Abstract
Background
Vitamin D has neuroprotective and immunomodulatory properties, and deficiency is associated with worse stroke outcomes. Little is known about effects of hypoxia-ischemia or hypothermia treatment on vitamin D status in neonates with hypoxic-ischemic encephalopathy (HIE). We hypothesized vitamin D metabolism would be dysregulated in neonatal HIE altering specific cytokines involved in Th17 activation, which might be mitigated by hypothermia.
Methods
We analyzed short term relationships between 25(OH) and 1,25(OH)2 vitamin D, vitamin D binding protein, and cytokines related to Th17 function in serum samples from a multicenter randomized controlled trial of hypothermia 33°C for 48h after HIE birth versus normothermia in 50 infants with moderate to severe HIE.
Results
Insufficiency of 25(OH) vitamin D was observed after birth in 70% of infants, with further decline over the first 72h, regardless of treatment. 25(OH) vitamin D positively correlated with antiinflammatory cytokine IL-17E in all HIE infants. However, Th17 cytokine suppressor IL-27 was significantly increased by hypothermia, negating the IL-27 correlation with vitamin D observed in normothermic HIE infants.
Conclusions
Serum 25(OH) vitamin D insufficiency is present in the majority of term HIE neonates and is related to lower circulating anti-inflammatory IL-17E. Hypothermia does not mitigate vitamin D deficiency in HIE.
INTRODUCTION
Vitamin D is an important neurosteroid during development and after CNS injury. Deficiency of vitamin D contributes to many diseases that involve systemic or CNS inflammation, and vitamin D deficient adults have worse outcomes after stroke (1, 2). Most vitamin D studies in neonates have focused on its role in mineral metabolism. Little is known about vitamin D status, immunomodulatory function, or effects of hypothermia on vitamin D binding protein (DBP) in neonatal hypoxic-ischemic encephalopathy (HIE).
The significance of vitamin D as an immunomodulator and regulator of pro-inflammatory Th17 lymphocytes has been well established in adult stroke. Adult patients have demonstrated an increased proportion of Th17 lymphocytes within 24 hours (3) and one week after stroke (4). These findings may be pertinent to neonatal HI, as naïve T cells develop into Th17 cells more readily in infants than adults, and may contribute to neonatal inflammatory response to HI injury (5). Vitamin D has been shown to reduce pro-inflammatory Th17 differentiation and proliferation, and IL-17 cytokine production (6), while promoting anti-inflammatory IL-10 and T regulatory cells (7). However, vitamin D degradation is increased in neuroinflammation (8), which may limit its effect as a Th17 immunomodulator after HI. In addition, vitamin D deficiency (<20 ng/ml) and insufficiency (<30 ng/ml) is widespread in human neonates (9). In the only other report on vitamin D status in neonatal HIE, Mutlu et al. demonstrated lower serum 25(OH) vitamin D (25(OH)D) concentrations in 31 cooled HIE infants in Turkey compared with healthy term control infants (10). In this study all HIE infants had serum 25(OH)D < 20ng/ml on day of life 1, and 30% infants had persistently low serum 25(OH)D on day 5.
Circulating concentrations of prohormone 25(OH)D are important for the maintenance of CNS concentrations of active 1,25(OH)2 vitamin D (1,25(OH)2D), which is synthesized in many extra-renal cells, including neuronal and glial cells that contain 1-α-hydroxylase (11). Thus, serum concentrations of 25(OH)D may be crucial for vitamin D’s neuroprotective and immune functions after HI injury, in addition to endocrine roles in calcium and phosphorus homeostasis (2, 12). We hypothesized that vitamin D metabolism would be increased with neonatal HIE, and that low serum 25(OH)D concentrations would adversely affect Th17 cytokines. We further hypothesized that hypothermia therapy would alter vitamin D metabolism and specific cytokines involved in Th17 activation. Using samples from neonates with moderate to severe HIE in the first 3 days after HI birth, we explored 25(OH)D and 1,25(OH)2D serum concentrations, vitamin D binding proteins (DBP and albumin), and circulating cytokines related to Th17 function in addition to calcium and phosphorus relationships.
METHODS
We investigated serum 25(OH)D, 1,25(OH)2D, DBP, albumin, Th17 related cytokines, calcium, and phosphorus concentrations in serum samples stored at −80°C from a multicenter randomized trial of systemic hypothermia in neonates with HIE (13). This study was approved by Institutional Review Boards at all seven participating centers (Medical University of South Carolina, Eastern Virginia Medical School, University of Virginia, Albany Medical Center, State University of New York, Medical College of Georgia, and University of Saskatchewan). Entry criteria and demographic data of this cohort have been published in detail (13). Briefly, newborn infants were at least 35 weeks gestation, 2,000g birth weight, and less than 6h after birth or HI injury, with signs of moderate to severe neonatal encephalopathy, and were randomization to either hypothermia (rectal temperature, Tr=33°C) or normothermia (Tr=36.5°C) treatment for 48h after parental consent. Infants with maternal chorioamnionitis, sepsis, and birth weight or head circumference less than 10% were excluded from the study.
Multiplex and Vitamin D Assays
Serum samples were collected at enrollment and every 12h for 72h. Hypothermia was initiated by transport teams at outside hospitals, and most hypothermia infants were cooled for several hours at the time of the first blood draw within 9h of birth. Study time points after enrollment correspond to time after birth (in parentheses) as follows: Enrollment 0 hours (0–9h), 12h (12–21h), 24h (22–33h), 36h (24–45h), 48h (46–57h), 60h (58–69h), and 72h (70–81h). Samples at the 60–72h after enrollment were obtained after rewarming. Samples were aliquoted and stored at −80°C until assay in duplicate on a BioPlex platform for the following analytes: IL-17A, IL-17E, IL-17F, IL-21, IL-22, IL-23, IL-27, and TNF-β (HTH17MAG-14K, EMD Millipore, Billerica, MA) as previously described (14). Concentrations were based on 5 parameter logistic fit of a 7 point standard curve. Serum levels of IL-17F, IL-22, IL-23, and TNF-β were below detectible limits, and IL-21 was only analyzed up to 12h due to low numbers of patients with detectible levels after this time.
25(OH)D and 1,25(OH)2D levels were measured in available samples from 0–72h after hypothermia initiation using a rapid, direct radioimmunoassay in Dr. Hollis’ laboratory with a lower limit of detection of 2ng/ml for 25(OH)D and 15pg/ml for 1,25(OH)2D, as previously described (15). DBP was measured on available serum samples using a 1:5000 dilution in a sandwich ELISA (GWB-DM3741, Genway Biotech, San Diego, CA) according to manufacturer’s protocol. Lower limit of detection of the DBP assay was 6.5ng/ml. On a subset of samples (n=17) from HIE infants at the Medical University of South Carolina, we performed a chart review and determined daily vitamin D intake in total parenteral nutrition (TPN) for the first 72h (pediatric multivitamins, ergocalciferol 400 IU/day =10 mcg/day or 417 ng/h).
Clinical Labs
Cord or initial neonatal gases were obtained at or within 1h of birth (13). Enrollment calcium, phosphorus, albumin and pH were all obtained after arrival at the tertiary care center, between 3–9h of life. Arterial blood gases were measured every 6h, calcium and phosphorus levels were measured every 12h, and albumin levels were measured every 24h over the first 4 days of life. Enteral nutrition was not started until 96h after HI birth.
Statistical Analysis
Data are reported as medians ± interquartile range (IQR) or graphically as medians ± 95% confidence intervals (CI). Mann-Whitney U test or Wilcoxon signed rank was used for Vitamin D comparisons as appropriate. Racial analyses were restricted to Caucasian and African American groups as others were too few to analyze. Correlations within individuals were analyzed using a Spearman rank correlation with reported significance p≤0.01, to account for multiple comparisons. The independent effects of 25(OH)D and 1,25(OH)2D on repeated laboratory measures, and the independent and interaction effects of hypothermia treatment, time and survival on laboratory measurements were assessed using mixed effects modeling with pairwise post-hoc analyses. The effects of albumin and DBP on 25(OH)D levels were also assessed using mixed model analysis with treatment and time in the model. Within the treatment groups, IL-27 was assessed over time using a mixed model. All analyses were performed using SPSS 21 (IBM Corp, Armonk, NY).
RESULTS
Demographics of the 50 patients included in this analysis were similar between hypothermia (n=28) and normothermia (n=22) groups (Table 1). Severe outcome (death or Bayley II psychomotor developmental indices ≤70 at 12 mo) was significantly more common in normothermia compared to hypothermia infants (Fisher’s Exact Test, p=0.036).
Table 1.
Study demographics
|
Hypothermia n = 28 |
Normothermia n = 22 |
Total n= 50 |
|
|---|---|---|---|
| Gender | |||
| Male | 16 (57 %) | 11 (50 %) | 26 (54 %) |
| Race | |||
| Caucasian | 16 (57 %) | 13 (59 %) | 29 (58 %) |
| African-American | 10 (36 %) | 7 (32 %) | 17 (34 %) |
| Other | 2 (7 %) | 2 (9 %) | 4 (8 %) |
| Sarnat | |||
| Stage I | 0 (0 %) | 1 (5 %) | 1 (2 %) |
| Stage II | 4 (14 %) | 4 (18 %) | 8 (16 %) |
| Stage III | 24 (86 %) | 17 (77 %) | 41 (82 %) |
| Outcome | |||
| Death | 9 (32 %) | 8 (36 %) | 17 (34 %) |
| Death or PDI≤70 @ 12 mo | 14 (50 %)* | 18 (77 %) | 32 (64 %) |
p=0.036 vs. normothermia, Fisher’s Exact Test
Vitamin D Deficiency Is Common And Declines Further Over 72h In Infants With Hypoxic-Ischemic Injury
Median serum 25(OH)D levels were not significantly different between normothermic (17.5ng/ml; IQR 7.5, 21.3ng/ml) and hypothermic HIE infants (16.8ng/ml; IQR 9.4, 20.8ng/ml) at any time point, nor between Sarnat stages 2 and 3 (p=0.897) or sex (p=0.742). Median serum 25(OH)D were significantly lower in African American versus Caucasian infants, regardless of treatment (Mann-Whitney, p=0.001, Figure 1a) (9). Several sites in this multicenter RCT were in more northern latitudes: there were 6 infants from the Canadian site (2 Native American, 4 Caucasian), 4 from Albany (4 Caucasian), and 2 from SUNY Brooklyn (1 African American, 1 Asian). The means and standard deviation of infants from northern latitude sites were Canada 24.7± 11.3 (range 12.3–39.7 ng/ml), Albany 18.5± 3.0 (range 14.7–21.5 ng/ml), and SUNY 13.7± 5.7 (range 9.7–17.7 ng/ml). Also comparing northern and southern latitude Native American/African American (northern 14.9± 4.9ng/ml, n=3; southern 10.4± 6.3 ng/ml, n=17) and Caucasian infants (northern 20.8± 9.9, n=8; southern 18.4± 6.9 ng/ml, n=23), the means were not significantly different in the two latitudes by race, though sample size was more limited in northern latitudes.
Figure 1.
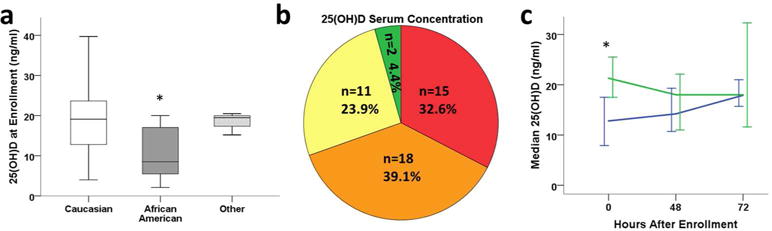
Circulating 25(OH)D at enrollment after HI injury (n=46). (1a) Box plots of median, 25th & 75th IQR, and ranges of serum 25(OH)D by race in HIE infants (n= 27 Caucasian, 16 African American, 3 other race; * p=0.001 vs Caucasian). (1b) Incidence of sufficiency (≥30 ng/ml, green), low-sufficiency (20–30 ng/ml, yellow), insufficiency (12–20 ng/ml, orange) and deficiency (<12 ng/ml, red) of 25(OH)D. (1c) Median (± 95%CI) serum 25(OH)D levels over 72 hours in HIE patients that either maintained serum 25(OH)D levels during the study period (n=17, blue) or had declining serum 25(OH)D (n=17, green) at 48–72 hours compared to enrollment (*, p=0.002).
Treatment groups were subsequently combined for analysis of 25(OH)D (n=46 infants with enrollment 25(OH)D concentrations). Circulating 25(OH)D at 0h was categorized within consensus guidelines for nutritional rickets but taking into account other levels that have implications for immune function (15, 16): sufficient (>30ng/ml; >75nmol/l), low-sufficient (>20–30ng/ml; 50–75nmol/l), insufficient (12–20ng/ml; 30–50nmol/l), and deficient (<12ng/ml; <30nmol/l).
25(OH)D was insufficient in 72% of HIE infants immediately after birth, and 33% of HIE infants were deficient (Figure 1b). Only 28% had 25(OH)D levels >20ng/ml, and 2 infants (4%) had 25(OH)D levels between 30 and 40ng/ml. All African American HIE infants had 25(OH)D levels ≤20ng/ml. These levels are in contrast to reported cord blood 25(OH)D concentrations in healthy, term neonates in whom 56% were deficient and mean 25(OH)D was 21.1±2.2ng/ml (15).
Serum 25(OH)D concentrations decreased further in 50% of HIE infants between enrollment and 48–72h (n=17 out of 34 infants with multiple measurements; Figure 1c).
We next evaluated a subset of infants (n=17) with serial 25(OH)D levels in whom documentation of exogenous parenteral administration of 25(OH)D in multivitamins was available, to determine the effect on serum 25(OH)D in the acute phase after HI birth. Nine infants received 400 IU (10 μg) ergocalciferol per day in TPN at a constant infusion rate of 417 ng/h, starting at a median of 35h (range 3–67h). Five out of nine infants had lower serum 25(OH)D at 48h, which continued to decline to 72h in two of these infants (Supplemental Figure S1, online). For all supplemented infants, the change in serum 25(OH)D varied from −1 to +3.7ng/ml at 72h, indicating little or no accumulation of 25(OH)D in the first 3 days after HIE.
Active Hormone 1,25(OH)2D Is Undetectable in a Third of HIE Infants
Circulating active 1,25(OH)2D concentrations are maintained by renal conversion of serum 25(OH)D by 1-alpha-hydroxylase, which is regulated by parathyroid hormone and calcium and phosphorus levels under normal physiologic conditions (17). We investigated 1,25(OH)2D levels after HIE on a more limited set of serum samples from 0–12h (n=16), 36–48h (n=14), and 60–72h (n=10). Six of 16 HIE infants (38%) had undetectable levels of 1,25(OH)2D within 12h of birth (<15pg/ml). Among those with detectable serum levels, 1,25(OH)2D concentrations were not different than that reported in healthy infants (median 37pg/ml (IQR 15, 50 pg/ml, 0–12h) (15), nor between treatment groups. Median serum 1,25(OH)2D gradually increased to 41pg/ml at 36–48h (IQR 15, 51 pg/ml, n=14) and 51 pg/ml at 60–72h (IQR 18, 85 pg/ml, n=10). The number of HIE infants with undetectable 1,25(OH)2D also declined over this period to 4 (28%) at 36–48h and 2 (20%) at 60–72 h. 1,25(OH)2D concentrations were not related to the serum 25(OH)D or DBP at any time point, which may be due to a smaller sample size for 1,25(OH)2D and/or preferential binding of DBP to 25(OH)D (12).
Low Vitamin D Binding Protein is Associated with Serum 25(OH)D Status
The bioavailability of circulating lipophilic vitamin D may be determined by binding either to DBP, which has high affinity for 25(OH)D, or to serum albumin, which is 100 times more abundant (12). In HIE, lower serum albumin and perhaps lower DBP might make more lipophilic 25(OH)D available for tissue uptake or renal conversion. Hypothermia treatment group had significantly lower serum albumin concentrations when compared to normothermia groups over the study period (p<0.0001). Albumin concentrations were positively correlated with serum 25(OH)D levels in the hypothermia group at both 0 and 72h (p≤0.016) (Figure 2a). Interestingly, in the normothermic HIE group, albumin and 25(OH)D levels were not significantly related.
Figure 2.
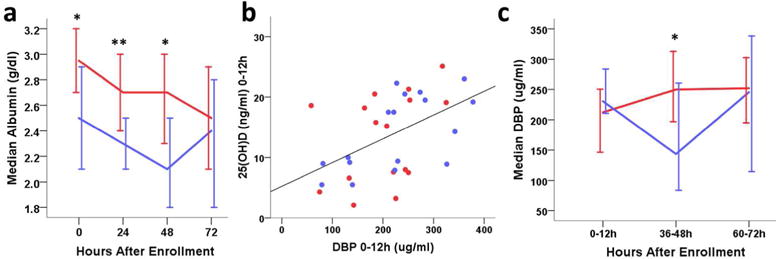
Circulating vitamin D binding proteins by hypothermia (blue) and normothermia (red) treatment. (2a) Median serum albumin over time (n=50, * p<0.01, **p<0.001); (2b) Serum DBP versus 25(OH)D concentrations at 0–12h after enrollment (n=33, rho=0.503, p=0.003); (2c) Median serum DBP over time by hypothermia and normothermia treatment. (*p<0.05).
Mean DBP was lower after moderate to severe HI birth (215±85ug/ml, n=33) in our study than previously reported in normal term and preterm infant cord blood (285 and 297 ug/ml, respectively) (18, 19), and positively correlated with serum 25(OH)D at 0–12h (Spearman’s Rho≥0.503, p≤0.006, n=33, Figure 2b). DBP did not differ by treatment group at 0–12h of age, or by those who had declining versus increasing serum 25(OH)D. However, during hypothermia treatment at 36–48h, circulating DBP was significantly lower in hypothermic HIE infants (p=0.004, Figure 2c). After rewarming (60–72h, n=27) DBP rebounded in the hypothermia group, equivalent to normothermic concentrations.
We performed mixed model analyses to determine whether DBP or albumin was more influential on serum 25(OH)D levels, with treatment in the model. At both 0–12 and 60–72h, DBP predicted 25(OH)D serum concentrations (p≤0.045), without significant contribution from albumin when DBP was included. At 36–48h, hypothermia treatment and DBP had an interaction effect in predicting serum 25(OH)D. This interaction effect is consistent with our simple correlational analyses in which hypothermia treatment decreased DBP at 36–48h, but did not have an independent effect on serum 25(OH)D concentrations in addition to its effect on DBP. These results are the first report of DBP levels after HIE and suggest a complex relationship of direct and indirect effects of HIE and hypothermia on DBP levels in these infants.
Vitamin D Correlates with Anti-inflammatory Th17 Regulatory Cytokines
Vitamin D is known to decrease Th17 activation in other inflammatory conditions (20). Th17 activated cells produce pro-inflammatory IL-17A and IL-17F, while IL-17E suppresses Th17 cell proliferation and activation (21). IL-27, produced by neonatal macrophages, also inhibits Th-17 induction and is regulated by vitamin D (22, 23). Therefore, we next examined vitamin D’s relationship to proinflammatory Th17 cytokines (IL-17A, IL-17F, IL-21, MIP3α) and anti-inflammatory IL-17E and IL-27 after moderate to severe HI injury.
Median serum cytokine levels for IL-17A, IL-21, MIP3α, and IL-17F were similar between treatment groups and were not associated with serum 25(OH)D levels immediately after HI birth (Supplemental Table S1, online). However, 25(OH)D levels at 48h showed a significant correlation with anti-inflammatory IL-17E serum concentrations at 60–72h (n= 20, Figure 3a).
Figure 3.
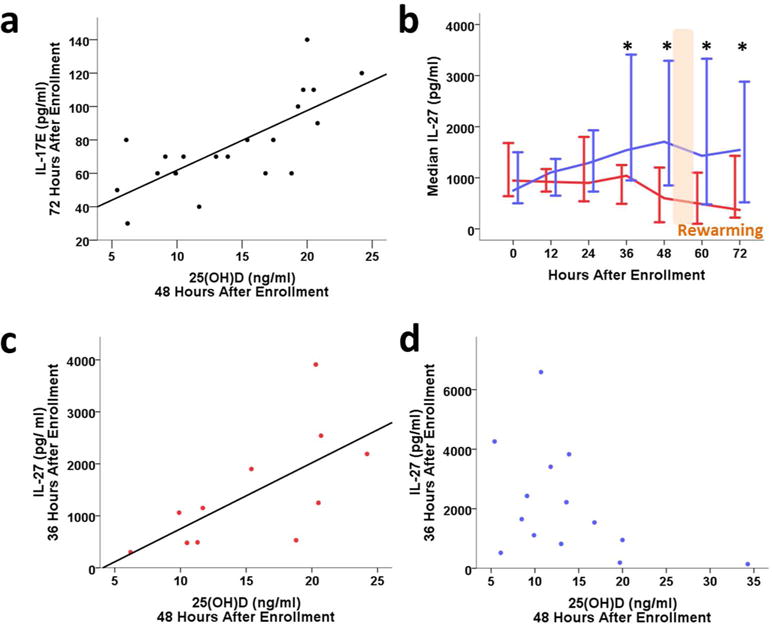
Anti-inflammatory cytokines IL-17E and IL-27 in HIE neonates. (3a) 25(OH)D at 48 hours correlates with IL-17E at 72 hours after enrollment (rho=0.555, p=0.001). (3b) Median IL-27 (± 95% CI, pg/ml) over time in hypothermia (blue) and normothermia (red) treatment groups. (*p<0.05, mixed model post-hoc analysis). (3c) IL-27 at 36 hours positively correlates with 25(OH)D in normothermic infants (n=11, rho=0.809, p=0.003). (3d) IL-27 at 36 hours does not correlate with 25(OH)D in hypothermic infants (n=14, rho= −0.442, p=0.114).
Hypothermia treatment had a significant main effect (p<0.0001) on IL-27 serum concentrations and an interaction effect with time (p=0.008), as illustrated in Figure 3B, where IL-27 levels were significantly higher in hypothermia treated infants before and after rewarming, from 36 to 72h (Figure 3b). Serum IL-27 strongly correlated with 25(OH)D concentrations at 48h in normothermic patients (Figure 3c), but not in the hypothermic infants (Figure 3d), consistent with a direct effect of hypothermia on production of IL-27, independent of 25(OH)D.
Vitamin D’s Relationship to Serum Phosphorus and Total Calcium Levels after HIE
Vitamin D’s role in calcium and phosphorus metabolism after moderate to severe HI has not been explored. Although there was marked variability in serum calcium, mean total and ionized calcium concentrations were significantly lower in hypothermic infants after rewarming at 60–72h compared with normothermic infants (p≤0.013), while serum phosphorus and pH were not different by treatment group (Supplemental Figure S2, online). Phosphorus concentrations were negatively correlated with pH (p<0.001) at 0h, supporting higher serum phosphorus as a marker of metabolic acidosis and perhaps injury severity.
Active 1,25(OH)2D controls circulating calcium and phosphorus levels under normal conditions, but after HIE neither were predicted by 1,25(OH)2D serum concentrations (n=16), using mixed model analysis. Total serum calcium was positively predicted by serum 25(OH)D at 0h (p=0.034), but not at 48 and 72h, perhaps due to intravenous calcium replacement therapy in 35 infants to correct initial hypocalcemia. Serum phosphorus was positively predicted by serum 25(OH)D concentration after pH had normalized at 48 and 72h (p≤0.025). This data suggests that vitamin D’s role in phosphorus homeostasis is functional 2–3 days after HI birth.
Survival relates to serum Phosphorus but not 25-OH Vitamin D
Infants who died in the neonatal period had significantly lower pH (p=0.0002) and higher serum phosphorus over the 72h study period than those who survived (p=0.00006, Figure 4a) with a survivaltime interaction for serum phosphorus (p=0.041). This difference in serum phosphorus between surviving and non-surviving HIE neonates was present at 0h (p≤0.002), and negatively correlated with pH (p=0.003, Figure 4b), supporting phosphorus as a marker of injury severity and poor outcome. Serum phosphorus concentrations of infants who survived were < 6.3mg/dL within 9h of birth.
Figure 4.
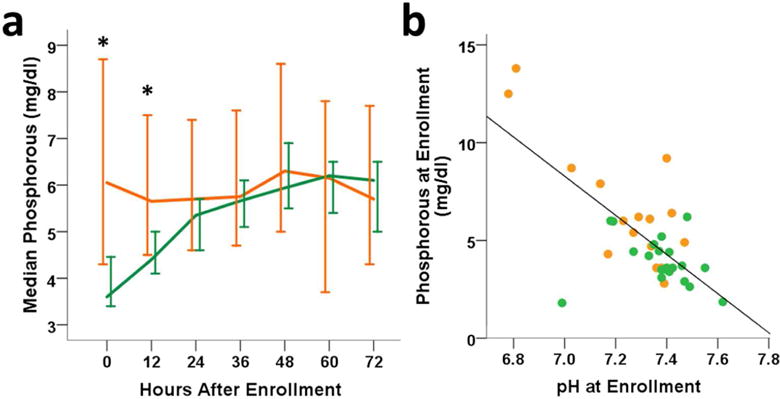
Phosphorous is significantly higher 3–9h after birth in infants who do not survive (orange) the neonatal period compared to survivors (green). (4a) Circulating median phosphorous (± 95%CI, * indicates p<0.05) over 72 hours after HI birth. (4b) Enrollment pH negatively correlates with phosphorous (rho= −0.477, p=0.003).
Infants who had 25(OH)D deficiency at 0h (<12ng/ml; n=17) had a 58% survival rate similar to those with low or insufficient 25(OH)D (12–30ng/ml; 65% survival, n=31, Fisher’s Exact Test, p=0.5). Both infants with sufficient 25(OH)D (>30ng/ml) survived (15). There was also no difference in survival between infants who maintained (63%, n=26) compared to those who had decreasing 25(OH)D over 72h (67%, n=11, Fisher’s Exact Test, p=1.0). Therefore, serum 25(OH)D did not demonstrate a direct relationship with survival in this cohort of infants with severe HIE, and the sample size was too limited for further analysis of developmental outcome.
DISCUSSION
25(OH)D insufficiency was present in the majority of our HIE infants, and half of these infants had decreasing 25(OH)D over the initial 72h of treatment, in spite of administration of 417ng/h ergocalciferol in TPN, which did not appreciably increase serum 25(OH)D concentrations. This data agrees with findings in a Turkish cohort of hypothermic mild to moderate HIE infants, in whom the incidence of serum 25(OH)D <5ng/ml increased from day 1 to 5, and was significantly greater at day 5 than in the healthy term controls (10). In our cohort, hypothermia treatment did not affect either 25(OH)D or 1,25(OH)2D serum concentrations compared to normothermic infants.
Vitamin D is a well-known regulator of inflammatory Tcells, and inhibits Th17 cell activation(6, 24). In our neonates low serum 25(OH)D was associated with reduced anti-inflammatory IL-17E after HIE. Another anti-inflammatory cytokine that may oppose Th17-induced inflammation, IL-27 was also correlated with 25(OH)D levels in normothermic HIE infants, providing additional evidence that vitamin D insufficient infants may have limited ability to mitigate post-HI Th17 inflammation. However, even in the face of low serum 25(OH)D, hypothermia treated patients had increased IL-27, indicating direct modulation of this serum cytokine by hypothermia treatment (14). These data suggest the immunoregulation of TH17 activation after HIE depends on the mediator, with IL-17E primarily affected by vitamin D deficiency, while IL-27 was primarily affected by hypothermia therapy.
Serum 25(OH)D also serves as the main source for local CNS production of 1,25(OH)2D. Similar to our study in neonates with HIE, serum 25(OH)D concentrations are known to decrease after stroke in adults (25). Although serum 25(OH)D has a two week half-life under physiologic conditions (26), inflammation contributes to accelerated depletion of serum 25(OH)D, which in turn limits 1,25(OH)2D production by leukocytes and neural cells (8, 11, 12). The net effect of increased vitamin D metabolism in inflamed CNS tissue is to reduce the circulating substrate, 25(OH)D (12), while circulating 1,25(OH)2D may initially be more stably maintained.
Urinary losses may contribute to low or decreasing serum 25(OH)D concentrations in HIE infants. HI-induced renal injury is common, involves tubular dysfunction with proteinuria, and takes days to resolve. Renal reabsorption of 25(OH)D-bound DBP occurs in proximal tubular cells upon binding to megalin, a transmembrane receptor found on many cell types, including brain capillary endothelial cells, neurons and astrocytes (27). Ischemia reperfusion down-regulates renal megalin expression (28), which may result in excessive urinary losses of vitamin D and DBP (29). As DBP binds 25(OH)D with higher affinity than 1,25(OH)2D, renal injury after HI birth may not have the same effect on serum 1,25(OH)2D (12). In fact, the tight correlation of serum 25(OH)D and DBP at 0–12h is consistent with either urinary loss of the bound 25(OH)D or tissue distribution. One of the limitations of this study is a lack of urine samples to verify urinary losses of DBP and 25(OH)D in our patients.
Taken together, our data and other studies of vitamin D in stroke suggest that HI injury increases the conversion of 25(OH)D to 1,25(OH)2D, and both 1,25(OH)2D and 25(OH)D may be subject to increased degradation as shown post-stroke and in neuroinflammatory conditions (8, 30). Along with increased urinary losses (12), uptake of 25(OH)D into tissues for intracellular production of 1,25(OH)2D, may also contribute to low and declining 25(OH)D serum concentrations (12, 31). Although we did not obtain serum samples from time points later than 72h, with evidence of insufficient serum 25(OH)D levels regardless of 25(OH)D supplementation, it is possible that at some point in the first days or week of life, the uptake of 25(OH)D and conversion to 1,25(OH)2D in neural and immune cells may be compromised in some HIE infants.
25(OH)D insufficiency is under-recognized in HIE, and is not part of standard of care HIE protocols in research or clinical practice(32, 33). Our data indicate the vitamin D insufficiency is present in more than 70% of HIE infants on admission, and may not be significantly improved by vitamin D supplementation in TPN. Both human and animal investigations indicate that pre-existing vitamin D deficiency exacerbates neurological injuries, and severe 25(OH)D deficiency is associated with increased stroke risk and worse outcomes in both adult humans and rodent models (1, 2, 30, 34, 35). Even in the absence of HI, vitamin D deficiency during a critical perinatal window in rodent models results in behavioral and learning deficits (36, 37). Thus, 25(OH)D insufficiency could be an aggravating factor after neonatal HIE. Although 25(OH)D concentrations were not independently associated with survival in our infants, only 2 of our infants had 25(OH)D levels ≥ 30ng/ml, and we were not able to analyze neurodevelopmental outcomes by degree of 25(OH)D insufficiency due to sample size. Vitamin D effects may also have been overshadowed by the marked severity of HI injury in this cohort, with greater than 70% of infants with severe HIE.
By convention we define ‘sufficiency’ and ‘insufficiency’ for circulating 25(OH)D levels according to previous reports in neonatal blood using established endocrine concentrations for bone growth and homeostasis (15). However, the circulating levels of 25(OH)D that are sufficient or deficient for neurodevelopment or other intracrine roles after injury have yet to be determined. Circulating 25(OH)D concentrations required for immune function appear to be significantly higher than for bone homeostasis (20 ng/ml) and prevention of rickets (10 ng/ml) (15). In addition, the relationship of DBP and whether it limits the tissue bioavailability of free, unbound 25(OH)D in the serum or enhances uptake in disease states, such as neonatal HI, have not been explored (12). DBP also has independent roles as a macrophage activating factor and scavenger of extracellular actin, unrelated to its vitamin D ligand function, and seems to be independently regulated by hypothermia at 36h, as we have shown with other serum chemotactic factors (14, 38, 39).
Vitamin D deficiency can result in increased Th17 cell activation (40), while treatment with vitamin D down-regulates Th17 activation (6). In HIE neonates, 25(OH)D levels correlated with circulating Th17 inhibitory cytokines IL-17E and IL-27, but for IL-27, this effect was overridden by hypothermia treatment itself. Hypothermia up-regulated anti-inflammatory IL-27 production and release from antigen presenting cells in the serum from 36 to72h, even while the total circulating leukocyte, neutrophil, lymphocyte, and monocyte counts were decreasing (39). Thus, increased circulating anti-inflammatory cytokines, such as IL-17E and IL-27, may contribute to the neuroprotective mechanisms of hypothermia and yet be impacted by 25(OH)D levels.
In conclusion, this study provides insights into 25(OH)D insufficiency and effects in infants with HIE and under hypothermic conditions over the first 72 hours. Without routine monitoring, vitamin D insufficiency goes largely undiagnosed in neonatal HIE. Our data indicate that 25(OH)D insufficiency is common on admission and may decline further during the acute phase after HI injury. As treatment with vitamin D has been shown to provide neuroprotection in animal models of neonatal HI and in a small pilot trial in human adults after stroke(41, 42), the potential for beneficial effects of vitamin D replacement in this at risk population deserve further study.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Neurologic Disorders and Stroke R01 NS38602.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
This work was published in abstract form and presented at the Pediatric Academic Societies Annual Meeting. Vancouver, BC, May 2014 and San Diego, CA, April 2015.
Clinical Trials Registration #: NCT02826941
References
- 1.Daumas A, Daubail B, Legris N, et al. Association between Admission Serum 25-Hydroxyvitamin D Levels and Functional Outcome of Thrombolyzed Stroke Patients. J Stroke Cerebrovasc Dis. 2016;25:907–913. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Park KY, Chung PW, Kim YB, et al. Serum Vitamin D Status as a Predictor of Prognosis in Patients with Acute Ischemic Stroke. Cerebrovasc Dis. 2015;40:73–80. doi: 10.1159/000434691. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Wang Y, Yu F, et al. Peripheral Th17/Treg imbalance in patients with atherosclerotic cerebral infarction. Int J Clin Exp Pathol. 2013;6:1015–1027. [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Zheng Y, Wu Y, Ni B, Shi S. Imbalance between IL-17A-Producing Cells and Regulatory T Cells during Ischemic Stroke. Mediators Inflamm. 2014;2014:813045. doi: 10.1155/2014/813045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol. 2012;42:311–319. doi: 10.1002/eji.201141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Shih DQ, Zhang X. Mechanisms underlying effects of 1,25-Dihydroxyvitamin D3 on the Th17 cells. Eur J Microbiol Immunol (Bp) 2013;3:237–240. doi: 10.1556/EuJMI.3.2013.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White JH. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch Biochem Biophys. 2012;523:58–63. doi: 10.1016/j.abb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Paintlia AS, Paintlia MK, Hollis BW, Singh AK, Singh I. Interference with RhoA-ROCK signaling mechanism in autoreactive CD4+ T cells enhances the bioavailability of 1,25-dihydroxyvitamin D3 in experimental autoimmune encephalomyelitis. Am J Pathol. 2012;181:993–1006. doi: 10.1016/j.ajpath.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basile LA, Taylor SN, Wagner CL, Quinones L, Hollis BW. Neonatal vitamin D status at birth at latitude 32 degrees 72′: evidence of deficiency. J Perinatol. 2007;27:568–571. doi: 10.1038/sj.jp.7211796. [DOI] [PubMed] [Google Scholar]
- 10.Mutlu M, Sariaydin M, Aslan Y, et al. Status of vitamin D, antioxidant enzymes, and antioxidant substances in neonates with neonatal hypoxic-ischemic encephalopathy. J Matern Fetal Neonatal Med. 2016;29:2259–2263. doi: 10.3109/14767058.2015.1081889. [DOI] [PubMed] [Google Scholar]
- 11.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132–137. doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005b;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins DD, Rollins LG, Perkel JK, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2012;32:1888–1896. doi: 10.1038/jcbfm.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker VP, Zhang X, Rastegar I, et al. Cord blood vitamin D status impacts innate immune responses. J Clin Endocrinol Metab. 2011;96:1835–1843. doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munns CF, Shaw N, Kiely M, et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016;101:394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer CK, Ye C, Hanley AJ, et al. The Relationship between Parathyroid Hormone and 25-Hydroxy Vitamin D during and after Pregnancy. J Clin Endocrinol Metab. 2016:jc20154060. doi: 10.1210/jc.2015-4060. [DOI] [PubMed] [Google Scholar]
- 18.Hoogenboezem T, Degenhart HJ, de Muinck Keizer-Schrama SM, et al. Vitamin D metabolism in breast-fed infants and their mothers. Pediatr Res. 1989;25:623–628. doi: 10.1203/00006450-198906000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Hanson C, Lyden E, Nelson A, et al. Response of vitamin D binding protein and free vitamin D concentrations to vitamin D supplementation in hospitalized premature infants. J Pediatr Endocrinol Metab. 2015;28:1107–1114. doi: 10.1515/jpem-2015-0089. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda U, Wakita D, Ohkuri T, et al. 1 alpha,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett. 2010;134:7–16. doi: 10.1016/j.imlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129:625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- 22.Kraft JD, Horzempa J, Davis C, Jung JY, Pena MM, Robinson CM. Neonatal macrophages express elevated levels of interleukin-27 that oppose immune responses. Immunology. 2013;139:484–493. doi: 10.1111/imm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeke F, Korf H, Overbergh L, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–227. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y. Abnormal bone and calcium metabolism in patients after stroke. Arch Phys Med Rehabil. 2000;81:117–121. doi: 10.1016/s0003-9993(00)90231-4. [DOI] [PubMed] [Google Scholar]
- 26.Jones KS, Assar S, Harnpanich D, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–3381. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvira-Botero X, Perez-Gonzalez R, Spuch C, et al. Megalin interacts with APP and the intracellular adapter protein FE65 in neurons. Mol Cell Neurosci. 2010;45:306–315. doi: 10.1016/j.mcn.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber A, Theilig F, Schweda F, Hocherl K. Acute endotoxemia in mice induces downregulation of megalin and cubilin in the kidney. Kidney Int. 2012;82:53–59. doi: 10.1038/ki.2012.62. [DOI] [PubMed] [Google Scholar]
- 29.Anderson RL, Ternes SB, Strand KA, Rowling MJ. Vitamin D homeostasis is compromised due to increased urinary excretion of the 25-hydroxycholecalciferol-vitamin D-binding protein complex in the Zucker diabetic fatty rat. Am J Physiol Endocrinol Metab. 2010;299:E959–967. doi: 10.1152/ajpendo.00218.2010. [DOI] [PubMed] [Google Scholar]
- 30.Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3 : a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Committee on F, Newborn. Papile LA, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146–1150. doi: 10.1542/peds.2014-0899. [DOI] [PubMed] [Google Scholar]
- 33.Olsen SL, Dejonge M, Kline A, et al. Optimizing therapeutic hypothermia for neonatal encephalopathy. Pediatrics. 2013;131:e591–603. doi: 10.1542/peds.2012-0891. [DOI] [PubMed] [Google Scholar]
- 34.Brondum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol. 2013;73:38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 35.Turetsky A, Goddeau RP, Jr, Henninger N. Low Serum Vitamin D Is Independently Associated with Larger Lesion Volumes after Ischemic Stroke. J Stroke Cerebrovasc Dis. 2015;24:1555–1563. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 36.Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187:343–350. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Wrzosek M, Lukaszkiewicz J, Jakubczyk A, et al. Vitamin D and the central nervous system. Pharmacological reports: PR. 2013;65:271–278. doi: 10.1016/s1734-1140(13)71003-x. [DOI] [PubMed] [Google Scholar]
- 38.Delanghe JR, Speeckaert R, Speeckaert MM. Behind the scenes of vitamin D binding protein: more than vitamin D binding. Best Pract Res Clin Endocrinol Metab. 2015;29:773–786. doi: 10.1016/j.beem.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins DD, Lee T, Chiuzan C, et al. Altered circulating leukocytes and their chemokines in a clinical trial of therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy*. Pediatr Crit Care Med. 2013;14:786–795. doi: 10.1097/PCC.0b013e3182975cc9. [DOI] [PubMed] [Google Scholar]
- 40.Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23:519–528. doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kajta M, Makarewicz D, Zieminska E, et al. Neuroprotection by co-treatment and posttreating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochemistry international. 2009;55:265–274. doi: 10.1016/j.neuint.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Prabhakar S, Modi M, et al. Effect of Vitamin D and calcium supplementation on ischaemic stroke outcome: a randomised controlled open-label trial. Int J Clin Pract. 2016;70:764–770. doi: 10.1111/ijcp.12866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


