Abstract
Williams-Beuren Syndrome (WBS) is a chromosomal microdeletion syndrome typically presenting with intellectual disability, a unique personality, a characteristic facial appearance, and cardiovascular disease. Several clinical features of WBS are thought to be due to haploinsufficiency of elastin (ELN), as the ELN locus is included within the WBS critical region at 7q11.23. Emphysema, a disease attributed to destruction of pulmonary elastic fibers, has been reported in patients without WBS who have pathogenic variants in ELN but only once (in one patient) in WBS. Here we report a second adult WBS patient with emphysema where the diagnosis of WBS was established subsequent to the discovery of severe bullous emphysema. Haploinsufficiency of ELN likely contributed to this pulmonary manifestation of WBS. This case emphasizes the contribution of rare genetic variation in cases of severe emphysema and provides further evidence that emphysema should be considered in patients with WBS who have respiratory symptoms, as it may be under-recognized in this patient population.
Keywords: Williams-Beuren syndrome, emphysema, elastin, intellectual disability
INTRODUCTION
Williams-Beuren syndrome (OMIM 194050), first reported over 50 years ago [Beuren 1962; Williams 1961], is a medical and developmental disorder caused by a loss of contiguous genes in the WBS critical region of chromosome 7 at 7q11.23. Deletions typically are 1.5–1.8 Mb in size and contain 26 to 28 genes, including elastin (ELN) [Pober 2010]. Individuals with WBS have characteristic facial features, cardiovascular abnormalities (particularly supravalvular aortic stenosis), developmental delay and intellectual disability, a social personality, and are at risk for a variety of medical complications [Pober 2010]. The loss of one allele of ELN may underlie several of the medical complications of WBS, such as arterial stenosis and the propensity for hernias and bowel and bladder diverticulae [Cherniske 2004; Pober 2008; Pober 2010; Pober and Morris 2007]. Elastin deficiency or dysfunction has also been implicated in the development of emphysematous lung disease. Elastin-deficient mice and elastin-haploinsufficient mice both develop emphysema-like histopathologic changes, though in haploinsufficient mice this pathology was dependent upon cigarette smoke exposure [Wendel 2000; Shifren 2007]. A rare variant in the terminal exon of ELN (c.2318 G>A, p.Gly773Asp) has been reported as a potential chronic obstructive pulmonary disease (COPD) risk factor in smokers [Kelleher 2005; Cho 2009]. A second variant, predicted to result in elastin haploinsufficiency (c.2T>C, p.Met1Thr), has been reported in a patient with severe pulmonary emphysema and “supra-aortic” stenosis [Louw 2012]. Emphysema is also seen in patients with autosomal dominant cutis laxa, caused by pathogenic variants in ELN that produce dysfunctional elastin products [Callewaert 2011; Rodriguez-Revenga 2004; Urban 2005; Hadj-Rabia 2013].
While patients with WBS are elastin haploinsufficient [Pober 2008], pulmonary manifestations of WBS are not commonly recognized. There has been only one previously-reported case of emphysema in this syndrome [Wan 2010]. Here, we present a second adult patient with both WBS and emphysema. The pulmonary involvement in this case was particularly severe and led to the diagnosis of WBS, illustrating the importance of considering the co-occurrence of these diagnoses.
CLINICAL REPORT
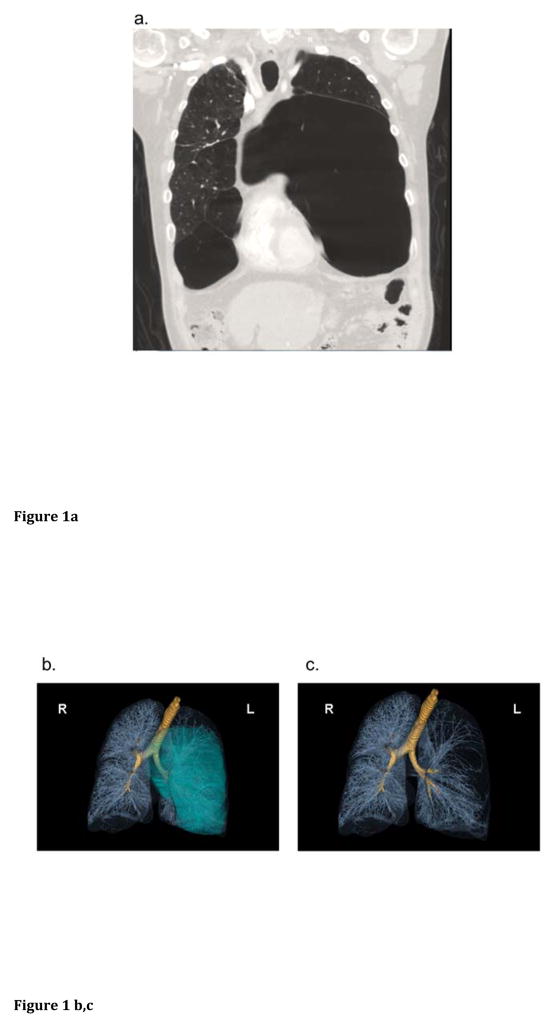
A 49-year-old life-long non-smoking man presented to an outside hospital with worsening dyspnea and a decline in physical activity over a period of weeks. Non-contrast computed tomography (CT) of the chest revealed significant panlobular and centrilobular emphysema with bullous destruction, the largest bulla measuring 25 cm in the left lung [Figure 1a]. An alpha-1 antitrypsin level was within the normal range (119 mg/dL). The severity of the emphysema in a non-smoker, together with a history of intellectual disability, prompted referral for genetic evaluation at a pulmonary genetics center.
Figure 1.
Bullous emphysema in WBS: a) Coronal section of the anterior thorax from reconstructions of a non-contrast chest CT obtained on initial presentation demonstrating bilateral, asymmetric lower lobe emphysematous bullae. b, c) 3 dimensional volume rendered images from repeat CT imaging several months later confirm a large left bulla (blue projection in panel b) extending across the midline into the right hemithorax, as well as causing distortion of vascular structures in the left lung (denoted by the sparse vascularity in panel c). d) SNP Array Result. The common, typical 1.5 Mb deletion in WBS is seen on chromosome 7, encompassing the WBS critical region.
The patient’s developmental history is significant for lack of speech noticed at 1.5 years of age, at which point he was deemed delayed in all of his developmental milestones. He ultimately graduated from a special-needs school. He lives with his father, with the assistance of his siblings. He worked as a bagger at a local supermarket for 26 years. His hobbies include playing the drums, collecting baseball cards, and watching sports.
The patient’s medical history is notable for inguinal and ventral hernia repairs, hypertension, a bladder polyp, and remote, idiopathic microscopic hematuria. Though a lifetime non-smoker, he was exposed to secondhand smoke in utero and in childhood. His family history is notable for COPD in his father, who was a smoker, and emphysema in his maternal grandfather. His mother died at age 84 from congestive heart failure and had a history of multiple aortic valve replacements, attributed to rheumatic fever, but no lung disease.
Physical examination revealed a pleasant and cooperative man, with repetitive rocking behavior when not directly engaged in conversation or tasks. His hair was grey, his eyes were blue and appeared normal, his nose had a bulbous tip. He had small teeth and a full lower lip, with mild prognathism [Figure 2]. His pulmonary exam was notable for significantly decreased breath sounds on the left side. His cardiac exam was normal. His abdominal exam showed diastasis recti and a widened surgical scar from his prior hernia repair. Musculoskeletal exam revealed an inability to supinate his left forearm fully, tightness at the hamstrings and heel cords, bilateral 5th finger camptodactyly, mild kyphosis but no scoliosis, and mild lower extremity lipedema. His skin had a normal degree of laxity and age-appropriate wrinkling. Neurological exam was unremarkable except for mild resting and intention tremor. He had a bright and engaging personality, was making small talk throughout the exam, and enjoyed telling jokes.
Figure 2.
Patient photos from childhood (a) and present day (b) demonstrate classic facial features of WBS including a short, up-turned nose, full cheeks, and a delicate chin in childhood, early greying in adulthood, full lips, and a bulbous nasal tip. a) Childhood photographs obtained with permission from the patient’s family. b) Present day photographs by Rick Guidotti of Positive Exposure, published with permission from the photographer and patient. Patient now uses continuous supplemental oxygen to maintain blood oxygen saturation levels about 88%. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-4833
Due to the history of intellectual disability and developmental delay in addition to the patient’s physical features, social personality, and connective tissue findings, a diagnosis of WBS was suspected. DNA was extracted from uncultured peripheral blood lymphocytes and chromosomal microarray analysis was performed at Brigham and Women’s Hospital using a high-density whole-genome microarray (Cytoscan HD, Affymetrix, Santa Clara, CA). Single-nucleotide polymorphism (SNP) array analysis revealed a 1.5 Mb single copy loss (deletion) encompassing the WBS critical region on chromosome 7 (arr[GRCh37 7q11.23 (72,691,242_74,142,190)x1]) [Figure 1d]. Given the severity of the patient’s pulmonary presentation, screening for additional pathogenic variants in genes known to cause cystic lung disease was performed. The exons and exon-intron boundaries of ELN and seven additional genes – EFEMP2, FBLN5, FLCN, LTBP4, SERPINA1, TSC1 and TSC2 – were sequenced using a commercially-available next generation sequencing assay, the PulmoGene Cystic Lung Disease Panel (Partners Laboratory for Molecular Medicine, Cambridge, MA). This effort confirmed the heterozygous whole gene deletion of ELN but found no additional clinically significant variants.
A chemistry panel revealed a normal calcium level at 9.8 mg/dL (reference range 8.8–10.7 mg/dL). An echocardiogram showed subvalvular narrowing of the pulmonic valve and right ventricular dilation with impaired systolic function. There was thickening of multiple aortic leaflets but no valvular or supravalvular aortic stenosis. An abdominal CT noted two small diaphragmatic hernias, scattered hepatic cysts, and colonic diverticulosis. Spirometric pulmonary function tests showed reduced forced expiratory volume, 1 second (FEV1, 0.76 L, 21% predicted), forced vital capacity (FVC, 2.42 L, 24% predicted), and FEV1/FVC ratio (31, 38% predicted) consistent with severe obstructive lung disease. A repeat chest CT revealed extensive, asymmetric bullous emphysema with basilar panlobular features [Figure 1b,c].
DISCUSSION
We present an adult patient whose diagnosis of Williams-Beuren syndrome (WBS) was suspected in the setting of severe bullous emphysema and intellectual disability. To our knowledge, this is only the second report of an adult with both emphysema and WBS. In contrast to the first case report, however, where the emphysema was detected incidentally on imaging in a patient with an established diagnosis of WBS, the emphysema noted in our patient was particularly severe and clinically impactful, and our patient represents the first example where the emphysema was the presenting manifestation leading to the diagnosis of WBS. It has been noted that WBS patients with a delayed diagnosis in adulthood typically lack the hallmark clinical features of this disorder such as supravalvular aortic stenosis and hypercalcemia [Pober and Morris 2007], as was the case with our patient. Such patients typically present with mild intellectual disability, psychiatric or emotional disorders, gastrointestinal disorders, intra-cardiac lesions or hypertension. However, as many of these problems are common in the general population, these individuals are often unrecognized [Pober and Morris 2007] and the diagnosis is only suspected when accompanied by unusual manifestations.
Genetic disorders such as alpha-1-antitrypsin deficiency, Marfan Syndrome, Loeys-Dietz Syndrome, or cutis laxa may be clinically suspected in patients who present with severe COPD at a young age, particularly non-smokers. To this list, we argue the addition of WBS. While the paucity of reports of emphysema in WBS might suggest it as a rare complication of the disorder, the prevalence of lung involvement may be underestimated. For example, a survey of 16 adolescents and young adults with WBS found that 50% endorsed symptoms of shortness of breath, 43.8% reported cough, and 31.3% reported wheezing requiring medical treatment [Wan 2010]. Pulmonary imaging was not available as part of this study and it is possible that mild emphysema was present in some of these subjects. It is also possible that severe emphysema in WS may not develop until later in adulthood, or that significant symptoms leading to the discovery of emphysema do not emerge until later in life. As with other features of WBS, there are likely multiple factors contributing to the variability in phenotype.
The molecular mechanisms linking elastinopathies and lung disease are not completely understood and likely vary by the type of pathogenic variant. The severe deficits in skin and vascular elasticity seen in patients with autosomal dominant cutis laxa are caused by pathogenic variants in the terminal exons of ELN [Hadj-Rabia 2013]. In contrast, patients with supravalvular aortic stenosis due to proximally situated (relatively 5′) pathogenic mutations do not commonly develop emphysema [Urbán 2000]. One family has been reported with a splice site variant in ELN purported to result in a dominant negative effect in a proband with supravalvular pulmonary artery stenosis and congenital emphysema, and haploinsufficiency in his father, who had aortic valve insufficiency but no pulmonary disease [Graul-Neumann 2008]. In this case, as with autosomal dominant cutis laxa, emphysema is likely a consequence of ELN variants that confer a dominant negative effect [Callewaert 2011; Hu 2010; Rodriguez-Revenga 2004; Urban 2005].
To this, both human and murine evidence suggest that elastin haploinsufficiency can also lead to the development of emphysema, but is likely not sufficient [Shifren 2007]. Eln −/− mice, who are completely deficient in elastin, spontaneously develop congenital emphysema-like pulmonary lesions [Wendel 2000], as do transgenic mice in which elastin expression is reduced to one-third of normal levels [Shifren 2007]. In contrast, haploinsufficient mice (Eln +/-) do not manifest pulmonary pathology spontaneously, and only develop severe emphysema upon exposure to cigarette smoke [Shifren 2007]. These data, together with observed co-segregation of reduced lung function and a loss of function ELN variant among smokers [Kelleher 2005], suggests elastin haploinsufficiency as a COPD susceptibility factor whose effects are mediated through interaction with exposure to cigarette smoke. Additional evidence for this is provided by a recently-reported family in which a pathogenic variant in ELN resulted in peripheral pulmonary stenosis and aortic stenosis but no pulmonary manifestations in a six year-old child and both supravalvular aortic stenosis and severe pulmonary emphysema in his father, who had a significant smoking history [Louw 2012]. While our patient was a life-long nonsmoker, he was exposed to environmental cigarette smoke, which may have contributed to his lung pathology.
Additional genetic factors may also make certain individuals with WBS more prone to the development of emphysema, such as coinheritance of common COPD-susceptibility genes [Boueiz 2016] or of genes implicated in other monogenic forms of cystic lung disease. We had considered the possibility of another genetic contribution to our patient’s significant pulmonary disease, and analysis of several genes associated with cystic lung disease revealed no additional variants that could contribute to his presentation. However, given the family history of COPD and emphysema, it remains possible that our patient inherited additional, more common COPD-susceptibility variants, or another unknown variant affecting connective tissue that could make him particularly prone to the development of emphysema.
In conclusion, our patient adds to the growing body of literature supporting a role for elastinopathy in the pathogenesis of cystic lung disease. Though considered rare, emphysema can be a significant complication of Williams Beuren syndrome and should be entertained as a possible diagnosis in patients with WBS who present with respiratory symptoms. Furthermore, we recommend advising all individuals with WBS to avoid smoking and to minimize exposure to secondhand smoke as much as possible. Additional research is needed to better characterize the pulmonary manifestations of WBS and to understand the phenotypic variability observed in the setting of haploinsufficiency of elastin. Further insight into the relationship between elastin and emphysema in WBS may also contribute to the understanding of genetic propensity towards emphysema in the general population. Finally, our case illustrates the need to maintain a high index of suspicion for rare genetic causes of lung disease, particularly in patients with disease of unusual severity, early onset, or in the absence of typical environmental risk factors.
Acknowledgments
The authors acknowledge research support from NIH 4 T32 HD 7466-20 (M.H.W.). They report no conflicts of interest. We thank Rick Guidotti of Positive Exposure (www.positiveexposure.org) for his wonderful photography session with our patient and also thank the family for procuring childhood photographs and early developmental information.
References
- Beuren AJ, Apitz J, Harmjanz D. Supravalvular Aortic Stenosis in Association with Mental Retardation and a Certain Facial Appearance. Circulation. 1962;26:1235–1240. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- Boueiz A, Lutz SM, Cho MH, Hersh CP, Bowler RP, Washko GR, Halper-Stromberg E, Bakke P, Gulsvik A, Laird NM, Beaty TH, Coxson HO, Crapo JD, Silverman EK, Castaldi PJ, DeMeo DL for COPDGene and ECLIPSE investigators. Genome-Wide Association Study of the Genetic Determinants of Emphysema Distribution. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201605-0997OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F, Mecham RP, Loeys B, Coucke PJ, De Paepe A, Urban Z. New Insights into the Pathogenesis of Autosomal Dominant Cutis Laxa with Report of Five ELN Mutations. Hum Mutat. 2011;32:445. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR. Multisystem Study of 20 Older Adults with Williams Syndrome. Am J Med Gen Part A. 2004;131:255–264. doi: 10.1002/ajmg.a.30400. [DOI] [PubMed] [Google Scholar]
- Cho MH, Ciulla DM, Klanderman BJ, Hersh CP, Litonjua AA, Sparrow D, Raby BA, Silverman EK. Analysis of Exonic Elastin Variants in Severe, Early-Onset Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Bio. 2009;40:751–755. doi: 10.1165/rcmb.2008-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graul-Neumann LM, Hausser I, Essayie M, Rauch A, Kraus C. Highly Variable Cutis Laxa Resulting from a Dominant Splicing Mutation of the Elastin Gene. Am J Med Genet Part A. 2008;146A:977–983. doi: 10.1002/ajmg.a.32242. [DOI] [PubMed] [Google Scholar]
- Hadj-Rabia S, Callewaert BL, Bourrat E, Kempers M, Plomp AS, Layet V, Bartholdi D, Renard M, De Backer J, Malfait F, Vanakker OM, Coucke PJ, De Paepe AM, Bodemer C. Twenty Patients Including 7 Probands with Autosomal Dominant Cutis Laxa Confirm Clinical and Molecular Homogeneity. Orphanet J Rare Dis. 2013;8:36. doi: 10.1186/1750-1172-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Shifren A, Sens C, Choi J, Szabo Z, Starcher BC, Knutsen RH, Shipley JM, Davis EG, Mecham RP, Urban Z. Mechanisms of Emphysema in Autosomal Dominant Cutis Laxa. Matrix Biol. 2010;29:621–628. doi: 10.1016/j.matbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher CM, Silverman EK, Broekelmann T, Litonjua AA, Hernandez M, Sylvia JS, Stoler J, Reilly JJ, Chapman HA, Speizer FE, Weiss ST, Mecham RP, Raby BA. A Functional Mutation in the Terminal Exon of Elastin in Severe, Early-Onset Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Bio. 2005;33:355–362. doi: 10.1165/rcmb.2005-0206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw JJ, Verleden G, Gewillig M, Devriendt K. Haploinsufficiency of Elastin Gene may Lead to Familial Cardiopathy and Pulmonary Emphysema. Am J Med Genet Part A. 2012;158A:2053–2054. doi: 10.1002/ajmg.a.35464. [DOI] [PubMed] [Google Scholar]
- Pober BR. Williams-Beuren Syndrome. N Engl J Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- Pober BR, Johnson M, Urban Z. Mechanisms and Treatment of Cardiovascular Disease in Williams-Beuren Syndrome. J Clin Invest. 2008;118:1606–1615. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober BR, Morris CA. Diagnosis and Management of Medical Problems in Adults with Williams–Beuren Syndrome. Am J Med Genet C Semin Med Genet. 2007;145C:280–290. doi: 10.1002/ajmg.c.30139. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Iranzo P, Badenas C, Puig S, Carrio A, Mila M. A Novel Elastin Gene Mutation Resulting in an Autosomal Dominant Form of Cutis Laxa. Arch Dermatol. 2004;140:1135–1139. doi: 10.1001/archderm.140.9.1135. [DOI] [PubMed] [Google Scholar]
- Shifren A, Durmowicz AG, Knutsen RH, Hirano E, Mecham RP. Elastin Protein Levels are a Vital Modifier Affecting Normal Lung Development and Susceptibility to Emphysema. Am J Physiol Lung Cell Mol Physiol. 2007;292:L778–L787. doi: 10.1152/ajplung.00352.2006. [DOI] [PubMed] [Google Scholar]
- Urban Z, Gao J, Pope FM, Davis EC. Autosomal Dominant Cutis Laxa with Severe Lung Disease: Synthesis and Matrix Deposition of Mutant Tropoelastin. J Invest Dermatol. 2005;124:1193–1199. doi: 10.1111/j.0022-202X.2005.23758.x. [DOI] [PubMed] [Google Scholar]
- Urbán Z, Michels VV, Thibodeau SN, Davis EC, Bonnefont JP, Munnich A, Eyskens B, Gewillig M, Devriendt K, Boyd CD. Isolated Supravalvular Aortic Stenosis: Functional Haploinsufficiency of the Elastin Gene as a Result of Nonsense-Mediated Decay. Hum Genet. 2000;106:577–588. doi: 10.1007/s004390000285. [DOI] [PubMed] [Google Scholar]
- Wan ES, Pober BR, Washko GR, Raby BA, Silverman EK. Pulmonary Function and Emphysema in Williams-Beuren Syndrome. Am J Med Genet Part A. 2010;152A:653–656. doi: 10.1002/ajmg.a.33300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired Distal Airway Development in Mice Lacking Elastin. Am J Respir Cell Mol Bio. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular Aortic Stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]