatial inhomogeneity is observed in the multi-band T1 maps.