Abstract
Hypertension and increased vascular stiffness are viewed as inevitable parts of aging. To elucidate whether the age-related decrease in vascular function is avoidable, we assessed the prevalence, correlates, and prognosis of healthy vascular aging (HVA) in 3196 Framingham Study participants aged ≥50 years. We defined HVA as absence of hypertension and pulse wave velocity <7.6 m/s (mean+2SD of a reference sample aged <30 years). Overall, 566 (17.7%) individuals had HVA, with prevalence decreasing from 30.3% in persons aged 50–59 to 1.0% in those aged ≥70 years. In regression models adjusted for physical activity, caloric intake, and traditional cardiovascular disease (CVD) risk factors, we observed that lower age, female sex, lower body mass index, use of lipid-lowering drugs, and absence of diabetes were cross-sectionally associated with HVA (P<0.001 for all). A unit increase in a cardiovascular health score (Life’s Simple 7) was associated with 1.55-fold (95% confidence interval [CI], 1.38–1.74) age- and sex-adjusted odds of HVA. Over a follow-up of 9.6 years, 391 CVD events occurred. In Cox regression models adjusted for traditional CVD risk factors, including blood pressure, HVA was associated with a hazard ratio of 0.45 (95% CI, 0.26–0.77) for CVD relative to absence of HVA. Although HVA is achievable in individuals acculturated to a Western lifestyle, maintaining normal vascular function beyond age 70 years is challenging. Although our data are observational, our findings support prevention strategies targeting modifiable factors and behaviors, and obesity, in particular, to prevent or delay vascular aging and the associated risk of CVD.
Keywords: Healthy vascular aging, vascular stiffness, aging, hypertension, blood pressure, epidemiology
Introduction
The traditional rule-of-thumb definition of normal systolic blood pressure as “100 plus your age” has not been acceptable after antihypertensive treatment was unequivocally shown to reduce cardiovascular disease (CVD) risk in elderly hypertensive patients a quarter century ago.1–3 Although hypertension at old age is no longer considered harmless, it is still highly prevalent. In middle-aged and elderly non-hypertensive individuals, the residual lifetime risk for hypertension was estimated to be 90%.4 Age-related increases in arterial stiffness and blood pressure are thereby widely accepted as an inevitable part of the aging process in acculturated societies, further exemplified by the widespread use of the term “essential” hypertension. However, age-associated increase in blood pressure is not common in some populations leading traditional hunter-gatherer lifestyles (although these data are limited by modest sample sizes).5–10
An individual’s vascular age can be crudely approximated from systolic blood pressure and pulse pressure, but arterial wall imaging or measurement of arterial stiffness can be used to obtain additional complementary information. Aortic pulse-wave velocity (PWV), for example, is a commonly used surrogate marker of arterial stiffness that is strongly related to CVD morbidity and may be considered a physiological method for quantifying arterial aging.11–14
The prevalence, correlates, and prognosis of “healthy vascular aging” (i.e., lack of age-related increases in arterial stiffness and blood pressure) in a contemporary Western cohort are incompletely understood. The aims of our study were 3-fold: 1) to operationalize the concept of healthy vascular aging using two easily measured vascular traits; 2) to assess the prevalence and correlates of healthy vascular aging in a cohort acculturated to a Western lifestyle; and 3) to evaluate the magnitude of CVD risk associated with unhealthy versus healthy vascular aging.
Methods
Participants
Because age-related arterial stiffening is absent in certain populations, we submit that the definition of normal vascular function should be the same in all age groups.5–9 To capture a distinct sample of individuals who have experienced healthy vascular aging, we considered only participants in late middle age or older (aged ≥50 years) who attended examination 7 (1998–2001) or 8 (2005–2008) of the Offspring cohort (n=3371) and examination 1 of the Third Generation (n=606; 2002–2005) cohort of the Framingham Heart Study (Figure S1). Offspring cohort participants were primarily drawn from examination cycle 7, or alternatively from examination 8 if data from examination 7 were missing. We excluded participants with prevalent CVD (see Cardiovascular Outcomes for detailed description), missing tonometry data, or missing covariates (Figure S1). To examine the association between American Heart Association’s (AHA) Life’s Simple 7 score and healthy vascular aging, we identified a subset of 2086 Offspring cohort participants (of 3197 included in the main analyses) who had data available for all components of the score. Boston University Medical Center’s Institutional Review Board approved all study protocols, and participants provided written informed consent. The characteristics and study protocols of the 2 cohorts have been previously published in detail.15, 16
Baseline Evaluation
At baseline, all participants provided a medical history and underwent a cardiovascular targeted physical examination, and laboratory assessment of CVD risk factors.15 We assessed the participants for cigarette smoking and diabetes mellitus (fasting glucose level of ≥7.0 mmol/L or the use of hypoglycemic medications). In addition, we measured blood pressure (mean of 2 auscultatory values obtained by a physician using a mercury column sphygmomanometer on the left arm of seated participants), PWV, body mass index (BMI), serum total cholesterol, and high-density lipoprotein cholesterol concentrations. We defined blood pressure as the mean of two readings. Participants underwent assessments of physical activity, using a questionnaire and calculation of the Framingham physical activity index, as well as assessments of diet, using data collected via the Harvard semi-quantitative food frequency questionnaire.17, 18
Measurements for Arterial Stiffness and Definition of Healthy Vascular Aging
We evaluated arterial stiffness with carotid-femoral PWV.19 PWV is directly related to aortic wall stiffness. We performed arterial tonometry as previously described after ≥5 minutes rest in the supine position.20, 21 All measurements were performed on the right side of the body. Arterial tonometry with concurrent electrocardiogram was obtained for the femoral and carotid arteries. We estimated the pulse wave transit distances by measuring the direct surface distance between the suprasternal notch and the carotid or femoral sites. We accounted for parallel transmission along the brachiocephalic and carotid arteries and around the aortic arch by taking the difference in distances from suprasternal notch to femoral and carotid sites. The methodology for pulse wave velocity measurement remained the same during all study cycles. PWV was defined as the corrected distance divided by the carotid-femoral transit time delay. We inverted PWV to reduce heteroscedasticity and multiplied it by −1 to restore direction of effect.
We defined healthy vascular aging for individuals age ≥50 years as having both of the following:
Non-hypertensive blood pressure, which constituted <140 mmHg systolic, <90 mmHg for diastolic blood pressure, and absence of hypertensive treatment.
PWV of <7.6 m/s, which was equivalent to the mean + 2 standard deviation (SD) value obtained from a reference sample of individuals who were aged <30 years, with optimal or normal blood pressures, and no additional CVD risk factors.22
Cardiovascular Health Score
For each participant, we calculated a cardiovascular health score by recoding 7 modifiable risk factors as dichotomous variables according to the American Heart Association’s (AHA) Life’s Simple 7 score, a metric that is used to measure and promote healthy lifestyle behaviours.23 The 7 components of the cardiovascular health score are: fasting blood glucose, cholesterol, resting blood pressure, BMI, self-reported smoking status, dietary quality, and physical activity (Table S1). We modified the score by excluding blood pressure as it is a part of the outcome variable in this study. Each component of the cardiovascular health was allocated a score of 1 indicating the AHA ideal category for that metric (versus 0 for non-ideal metric); thus, the cardiovascular health score could vary from a minimum of 0 (indicating poor cardiovascular health) to a maximum of 6 (reflecting ideal cardiovascular health).
Cardiovascular Outcomes
We used 2 composite CVD endpoints in our study: hard CVD events and CVD events. Hard CVD was a composite endpoint of cardiovascular death, fatal or nonfatal myocardial infarction, and stroke. CVD was a composite endpoint of angina pectoris, unstable angina (prolonged ischemic episode with documented reversible ST-segment changes), transient ischemic attack, heart failure, intermittent claudication, and all endpoints included in hard CVD. We obtained medical records for all hospitalizations and physician visits related to CVD during follow-up, which were reviewed by an adjudication panel consisting of 3 investigators. Criteria for these CVD events have been described previously.24
Statistical Methods
We assessed baseline characteristics according to healthy vascular aging status, overall and by sex. We compared prevalence of healthy vascular aging among age groups using the chi-squared test.
We examined cross-sectional correlates of healthy vascular aging using multivariable stepwise logistic regression with backward selection, retaining covariates with P<0.10. In addition to cohort (Offspring vs. Third Generation), we derived the other covariates for the logistic regression models from the Framingham 10-year CVD risk score (i.e., age, sex, cohort, BMI, smoking status, diabetes mellitus, serum total cholesterol and high-density lipoprotein cholesterol). We included total caloric intake and the Framingham Physical Activity Index in a separate model (2671 participants had those data). We standardized continuous variables and tested for statistical interactions with age (using median age) and sex interactions by entering corresponding cross-product terms into the models. We also performed a sensitivity analysis in 3111 individuals with data available using a 3-category variable to define hyperglycemia: normal glycemia (plasma fasting glucose <5.6 mmol/L), impaired fasting glucose (plasma fasting glucose 5.6–6.9 mmol/L), and diabetes (plasma fasting glucose ≥ 7.0 mmol/L or use of hypoglycemic medications). To characterize individuals with HVA in more detail beyond traditional risk factors, we determined serum high sensitivity C-reactive protein, interleukin-6, estimated glomerular filtration rate, homeostatic model assessment insulin resistance index (HOMA-IR), and urine albumin:creatinine ratio levels, and a genetic risk score for coronary artery disease in 1418–3190 individuals with such data available. We used generalized linear models to compare age- and sex-adjusted means of genetic, inflammatory, renal, and metabolic biomarker levels between individuals with and without healthy vascular aging. The methods for determining these biomarkers are described in more detail in the Supplementary Methods. Next, we calculated age- and sex-adjusted odds ratios for healthy vascular aging comparing participants who had a health scores of 2 through 6 to those with scores of 0–1.
We examined associations between healthy vascular aging and CVD outcomes using proportional hazards regression (Cox) models: plotting age-, sex- and cohort-adjusted cumulative incidence functions25 and also fitting multivariable-adjusted models. We performed a sensitivity analysis for risk of CVD outcomes using 3 alternative definitions for healthy vascular aging: 1) blood pressure <140/90 mmHg and PWV <8.1 m/s (corresponding to the 95th percentile for PWV in a healthy Framingham Heart Study reference sample <50 years);26 2) blood pressure <120/80 mmHg and PWV <7.6 m/s; and 3) blood pressure <140/90 mmHg and absence of coronary artery calcification (Agatston score 0). The latter analysis was performed in a subgroup of 1332 participants who underwent coronary artery calcium scoring, as previously described.27 All analyses were performed with SAS software version 9.4 (Cary, North Carolina, USA). We considered a two-sided p<0.05 to be statistically significant.
Results
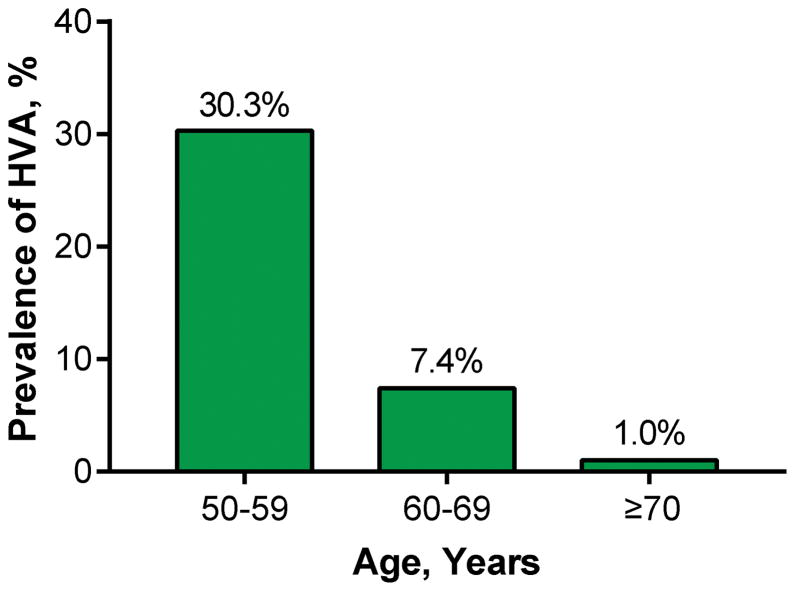
Characteristics of the sample (n=3196, mean age 62±9 years, 56% women) according to healthy vascular aging status are presented in Table 1. The characteristics of the included and excluded participants are shown in Table S2. Of the 490 persons who were excluded because of prevalent CVD, only 14 (2.9%) were non-hypertensive and had a PWV <7.6 m/s. Among participants, 566 (17.7%) had healthy vascular aging. Men and women with healthy vascular aging had more favorable CVD risk profiles than others (Table 1). Prevalence of healthy vascular aging decreased from 30.3% in persons aged 50–59 to 1.0% in those aged ≥70 years (Figure 1, P<0.001).
Table 1.
Baseline Characteristics According to Healthy Vascular Aging Status.
| Characteristic | Overall | Men | Women | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HVA (n=566) | No HVA (n=2630) | HVA (n=164) | No HVA (n=1240) | HVA (n=402) | No HVA (n=1390) | |
| Cohort | ||||||
| Offspring | 402 (71) | 2259 (86) | 109 (66) | 1042 (84) | 293 (73) | 1217 (88) |
| Third Generation | 164 (29) | 371 (14) | 55 (34) | 198 (16) | 109 (27) | 173 (12) |
| Age, y | 55.0±4.7 | 63.0±8.6 | 54.8±4.9 | 62.4±8.6 | 55.1±4.7 | 63.6±8.6 |
| Women | 402 (71) | 1390 (53) | 0 (0) | 0 (0) | 402 (100) | 1390 (100) |
| Systolic BP, mmHg | 113±11 | 130±18 | 116±10 | 130±16 | 111±11 | 130±19 |
| Diastolic BP, mmHg | 71±8 | 76±10 | 74±7 | 77±10 | 70±8 | 74±10 |
| PWV, m/s | 6.8±0.5 | 10.4±3.2 | 6.9±0.5 | 10.6±3.3 | 6.8±0.5 | 10.3±3.2 |
| HTN Medication | 0 (0) | 1103 (42) | 0 (0) | 531 (43) | 0 (0) | 572 (41) |
| BMI, kg/m2 | 25.1±4.0 | 28.3±5.1 | 26.2±3.2 | 28.8±4.4 | 24.7±4.2 | 27.9±5.6 |
| Diabetes mellitus | 3 (0.5) | 286 (10.9) | 1 (0.6) | 156 (12.6) | 2 (0.5) | 130 (9.4) |
| Current Smoker | 83 (15) | 275 (10) | 18 (11) | 135 (11) | 65 (16) | 140 (10) |
| Cholesterol, mmol/l | 5.2±0.9 | 5.1±1.0 | 5.1±0.9 | 4.9±0.9 | 5.2±0.9 | 5.4±1.0 |
| HDL cholesterol, mmol/l | 1.7±0.5 | 1.4±0.5 | 1.3±0.3 | 1.2±0.4 | 1.8±0.5 | 1.6±0.5 |
| Lipid-lowering medication | 64 (11) | 712 (27) | 26 (16) | 353 (29) | 38 (9) | 359 (26) |
| Physical activity index | 37.6±6.4 | 37.9±6.6 | 38.5±7.3 | 38.5±7.3 | 37.3±5.9 | 37.3±5.8 |
| Total caloric intake, kcal/d | 1855±591 | 1868±662 | 2078±642 | 2000±727 | 1769±548 | 1751±574 |
Data are shown as mean±SD for continuous variables and as n (%) for categorical variables. Data on physical activity index and total caloric intake were available for 2887 and 2833 participants, respectively. HVA, healthy vascular aging; BP, blood pressure; PWV, pulse wave velocity; HTN, hypertension; BMI, body mass index; HDL, high density lipoprotein.
Figure 1.
Prevalence of Healthy Vascular Aging in Age Groups of 50–59 (n=1610), 60–69 (n=969), and ≥70 years (n=617). HVA, healthy vascular aging.
In backward selection logistic regression modeling (Table 2), we observed that younger age, female sex, lower BMI, use of lipid-lowering drugs, and absence of diabetes (P<0.001 for all) were cross-sectionally associated with healthy vascular aging. Current smoking, serum total cholesterol, and serum high-density lipoprotein cholesterol were not retained. No statistically significant interactions were observed between age or sex and any of the significant correlates of healthy vascular aging in the multivariable model (P≥0.27 for all). In a subsample of 2671 participants with data available, we also included physical activity index and total caloric intake in the model for healthy vascular aging. Neither variable reached statistical significance (P≥0.10 for both). In a sensitivity analysis using a 3-category variable for hyperglycemia, impaired fasting glucose (odds ratio 0.63; 95% confidence interval [CI], 0.50–0.81; P=0.03) and diabetes (odds ratio 0.10; 95% CI, 0.03–0.32; P<0.001) were associated with lower odds of healthy vascular aging compared to normal glycemia. The age- and sex-adjusted mean genetic, inflammatory, renal, and metabolic biomarker levels according to healthy vascular aging status are presented in Table S2. We observed that individuals with healthy vascular aging had lower interleukin-6, high sensitivity C-reactive protein, and HOMA-IR levels than participants with no healthy vascular aging. No between-group differences were observed in renal function or in the genetic risk score for coronary artery disease.
Table 2.
Multivariable Logistic Regression for Odds of Healthy Vascular Aging Versus No Healthy Vascular Aging.
| Characteristic | OR for HVA (95% CI) | P value |
|---|---|---|
| Age, y | 0.20 (0.17–0.24) | <0.001 |
| Female sex | 2.10 (1.68–2.64) | <0.001 |
| BMI, kg/m2 | 0.50 (0.44–0.57) | <0.001 |
| Diabetes mellitus | 0.12 (0.04–0.39) | <0.001 |
| Lipid-lowering therapy | 0.68 (0.50–0.93) | 0.01 |
| Total cholesterol, mmol/l | 0.90 (0.80–1.005) | 0.06 |
566 individuals had healthy vascular aging whereas 2630 did not. Odds ratios for BMI, total cholesterol, and age are reported per 1-SD increase for comparability. Odds ratios are adjusted for cohort. High-density lipoprotein cholesterol and smoking did not meet the threshold P value of 0.10 and were not retained in in the backward selection process. HVA, healthy vascular aging; OR, odds ratio; CI, confidence interval; BMI, body mass index.
The odds ratios for healthy vascular aging according to the cardiovascular health score in 2086 participants (mean age 63.2±8.3, 57.1% women) of the Offspring cohort are shown in Table 3. Compared with participants who achieved 0–1 goals, the odds ratio for healthy vascular aging was significantly increased in participants who achieved 3 goals (odds ratio, 3.11; 95% CI, 1.67–5.79). Participants who achieved 6 goals had 10.23-fold (95% CI, 3.85–27.16) odds of healthy vascular aging. A 1-unit increase in the cardiovascular health score corresponded to 1.55-fold (95% CI, 1.38–1.74) odds of healthy vascular aging.
Table 3.
Association of Cardiovascular Health Score with Healthy Vascular Aging (n=2086).
| Cardiovascular Health Score* | No. of Participants | No. with HVA | OR (95% CI) | P Value |
|---|---|---|---|---|
| Per 1-unit increase | 2086 | 312 | 1.55 (1.38–1.74) | <0.001 |
| Goals achieved | ||||
| 0–1 | 221 | 14 | 1.00 (reference) | - |
| 2 | 562 | 51 | 1.62 (0.85–3.09) | 0.15 |
| 3 | 625 | 95 | 3.11 (1.67–5.79) | <0.001 |
| 4 | 435 | 70 | 2.89 (1.52–5.50) | 0.001 |
| 5 | 209 | 68 | 7.36 (3.75–14.44) | <0.001 |
| 6 | 34 | 14 | 10.23 (3.85–27.16) | <0.001 |
One additional point for each low-risk health metric achieved (physical activity, diet, BMI, smoking, total cholesterol, and fasting glucose). All analyses are adjusted for sex, age, and cohort. HVA, healthy vascular aging; OR, odds ratio; CI, confidence interval.
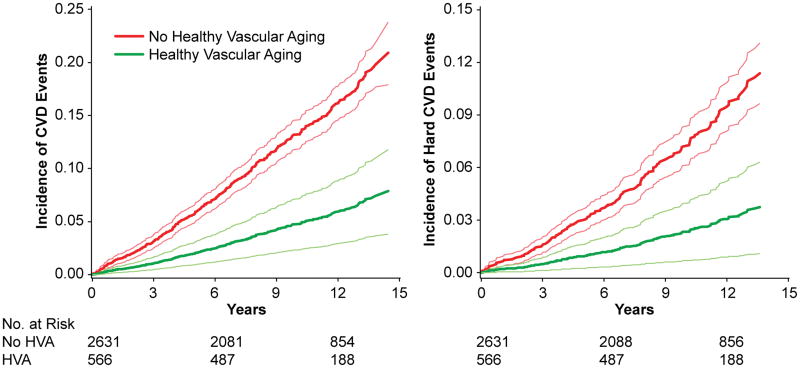
During follow-up (median 9.6 years), 391 developed CVD, which included 207 hard CVD events. The direct-adjusted plots for incidence of CVD and hard CVD are shown in Figure 2. Individuals with healthy vascular aging had considerably lower age- and sex-adjusted risk of CVD (hazard ratio, 0.34; 95% CI, 0.20–0.57) and hard CVD (hazard ratio, 0.31; 95 CI, 0.15–0.64) in relation to individuals without healthy vascular aging (Table 4). After adjusting for other traditional CVD risk factors, including systolic blood pressure, healthy vascular aging was associated with a 0.45-fold (95% CI, 0.26–0.77) risk of CVD events and a 0.46-fold risk (95% CI, 0.22–0.96) of hard CVD events. No statistically significant interactions were observed between age or sex and healthy vascular aging in the multivariable model (P≥0.34 for all). We also performed 3 separate sensitivity analyses, in which healthy vascular aging was defined as 1) blood pressure <140/90 mmHg and PWV <8.1 m/s; 2) blood pressure <120/80 mmHg and PWV <7.6 m/s; or 3) blood pressure <140/90 mmHg and coronary artery calcium score of 0 (Table S3). In these sensitivity analyses, the multivariable-adjusted associations between healthy vascular aging and CVD outcomes were statistically non-significant, except for the relationship between healthy vascular aging defined as blood pressure <120/80 mmHg and PWV <7.6 m/s, and CVD.
Figure 2.
Direct-Adjusted Incidence Curves for Cardiovascular Events. Curves are adjusted for age, sex, and cohort. Thin, light lines represent 95% confidence limits. HVA, healthy vascular aging; CVD, cardiovascular disease.
Table 4.
Risk of Cardiovascular Events Related to Healthy vs. unhealthy Vascular Aging (n=3196).
| Model | CVD (391 events) | Hard CVD (207 events) | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Model 1: Adjusted for sex, age, and cohort. | 0.34 (0.20–0.57) | <0.001 | 0.31 (0.15–0.64) | 0.002 |
| Model 2: Model 1+BMI, HDL-C, cholesterol, DM, lipid meds and smoking | 0.40 (0.23–0.68) | 0.001 | 0.37 (0.18–0.77) | 0.008 |
| Model 3: Model 2+systolic blood pressure | 0.45 (0.26–0.77) | 0.004 | 0.46 (0.22–0.96) | 0.04 |
Only 15 CVD and 8 hard CVD events occurred in participants with healthy vascular aging. CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; BMI, body mass index; HDL-C, high–density lipoprotein cholesterol; DM, diabetes mellitus.
Discussion
In the present investigation, we operationalize the theoretical construct of healthy vascular aging using blood pressure and PWV. Although modest improvements in risk discrimination may be achieved using multivariable risk scores to define HVA (incorporating additional measures of vascular aging), we opted to use these two easily obtained metrics of vascular health for simplicity of interpretation of results. We observed that every sixth person aged ≥50 years had experienced healthy vascular aging, defined as absence of hypertension and CFPWV <7.6 m/s. However, healthy vascular aging was virtually nonexistent in persons aged ≥70 years. Modifiable risk factors included in AHA’s Life’s Simple 7 score, and particularly low BMI, having no diabetes, and hypercholesterolemia (lipid-lowering therapy and borderline significance for serum total cholesterol), were associated with healthy vascular aging. Individuals with healthy vascular aging had a markedly lower risk of CVD events, even after accounting for traditional CVD risk factors.
The concept of healthy vascular aging may not have received notable attention previously because of the very strong correlation between age and arterial stiffness.19, 28 A large part of recent research on vascular aging has therefore focused on “early vascular aging”, an even more rapid course of vascular aging that results in premature CVD manifestations.28 However, both past and more contemporary research suggest that vascular aging, just as isolated systolic hypertension, should no longer be considered a part of normal aging. Studies performed on hunter-gatherers have demonstrated the low rates of age-related increases in blood pressure,5–10 pulse wave velocity,7 and CVD6 in these populations. Although some of these studies included very few individuals aged >50 years,10 their results also showed that blood pressure levels and arterial stiffness in these populations increased with the level of Western acculturation and urbanization.6, 7, 9 These interesting findings seem to be mainly driven by environmental, instead of genetic, factors as blood pressure levels and arterial stiffness in these populations were shown to increase with the level of Western acculturation and urbanization.6, 7, 9 Our findings on the statistically non-significant differences in the genetic coronary artery disease risk scores also are consistent with this premise. More contemporary results from the Systolic Blood Pressure Intervention Trial (SPRINT) also imply that an individual’s blood pressure should remain optimal even after middle age. In the SPRINT trial with a study population of hypertensive patients without diabetes aged ≥50 years, targeting a systolic blood pressure <120 mmHg, as compared with <140 mmHg, resulted in lower rates of major cardiovascular events and death from any cause. The SPRINT results were also similar in subgroups by age (<75 and ≥75 years), suggesting that “lower is better” applies to blood pressure values in all age groups and to treated and untreated individuals without diabetes. Healthy vascular aging should, therefore, be considered a universal goal. Our results demonstrate that healthy vascular aging, defined as non-hypertension and PWV <7.6 m/s at age ≥50 years, is achievable and provides an effective method to distinguish between individuals with low versus high cardiovascular risk. However, maintaining a youthful vascular function beyond age 70 is extremely challenging, which underscores the need for lifelong prevention of arterial stiffening.
Our results demonstrate that maintaining a lifestyle and a cardiovascular risk profile that are in accord with the Life’s Simple 7 health factors are important for preventing arterial aging.23 Besides non-modifiable risk factors, obesity and diabetes were the strongest correlates of healthy vascular aging in our study. We also observed lower levels of inflammatory biomarkers and HOMA-IR in individuals with healthy vascular aging. Our findings, therefore, are consistent with the notion that insulin resistance and chronic low-grade inflammation, both closely related to obesity and diabetes, could be potential contributors to vascular aging.29 Somewhat surprisingly, we did not observe an independent association between some cardiovascular risk factors, such as smoking, diet, and exercise, and healthy vascular aging. In addition to lack of statistical power and small effect sizes, these findings may be also explained by the mediating effects of BMI on the relations of diet and/or exercise and healthy vascular aging. Furthermore, the association between smoking and vascular function is not straightforward as paradoxically lower rates of hypertension and decreased arterial stiffness have been observed among smokers in some epidemiological studies.30, 31 Although smoking, diet, and exercise may not be equally strong correlates of healthy vascular aging as obesity, diabetes, or hypercholesterolemia, our results showed a gradual stepwise increase in the odds of healthy vascular aging across the whole range of Life’s Simple 7 score. Our results are, therefore, suggestive that controlling all the modifiable factors included in Life’s Simple 7 is important for achieving healthy vascular aging. Specifically, our results should not be interpreted to indicate that smoking, diet or physical activity are not related to vascular aging. We have previously reported that physical activity (measured by accelerometry) is associated with lower vascular stiffness.32
In a previous study, the Multi-Ethnic Study of Atherosclerosis researchers examined a group of 165 individuals that had survived to ≥70 years free of clinical CVD and also had limited subclinical vascular disease by a combination index of 3 separate parameters (coronary artery calcium <25th percentile, carotid intima-media thickness <25th percentile, and ankle-brachial index >0.9).33 In accordance with our results, the authors found that younger age, female sex, and lower BMI were most strongly (P value of <0.001) associated with the combination index. In addition to these 3 factors, diabetes also seems to markedly decrease the odds of healthy vascular aging – individuals with diabetes had over 8-fold lower odds of healthy vascular aging than non-diabetics in our study. Considering that >80% of people with type 2 diabetes are also obese, our results highlight the importance of maintaining normal body weight in preventing cardiovascular aging.34 Despite the marked reductions in the prevalence of cardiovascular risk factors such as smoking and hypercholesterolemia in developed countries, there has been little success in preventing obesity, the most important risk factor for diabetes.35, 36 Over the past 30 years, age-standardized mean global BMI has increased from 22 kg/m2 to 24 kg/m2, which conjointly with population growth and ageing, has led to a near quadrupling of the number of adults with diabetes worldwide.37, 38 The impact of the global obesity epidemic is also reflected by recent plateauing of hypertension rates in the United States.39 Healthy vascular ageing will therefore likely remain infrequent unless adverse trends in population nutrition and BMI occur.40
We observed a 3-fold lower age- and sex-adjusted risk, and an over 2-fold lower multivariable-adjusted risk of CVD in persons with healthy vascular aging relative to absence of healthy vascular aging. Because the final model (Model 3 in Table 4) also included systolic blood pressure as a covariate, these results mainly reflect the poor CVD prognosis associated with arterial stiffening.12, 19 Although these estimates are not robust enough for healthy vascular aging to be used as a screening method, they still accentuate the importance of vascular stiffening and arterial aging in the pathogenesis of CVD. In sensitivity analyses, we examined the association between healthy vascular aging and CVD outcomes using three alternate definitions of healthy vascular aging. Using a definition of blood pressure 140/90 mmHg and a higher PWV threshold of <8.1 m/s, the risk estimates of CVD related to healthy vascular aging were statistically nonsignificant, possibly reflecting lesser statistical power due to very few events in the group with HVA; of note, points estimates for HVA in these analyses were all less than 1.00 and the p values were borderline statistical significant (<0.10). With the other two alternate definitions of healthy vascular aging (a blood pressure threshold of 120/80 mmHg instead of 140/90 mmHg, or coronary calcium scoring instead of pulse wave velocity), the risk estimates were, although mainly nonsignificant, comparable to those observed in the primary analyses. However, because of the low number of CVD events among individuals with healthy vascular aging we are lack statistical power to make any definite conclusions. In spite the fact that arterial stiffening, and the increased CVD risk associated with it, can be detected with relative ease, treating it remains a considerable challenge for physicians. Lifestyle factors and certain antihypertensive drugs have been shown to reduce arterial stiffness mainly through lowering blood pressure, but there are currently no drugs that specifically target arterial stiffening.41, 42 The most promising drug candidates in this area are nitric oxide donors, drugs interfering with the arterial extracellular matrix, selective angiotensin type 2 receptor agonists, and agents reducing arterial calcification, but clinical data are still pending.41–43 However, given the notable CVD risk associated with arterial stiffening, the clinical need for “destiffening” therapies remains significant.
Our study has certain limitations. First, our study sample consisted of predominantly white individuals and our results may not be generalizable to the other races/ethnicities. This is a major limitation of our investigation given the known racial disparities in hypertension and hypertension-related outcomes.44 Second, data on nutrition and physical exercise were not available for all individuals. Third, validated self-report questionnaires may not be the most accurate methods for assessing food intake and physical activity, despite being the most commonly used methods in epidemiological settings. Fourth, we excluded from the present analyses individuals who died before reaching 50 years of age and had prevalent CVD. Nearly all individuals who were excluded due to prevalent CVD had unhealthy vascular aging. We therefore studied healthy vascular aging in the context of generally fairly healthy aging (i.e. absence of clinical disease). Fifth, some of the analyses in our study are cross-sectional (precluding any causal inferences) and raising the additional possibility of reverse causation between healthy vascular aging and some of the exposure variables (such as lower ability to exercise in individuals with arterial stiffening).
Supplementary Material
Perspectives.
1 in 6 individuals experienced healthy vascular aging in our sample aged ≥50 years. Although healthy vascular aging is achievable in individuals acculturated to a Western lifestyle, our results demonstrate that maintaining normal vascular function beyond age 70 is extremely challenging. Our data, which are observational, thereby precluding causal inferences, are consistent with the notion that prevention strategies targeting modifiable factors and behaviors included in Life’s Simple 7, and obesity in particular, may prevent or delay vascular aging and the associated risk of CVD.
Novelty and Significance.
1) What is New?
Although hypertension and increased vascular stiffness are seen as inevitable parts of the aging process in acculturated societies, these traits are not common in hunter-gatherer populations (although prior studies are limited by the small number of individuals over age 50 years who were studied).
To elucidate whether the age-related decrease in vascular function is unavoidable, we assessed the prevalence, correlates, and prognosis of healthy vascular aging (HVA) in a western community-dwelling cohort.
2) What is Relevant?
The prevalence of HVA, defined as absence of hypertension and pulse wave velocity <7.6 m/s, decreased from 30% in persons aged 50–59 to 1% in those aged ≥70 years.
Lower body mass index, absence of diabetes, and a higher American Heart Association’s Life’s Simple 7 score were the strongest modifiable correlates of HVA.
HVA was associated with a 55% lower multivariable-adjusted risk for cardiovascular disease relative to absence of HVA.
3) Summary
Although healthy vascular aging is achievable in individuals acculturated to a Western lifestyle, our results demonstrate that maintaining normal vascular function beyond age 70 is extremely challenging. Our observational data are consistent with the notion that prevention strategies targeting modifiable factors and behaviors included in Life’s Simple 7, and obesity in particular, may prevent or delay vascular aging and the associated risk of CVD.
Acknowledgments
We thank the participants of the Framingham Heart Study for invaluable contributions to this work.
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contracts N01-HC-25195 and HHSN268201500001I) and NIH grants 1R01HL126136-01A1; 5R01HL107385-04; 1R01HL60040; 1RO1HL70100; HL094898; DK082447; HL107385; HL104184; and HL126136.
Footnotes
Disclosures
Dr. Mitchell is owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck, Servier, and Philips.
References
- 1.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 2.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 3.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 5.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 6.Page LB, Damon A, Moellering RC., Jr Antecedents of cardiovascular disease in six Solomon Islands societies. Circulation. 1974;49:1132–1146. doi: 10.1161/01.cir.49.6.1132. [DOI] [PubMed] [Google Scholar]
- 7.Lemogoum D, Ngatchou W, Janssen C, Leeman M, Van Bortel L, Boutouyrie P, Degaute JP, Van de Borne P. Effects of hunter-gatherer subsistence mode on arterial distensibility in Cameroonian pygmies. Hypertension. 2012;60:123–128. doi: 10.1161/HYPERTENSIONAHA.111.187757. [DOI] [PubMed] [Google Scholar]
- 8.Truswell AS, Kennelly BM, Hansen JD, Lee RB. Blood pressures of Kung bushmen in Northern Botswana. Am Heart J. 1972;84:5–12. doi: 10.1016/0002-8703(72)90299-2. [DOI] [PubMed] [Google Scholar]
- 9.Sever PS, Gordon D, Peart WS, Beighton P. Blood-pressure and its correlates in urban and tribal Africa. Lancet. 1980;2:60–64. doi: 10.1016/s0140-6736(80)92940-2. [DOI] [PubMed] [Google Scholar]
- 10.Gurven M, Blackwell AD, Rodriguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension. 2012;60:25–33. doi: 10.1161/HYPERTENSIONAHA.111.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 16.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RBS, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 22.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, Wolf PA, Garrison RJ, editors. Framingham Heart Study, 30 Year Follow-Up. Bethesda, MD: US Department of Health and Human Services; 1987. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. [Google Scholar]
- 25.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35:669–674. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann U, Siebert U, Bull-Stewart A, Achenbach S, Ferencik M, Moselewski F, Brady TJ, Massaro JM, O’Donnell CJ. Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort--consequences for progression studies. Eur J Radiol. 2006;57:396–402. doi: 10.1016/j.ejrad.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag. 2008;4:547–552. doi: 10.2147/vhrm.s1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cefalu WT. Inflammation, insulin resistance, and type 2 diabetes: back to the future? Diabetes. 2009;58:307–308. doi: 10.2337/db08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green MS, Jucha E, Luz Y. Blood pressure in smokers and nonsmokers: epidemiologic findings. Am Heart J. 1986;111:932–940. doi: 10.1016/0002-8703(86)90645-9. [DOI] [PubMed] [Google Scholar]
- 31.Omvik P. How smoking affects blood pressure. Blood Press. 1996;5:71–77. doi: 10.3109/08037059609062111. [DOI] [PubMed] [Google Scholar]
- 32.Andersson C, Lyass A, Larson MG, Spartano NL, Vita JA, Benjamin EJ, Murabito JM, Esliger DW, Blease SJ, Hamburg NM, Mitchell GF, Vasan RS. Physical activity measured by accelerometry and its associations with cardiac structure and vascular function in young and middle-aged adults. J Am Heart Assoc. 2015;4:e001528. doi: 10.1161/JAHA.114.001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michos ED, Rice KM, Szklo M, Burke GL, Siscovick DS, Tracy RP, Barr RG, Nettleton JA, Greenland P, Jacobs DR, Jr, Post W. Factors associated with low levels of subclinical vascular disease in older adults: Multi-Ethnic Study of Atherosclerosis. Prev Cardiol. 2009;12:72–79. doi: 10.1111/j.1751-7141.2008.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Nunez L, Gudbjornsdottir S, Eliasson B. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52:65–73. doi: 10.1007/s00125-008-1190-x. [DOI] [PubMed] [Google Scholar]
- 35.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 36.Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek CJ, Singh GM, Lin JK, Stevens GA, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Cholesterol) National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 37.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 40.Mellen PB, Gao SK, Vitolins MZ, Goff DC. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114. [DOI] [PubMed] [Google Scholar]
- 42.Wu CF, Liu PY, Wu TJ, Hung Y, Yang SP, Lin GM. Therapeutic modification of arterial stiffness: An update and comprehensive review. World J Cardiol. 2015;7:742–753. doi: 10.4330/wjc.v7.i11.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59:291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158. [DOI] [PubMed] [Google Scholar]
- 44.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348:135–138. doi: 10.1097/MAJ.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.