Abstract
BACKGROUND
Neuroimaging studies of attention-deficit/hyperactivity disorder (ADHD) have most commonly reported volumetric abnormalities in the basal ganglia, cerebellum, and prefrontal cortices. Few studies have examined the relationship between ADHD symptomatology and brain structure in population-based samples. Herein, we investigate the relationship between dimensional measures of ADHD symptomatology, brain structure, and reaction time variability—an index of lapses in attention. We also test for associations between brain structural correlates of ADHD symptomatology and maps of dopaminergic gene expression.
METHODS
Psychopathology and imaging data were available for 1,538 youths. Parent ratings of ADHD symptoms were obtained using the Development and Well-Being Assessment (DAWBA) and the Strengths and Difficulties Questionnaire (SDQ). Self-reports of ADHD symptomatology were assessed using the youth version of the SDQ. Reaction time variability was available in a subset of participants. For each measure, whole brain voxel-wise regressions with gray matter volume (GMV) were calculated.
RESULTS
Parent ratings of ADHD symptoms (DAWBA and SDQ), adolescent self-reports of ADHD symptoms on the SDQ, and reaction time variability were each negatively associated with GMV in an overlapping region of the ventromedial prefrontal cortex (vmPFC). Maps of DRD1 and DRD2 gene expression were associated with brain structural correlates of ADHD symptomatology.
CONCLUSIONS
This is the first study to reveal relations between vmPFC structure and multi-informant measures of ADHD symptomatology in a large population-based sample of adolescents. Our results indicate that vmPFC structure is a biomarker for ADHD symptomatology. These findings extend previous research implicating the default mode network and dopaminergic dysfunction in ADHD.
Keywords: attention-deficit/hyperactivity disorder, neuroimaging, ventromedial prefrontal cortex, inattention, reaction time variability, multi-informant
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is among the most prevalent neuropsychiatric disorders in youths, with roughly 3–7% of school-aged children meeting diagnostic criteria (1). Longitudinal studies indicate that functionally impairing symptoms continue into adolescence and adulthood in approximately 60–80% of cases diagnosed during childhood (2, 3). Extant morphometry studies on ADHD have implicated a number of anatomically related brain areas; however, findings have been somewhat inconsistent, with no common structural abnormality emerging across studies. In adults and youths, structural abnormalities have been reported in the basal ganglia (4–10), prefrontal cortex (10–12), cerebellum (5, 10, 13, 14), anterior cingulate cortex (15), and less frequently reported in the hippocampus, amygdala, and thalamus (16, 17). Several factors, however, may serve to obscure underlying brain-behavior relations in the study of ADHD symptomatology, including the use of categorical diagnoses, the lack of multi-informant behavioral ratings, and small sample sizes. In order to aptly characterize the neuroanatomical substrates of ADHD symptomatology, it is critical to demonstrate convergence across dimensional, multi-informant behavioral data using large population-based samples. If possible, findings should also demonstrate convergence with other established features of ADHD symptomatology across different domains—including measures of cognition, as well as neurochemistry.
Over the last few decades, empirically based assessment of psychopathology has revealed aspects of dimensionality with regard to many psychiatric conditions, including ADHD (18). Such findings have been somewhat difficult to reconcile with the categorical taxonomy espoused by the Diagnostic and Statistical Manual of Mental Disorders (DSM). Although numerous studies have tested for brain differences between ADHD patients and typically developing controls, few studies have investigated brain correlates of attention problems in the general population. Following from a dimensional conceptualization of psychopathology, it is reasonable to postulate that both clinical and normative levels of a given psychiatric syndrome will be underpinned by overlapping neural substrates. Mous et al. (2014) recently reported that cortical thickness in bilateral postcentral gyri was negatively associated with parent-reported attention problems in a population-based sample of 444 6- to 8-year-old children (19). Ducharme et al. (2012) found that subclinical attention problems in typically developing youths, ranging from 6 to 18 years of age, were associated with a decreased rate of cerebral cortical thinning within prefrontal and parietal cortical regions—brain areas that have been implicated in the pathophysiology of clinically significant attention problems (i.e., ADHD) (20–22). Similarly, Shaw et al. (2011) reported an association between subclinical symptoms of hyperactivity and impulsivity in typically developing youths and delayed cortical thickness maturation (23). Such evidence supports the use of dimensional measures of psychopathology, as emphasized by the National Institute of Mental Health’s Research Domain Criteria program (24). Taken together, there is compelling evidence that subclinical variation in ADHD symptomatology is tied to brain structure and development—and that these associations may be obfuscated by a strict categorical DSM approach.
In the assessment of developmental psychopathology, informants represent an important source of variance (18). The current DSM taxonomy does not offer clear, standardized methods for synthesizing reports from multiple informants. Martel et al. (2015) recently reported that information from multiple informants increases the validity of assessing ADHD, and that averaging ratings is the optimal method for integrating multi-informant data (25). Dimensional ratings from multiple informants also allow for more sophisticated methods of integrating data, such as latent variable approaches. Unfortunately, few neuroimaging studies have utilized dimensional assessments of ADHD symptomatology from multiple informants.
In addition to using quantitative, multi-informant behavioral ratings, we aimed to demonstrate convergence across different domains with measures previously associated with ADHD symptomatology. Reaction time variability refers to the degree of intra-individual variation in responding to a target stimulus, and increased reaction time variability on attention tasks has been commonly reported in ADHD youths (26, 27). Lesion studies indicate that frontal lobe damage is accompanied by increased reaction time variability (28). There is also evidence that individual differences in reaction time variability predict inhibitory success (29). Further, subjects with increased reaction time variability exhibit greater activation within inhibitory regions of the brain during tasks of response inhibition (29). Thus, reaction time variability may serve as an objective neurocognitive marker for ADHD symptomatology. It remains unclear, however, the extent to which such cognitive measures are related to parent and self-report ratings of ADHD symptomatology, as well as brain structure, in the general population.
Finally, patterns of gene expression may provide additional support in identifying potential brain-based markers for ADHD symptomatology. The brain’s dopaminergic system has been strongly implicated in a wide variety of cognitive functions including attention, and repeatedly linked to the pathophysiology of ADHD symptomatology. Indeed, a number of medications that have proven efficacious in the treatment of ADHD work by blocking dopamine reuptake and/or stimulating dopamine release, increasing extracellular dopamine levels. It is reasonable to postulate that regions of the brain that are volumetrically related to ADHD symptomatology will be tied the expression of genes encoding for dopaminergic receptors.
Herein, we investigate the relationship between dimensional measures of ADHD symptomatology and brain structure in a large population-based sample of adolescents, utilizing multi-informant behavioral ratings. In a subset of participants, we also investigate relations between reaction time variability, measures of ADHD symptomatology, and brain structure. Finally, utilizing publicly available gene expression data collected as part of the Allen Human Brain Atlas (30), we test the extent to which the relationship between brain structure and ADHD symptomatology is correlated with patterns of dopaminergic gene expression. To our knowledge, the following represents the first voxel-based morphometry (VBM) of ADHD symptomatology using a population-based sample of youths.
METHODS AND MATERIALS
Sample
Neuroimaging and behavioral data were obtained from the IMAGEN study conducted across 8 European sites in France, the United Kingdom, Ireland, and Germany, which includes 2,223 adolescents recruited from schools at age 14 years (SD = 0.41 year; age range = 12.9–15.7 years). A detailed description of recruitment and assessment procedures has been published elsewhere (31). In the present study, a total of 1,538 participants possessed multi-informant psychopathology data, quality controlled neuroimaging data, and complete demographic data (Table 1). Behavioral data for the stop signal task (SST) were only available in a subset of participants (N = 767).
TABLE 1.
Summary statistics for predictor variables.
| Age (in years) (Mean ± SD) | 14.53 ± 0.41 |
| Gender | 51% F (785), 49% M (753) |
| SES (Mean ± SD) | 17.80 ± 4.06 |
| Verbal IQ (Mean ± SD) | 110.94 ± 14.88 |
| Performance IQ (Mean ± SD) | 108.16 ± 14.87 |
| DAWBA Symptom Count (Mean ± SD) | 4.05 ± 5.79 |
| H/I Score on Parent SDQ (Mean ± SD) | 2.94 ± 2.27 |
| H/I Score on Youth SDQ (Mean ± SD) | 3.94 ± 2.15 |
| Reaction Time Variability (Mean SD ± SD) | 101.49 ± 24.96 (N = 767) |
N= 1,538 unless otherwise noted; H/I = Hyperactive/Inattentive scale
Psychopathology Assessment
The Development and Well-Being Assessment (32) is a computer-based package of questionnaires, interviews, and rating techniques used to assess adolescent psychopathology. In the present study, ADHD symptom counts were derived from the parent version of the DAWBA—youths did not complete the DAWBA ADHD module. In addition to total symptom count, the parent version of the DAWBA yielded separate symptom counts for both Hyperactivity/Impulsivity and Inattention.
Self-report and parent report versions of the Strengths and Difficulties Questionnaire (SDQ) were also used to assess symptoms of hyperactivity and inattention (33). In addition to the Hyperactive/Inattentive scale, the Emotional scale on the youth SDQ was utilized to assess mood and anxiety symptomatology. The SDQ is a reliable and valid measure of youth emotional and behavior symptoms, on which scores are predictive of increased probability of clinician-rated psychiatric disorders and retest stability over 4–6 months (34).
Behavioral Measures
Behavioral data from the functional imaging stop signal task (SST) were utilized in the present study. Associations were tested between ADHD symptom scores (both DAWBA and SDQ) and several SST measures including mean reaction time, stop signal reaction time (SSRT), and reaction time variability. The standard deviation of “Go” reaction time on the SST was used to assess reaction time variability in participants.
Demographic Measures
The puberty development scale (PDS) was used to assess the pubertal status of participants (35). The socioeconomic status (SES) score was derived by summing the following variables: Mother’s Education Score, Father’s Education Score, Family Stress Unemployment Score, Financial Difficulties Score, Home Inadequacy Score, Neighborhood Score, Financial Crisis Score, Mother Employed Score, and Father Employed Score (36).
MRI acquisition
MRI scanning was conducted at the eight IMAGEN assessment sites using 3T whole body MRI systems (31). See supplemental information for further details.
Structural MRI
High-resolution anatomical MRIs were acquired with a three-dimensional T1-weighted magnetization prepared gradient echo sequence (MPRAGE) based on the ADNI protocol (http://www.loni.ucla.edu/ADNI/Cores/index.shtml).
MRI data preprocessing
Preprocessing of the structural T1-weighted data was performed with Statistical Parametric Mapping version 8 (Wellcome Department of Neuroimaging, London, United Kingdom, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), using standard automated pipelines (31). See supplemental information for details.
Statistical Analyses
Whole brain voxel-wise analyses were conducted using the general linear model, performed with the VBM toolbox of SPM8. Age, gender, total gray matter volume (GMV), site, pubertal development, Performance IQ, Verbal IQ, and SES were controlled for in each model. For all analyses, an initial height threshold of p ≤ .005 was implemented at the voxel level, with a corrected family-wise error (FWE; p ≤ .05) subsequently applied to identify significant clusters. It should be noted that for the VBM analyses outlined below, results were not meaningfully altered when adopting an initial height threshold of p ≤ .001.
Latent Variable Analysis
Latent variable analysis has been proposed as a powerful method for incorporating multi-informant reports of psychopathology as predictor variables in regression modeling (37). In the present study, confirmatory factor analysis was carried out using the package Lavaan in R (38). In particular, a latent ADHD symptom variable was derived from the observed multi-informant measures. Observed measures included parent- and self-reports of ADHD symptomatology on the SDQ, as well as the ADHD symptom counts based on the parent version of the DAWBA.
Gene Expression
Finally, relations between the structural correlates of ADHD symptomatology and gene expression were examined. In order to test for associations between gene expression and brain structural correlates of ADHD symptoms, we used a brain map derived from regressions between GMV and the mean composite of multi-informant behavioral ratings. This decision was based on previous research indicating that averaging symptom ratings across multiple informants appears optimal relative to other approaches such as structural equation modeling (25). The unthresholded t-statistic map, resulting from regressing regional gray matter volume against the mean composite of multi-informant ratings, was subsequently tested for associations with patterns of gene expression. Using the alleninf toolbox (39) and gene expression data collected for the Allen Human Brain Atlas (30), we tested for an association with several dopaminergic genes that have been previously implicated in ADHD symptomatology: DRD1, DRD2, and DRD4. In brief, the toolbox extracts the MNI coordinates of each gene expression sampling site, draws a spherical ROI (r = 4 mm) and averages values of the statistical map within each spherical ROI. Average ROI values are then correlated with normalized gene expression values. Further details can be found elsewhere (39).
RESULTS
Demographic and Behavioral Measures
Demographic information for participants is provided in Table 1. Correlations between multi-informant ratings (including mean composite and latent ADHD measures) and reaction time variability are listed in Table 2.
TABLE 2.
Bivariate correlations between behavioral and cognitive measures of ADHD symptomatology.
| Parent SDQ | DAWBA | Reaction Time Variability | Composite ADHD | Latent Variable | ||
|---|---|---|---|---|---|---|
| Youth SDQ | Pearson Correlation | *.430 | *.359 | *.139 | *.736 | *.518 |
| Sig. (2-tailed) | p <.001 | p <.001 | p <.001 | p <.001 | p <.001 | |
| N | 1538 | 1538 | 767 | 1538 | 1538 | |
| Parent SDQ | Pearson Correlation | *.661 | *.139 | *.861 | *.973 | |
| Sig. (2-tailed) | p <.001 | p <.001 | p <.001 | p <.001 | ||
| N | 1538 | 767 | 1538 | 1538 | ||
| DAWBA | Pearson Correlation | *.108 | *.832 | *.802 | ||
| Sig. (2-tailed) | p <.005 | p <.001 | p <.001 | |||
| N | 767 | 1538 | 1538 | |||
| Reaction Time Variability | Pearson Correlation | *.159 | *.149 | |||
| Sig. (2-tailed) | p <.001 | p <.001 | ||||
| N | 767 | 767 | ||||
| Composite ADHD | Pearson Correlation | *.944 | ||||
| Sig. (2-tailed) | p <.001 | |||||
| N | 1538 | |||||
Both parent and youth ratings of ADHD symptomatology were inversely correlated with SES (ranging from r = −0.095 to r = −0.193), as well as Performance and Verbal IQ (ranging from r = −0.107 to r = −0.231). In addition, parent ADHD ratings on the DAWBA and SDQ were inversely correlated with pubertal stage (ranging from r = −0.107 to r = −0.112). Males, on average, also possessed significantly higher ADHD symptom ratings, but only on the DAWBA (t = 5.86, p < 0.001) and parent SDQ (t = 6.66, p < 0.001).
Imaging Analyses
DAWBA Symptom Count
Regressing regional gray matter volume against total ADHD symptom count—based on parent reporting on the DAWBA—revealed a negative association in bilateral ventromedial and orbital lateral prefrontal cortices (3424 voxels, x = −4, y = 30, z = −20; peak Z score = 4.12) (Figures 1 and 2). The effect size for this association was f2 = 0.01. No other associations survived correction for multiple comparisons.
FIGURE 1.
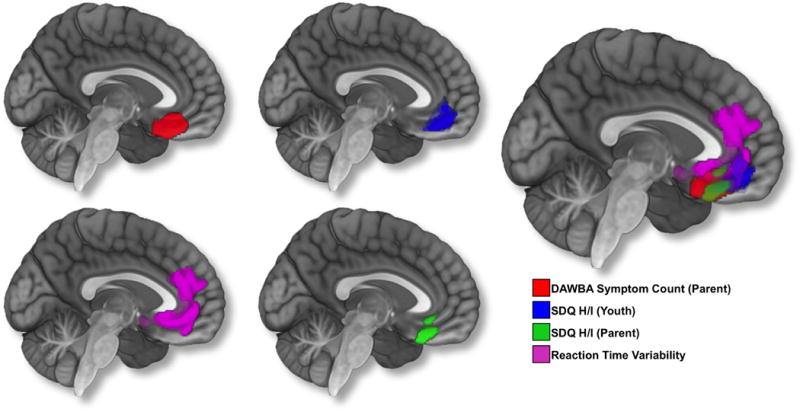
Results from whole brain voxel-wise analyses. Age, gender, total gray matter volume (GMV), site, pubertal development, Performance IQ, Verbal IQ, and socio-economic status were controlled for in the analyses. An initial height threshold of p ≤ .005 was implemented at the voxel level, with a corrected family-wise error (FWE; p ≤ .05) subsequently applied to identify significant clusters. To visualize overlap in findings. the image on the right is a composite of all associations.
FIGURE 2.
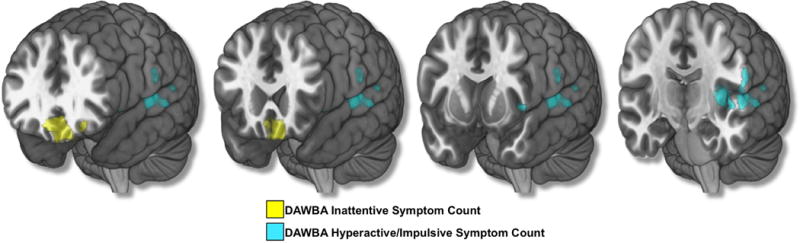
Results from whole brain voxel-wise analyses using Hyperactive/Impulsive and Inattentive symptom counts. Age, gender, total gray matter volume (GMV), site, pubertal development, Performance IQ, Verbal IQ, and socio-economic status were controlled for in the analyses. An initial height threshold of p ≤ .005 was implemented at the voxel level, with a corrected family-wise error (FWE; p ≤ .05) subsequently applied to identify significant clusters.
Follow up analyses were conducted using Hyperactive/Impulsive and Inattentive symptom counts on the DAWBA. Hyperactive/Impulsive and Inattentive symptom counts on the DAWBA were positively correlated (r = 0.57, p = 2.93×10−130). When analyzed separately, Inattentive symptoms were negatively associated with gray matter volume in bilateral ventromedial and left orbital lateral prefrontal cortices (2906 voxels, x = −4, y = 28, z = −20; peak Z score = 4.58), and Hyperactive/Impulsive symptoms were negatively associated with gray matter in the left superior temporal gyrus (STG), left posterior insula, and left parietal operculum (2006 voxels, x = −63, y = −16, z = 4; peak Z score = 4.16) (Figure 2). No other associations survived correction for multiple comparisons. Results were not altered when covarying for self-reported mood and anxiety symptomatology on the SDQ.
Parent SDQ
Regression analysis revealed a negative association between the Hyperactivity/Inattention subscale score on the parent version of the SDQ and gray matter volume in bilateral ventromedial prefrontal cortices (1887 voxels, x = 10, y = 38, z = −15; peak Z score = 4.86) (Figures 1 and 2). The effect size for this association was f2 = 0.01. No other associations survived correction for multiple comparisons. Again, results were not changed when controlling for self-reported mood and anxiety symptomatology on the SDQ.
Youth SDQ
Regressing regional gray mater volume against the Hyperactivity/Inattention subscale score on the youth self-report version of the SDQ revealed a negative association in bilateral ventromedial and right orbital lateral prefrontal cortices (2576 voxels, x = 9, y = 33, z = −15; peak Z score = 3.73) (Figure 1). The effect size for this association was f2 = 0.02. No other associations survived correction for multiple comparisons. Results were not changed when controlling for self-reported mood and anxiety symptomatology on the SDQ.
Latent Variable
Regional gray matter volume was regressed against factor loadings on the latent ADHD symptom variable, derived using confirmatory factor analysis. Factor loadings on the latent variable were negatively associated with gray matter volume in bilateral ventromedial prefrontal cortices (3210 voxels, x = 9, y = 33, z = −17; peak Z score = 4.96) (See Supplementary Figure 1). The effect size for this association was f2 = 0.01. The Dice Similarity Coefficient (DSC) was calculated in order to quantify the spatial overlap between results obtained using the latent ADHD variable and each of the three ADHD behavioral rating measures described above (DAWBA, parent SDQ, youth SDQ). Based on image validation literature, a good overlap occurs when DSC >0.70 (40). The DSC was high when comparing results obtained with the latent ADHD variable and both the DAWBA and parent SDQ (0.72 and 0.73, respectively); however, the DSC was considerably lower when comparing with the youth SDQ (0.29).
Supplementary Figure 1 depicts the relationship between regional gray matter volume and the CFA-derived latent ADHD variable, as well as a composite ADHD score calculated by averaging across multi-informant data on the SDQ and DAWBA. The correlation between CFA-derived latent ADHD variable and the composite ADHD score was very high, r = 0.94, p < 0.001. Again, the DSC was calculated in order to quantify the spatial overlap between the multi-informant averaging method and the latent variable approach. The DSC for the two maps was equal to 0.71.
Stop Signal Task
Mean reaction time and SSRT were not related to measures of ADHD symptomatology. However, each measure of ADHD symptomatology was positively correlated with reaction time variability (r = 0.11 – 0.14) (See Table 2). Regressing regional gray matter volume against reaction time variability in a subset of participants with available behavioral data (N = 767) revealed a negative association in bilateral medial prefrontal cortices, including dorsal portions of the anterior cingulate gyrus (5007 voxels, x = 9, y = 50, z = 12; peak Z score = 3.85). The effect size for this association was f2 = 0.03. No other associations survived correction for multiple comparisons (Figure 1). Figure 3 depicts the region in which gray matter volume was negatively associated with reaction time variability, as well as all each of the three behavioral rating measures (center-of-gravity for region of overlap, x = 3.13, y = 34.42, z = −14.84).
FIGURE 3.
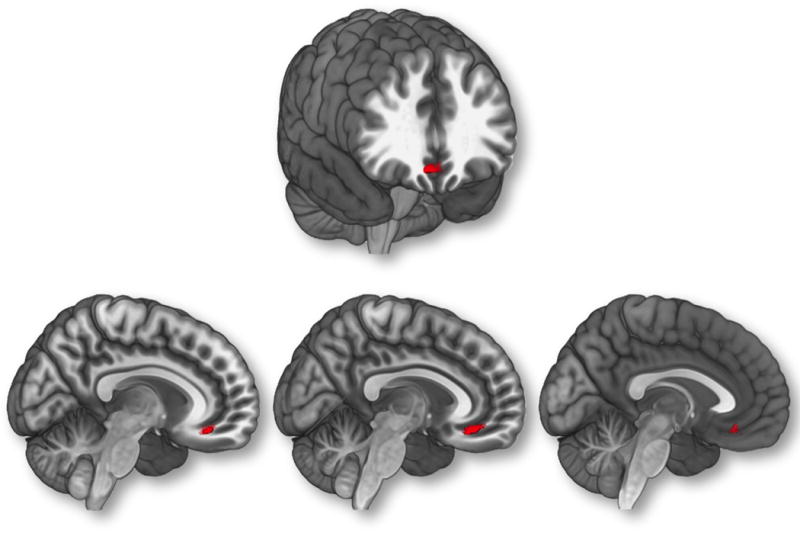
Figure illustrates region of overlap between parent DAWBA, parent SDQ, youth SDQ, and reaction time variability. Age, gender, total gray matter volume (GMV), site, pubertal development, Performance IQ, Verbal IQ, and socio-economic status were controlled for in the analyses. An initial height threshold of p ≤ .005 was implemented at the voxel level, with a corrected family-wise error (FWE; p ≤ .05) subsequently applied to identify significant clusters.
Gene Expression
The statistical map resulting from regressing regional gray matter volume against the mean composite of multi-informant ratings (Supplementary Figure 2) was associated with DRD1 (r = −0.27, p = 2.0 × 10−54) and DRD2 expression (r = 0.09, p = 1.9 × 10−7) (Figures 4 and 5). DRD4 expression was not associated with brain areas that were related to ADHD symptomatology.
FIGURE 4.
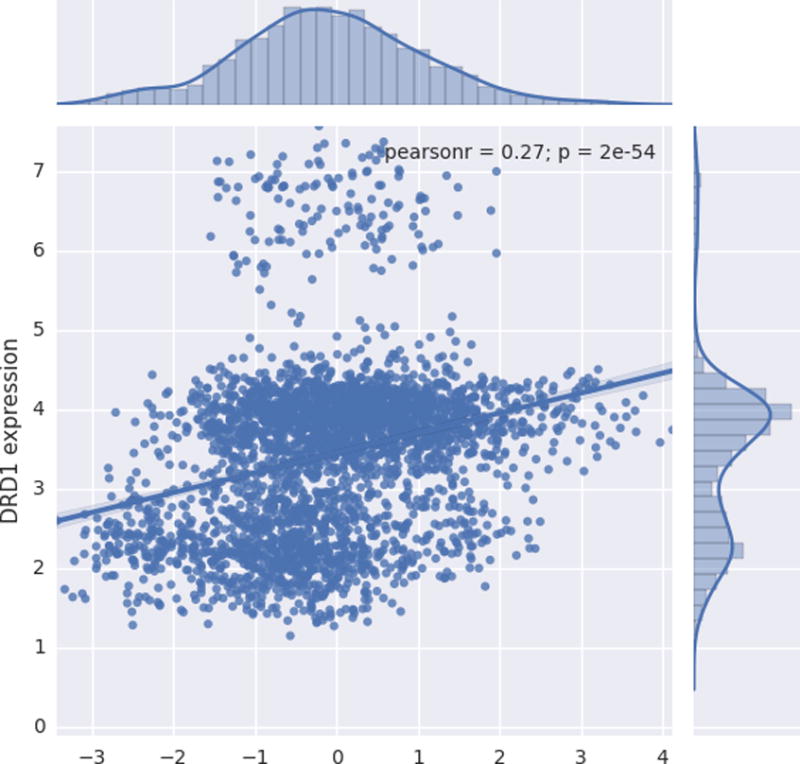
Scatter plot depicting the relationship between normalized gene expression values (y axis) and t-statistic value (x axis) corresponding to the association between gray matter volume (GMV) and multi-informant average of ADHD symptoms. Positive t values indicate an inverse association between GMV and symptomatology, whereas negative t values represent a positive association between GMV and ADHD symptomatology.
FIGURE 5.
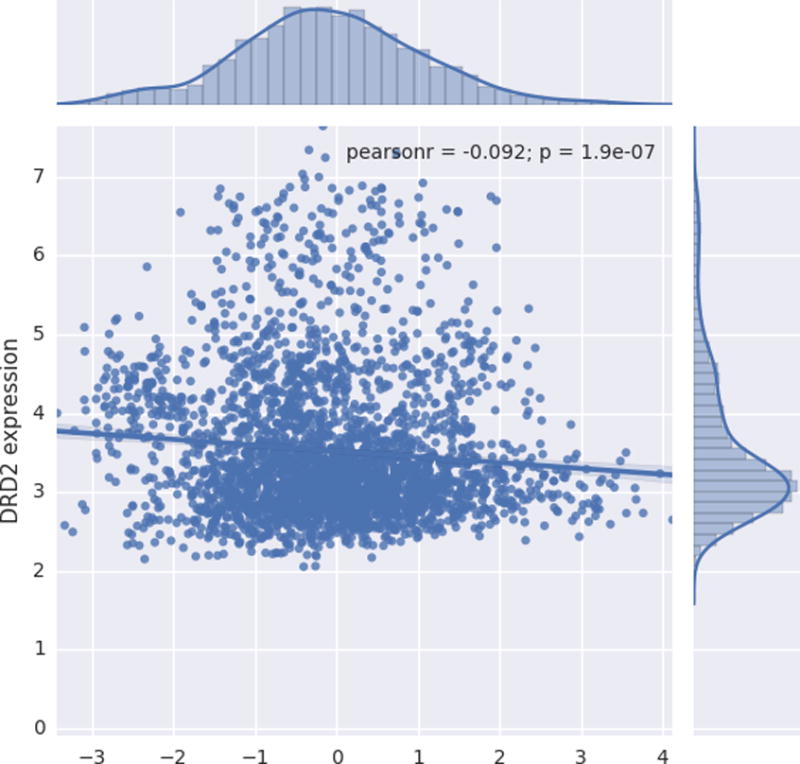
Scatter plot depicting the relationship between normalized gene expression values (y axis) and T-statistic value (x axis) corresponding to the association between gray matter volume (GMV) and multi-informant average of ADHD symptoms. Positive t values indicate an inverse association between GMV and symptomatology, whereas negative t values represent a positive association between GMV and ADHD symptomatology.
DISCUSSION
To our knowledge, this is the largest population-based structural imaging study on ADHD symptomatology to date. Parent and youth ratings of ADHD symptomatology were all negatively associated with gray matter volume in an overlapping portion of the vmPFC. Critically, our findings were not changed when measures of mood and anxiety symptomatology were controlled for in analyses. When analyzing Hyperactivity/Impulsivity and Inattention symptom counts separately, we found that Inattention symptoms were associated with reduced volume in bilateral vmPFC, whereas Hyperactive/Impulsive symptoms were related to reduced gray matter volume in the left superior temporal gyrus, parietal operculum, and posterior insula. Thus, reduced gray matter volume in ventromedial prefrontal cortices appears to be particularly tied to aspects of inattentive symptomatology in adolescents. In line with this interpretation, we found that reaction time variability—posited to reflect lapses in attention as opposed to hyperactivity—was negatively associated with gray matter volume in an overlapping region of the vmPFC. Given convergence across dimensional, multi-informant behavioral ratings, and a measure of neurocognitive functioning that has been previously tied to ADHD, our findings indicate that vmPFC structure is a brain-based marker for attention problems in adolescents.
In the largest VBM study to date on adult ADHD, a significant negative correlation was revealed between vmPFC gray matter volume and dimensional measures of ADHD symptomatology (41). Specifically, when analyzing patients and controls together, the authors found an inverse relationship between dimensional measures of ADHD symptomatology and vmPFC gray matter volume. Inattentive symptoms, in particular, were negatively correlated with gray matter volume in the vmPFC (41). Strikingly, the findings of Maier et al. (2015) largely overlap with the results of the present study. Taken together, reduced gray matter in the vmPFC may serve as a marker for attention problems in both adolescent and adult populations.
Increased reaction time variability on tasks of vigilant attention has been a common finding when comparing children with ADHD versus typically developing controls (26, 27). This finding has led others to hypothesize that increased reaction time variability is tied to aberrant default mode network (DMN) activity (42). In particular, the default-mode interference hypothesis posits that activity in the DMN, which is typically attenuated during goal-directed tasks, can persist into periods of task-related processing and, as a result, compete with task-specific neural processing (42). The vmPFC represents a primary hub in the brain’s default mode network (DMN)—a network posited to play a central role in mind-wandering and task-unrelated thought. Although speculative, it is possible that the volumetric reductions in the vmPFC may be linked to DMN dysfunction. In a recent study by Salavert et al. (2015), ADHD participants exhibited reduced deactivation of the medial prefrontal cortex during a working memory task. The authors suggest that failure to deactivate the medial prefrontal cortex is tied to lapses of attention, and that this may be a central feature of ADHD symptomatology. It is possible that findings in the present study are tied to DMN dysfunction, and, more specifically, reduced vmPFC gray matter volume may be related to an impaired ability to deactivate portions of the DMN. Future studies are needed to test this possibility.
It is noteworthy that, despite modest correlations between multi-informant behavioral ratings (r = 0.36–0.66), we observed striking convergence with regard to brain structural correlates. Similarly, reaction time variability was modestly correlated with behavioral ratings of ADHD symptomatology (r = 0.11–0.14), and, again, we observed considerable overlap with regard to anatomical correlates. These findings suggest that the vmPFC is tied to both parental and youth self-reports of ADHD symptomatology, as well as an objective behavioral measure of ADHD symptomatology (i.e., reaction time variability).
In the present study, two different methods were used to analyze multi-informant ratings of adolescent attention problems. First, a composite ADHD symptom score was created by averaging multi-informant behavioral ratings. Averaging multi-informant ratings of ADHD symptomatology has been found by others to be the optimal method for integrating multi-informant data (25). Second, confirmatory factor analysis was used to derive a latent ADHD symptom variable based on multi-informant DAWBA and SDQ data. To our knowledge, this is the first neuroimaging study to directly compare these two methods of handling multiple-informant behavioral data. In short, we found that these two methods yielded very similar results when relating to regional gray matter volume, producing statistical maps with a high degree of spatial overlap.
We found that the statistical map representing the relationship between GMV and ADHD symptomatology was significantly correlated with patterns of dopaminergic gene expression. More specifically, areas of the brain in which ADHD symptomatology was inversely associated with GMV tended to be regions that expressed the gene that encodes for the D1 dopamine receptor, DRD1. This finding is particularly intriguing given that D1 receptor density in the cortex has been recently linked to the functional decoupling of DMN and task-positive networks in humans (43). Thus, D1 receptor density in the vmPFC—a major hub of the DMN—may be particularly relevant with regard to ADHD symptomatology, affecting the dynamic interplay between task-negative and task-positive networks. Candidate gene studies have also tied DRD1 to symptoms of inattention (44). Brain areas showing reduced volume at higher levels of ADHD symptomatology were characterized by relatively low DRD2 expression. It is unclear why DRD1 and DRD2 expression patterns were differentially associated with our VBM results. It is possible that these findings can be explained, in part, by the differential distribution of D1 and D2 receptors in the human brain (45). Previous research indicates a dorsolateral-ventromedial gradient with regard to the respective distribution of D1 and D2 receptors, with D1 receptors being more prevalent in ventromedial regions of the striatum and cortex (45, 46). This differential distribution of D1 and D2 receptors is also believed to reflect a distinction between “direct” and “indirect” cortico-striatal-thalamic-cortical circuits—with D1 receptors playing a more important role in “direct” circuits that serve to disinhibit thalamic activity and initiate behavior, and D2 receptors playing a greater role in “indirect” circuits that serve to inhibit neuronal activity (46–48). Although speculative, the results of our follow-up gene expression analyses may reflect a relative imbalance between D1 and D2 systems. However, it is important to emphasize that these gene expression analyses are meant to be hypothesis-generating in nature. It is critical that future studies more directly test these possibilities.
Before concluding, it is important to address limitations of the present study. First, given that we have focused on regional gray matter volume in our analyses, we are unable to definitively comment on the neurophysiological underpinnings of our VBM findings. Similarly, we are unable to comment on possible ties to aberrant structural and/or functional connectivity. Second, patterns of gene expression are related to age and developmental stage, and our gene expression analyses do not capture these age-related influences. Third, it is worth noting that, across all analyses, observed effect sizes were small. This may reflect that the vmPFC findings in the present study constitute one part in the full elucidation of ADHD’s brain-based correlates. Fourth, we observed an apparent dissociation with regard to the neuroanatomical correlates of hyperactive/impulsive and inattentive symptomatology. It is possible, however, that differences in statistical power may have influenced these results—in particular, more variance was observed in inattentive symptoms (M = 2.64, SD = 3.86) relative to hyperactive/impulsive symptoms (M = 1.42, SD = 2.65).
Taken together, reduced volume in the vmPFC may serve as a relatively stable biomarker for inattention. The present study lays the foundation for informing early intervention and prevention efforts. One exciting future direction will be to examine the extent to which vmPFC structure during adolescence predicts subsequent symptom trajectories into adulthood. This work has the exciting potential to identify brain-based markers for future outcomes, helping to target youths at greatest risk for poor outcomes.
Supplementary Material
Acknowledgments
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the FP7 projects IMAGEMEND(602450; IMAging GEnetics for MENtal Disorders), AGGRESSOTYPE (602805) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grants ‘‘Developmental pathways into adolescent substance abuse’’ (93558) and Consortium on Vulnerability to Externalizing Disorders and Addictions [c-VEDA] (MR/N000390/1), the Swedish funding agencies VR, FORTE and FORMAS, the Medical Research Council and the Wellcome Trust (Behavioural and Clinical Neuroscience Institute, University of Cambridge), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1), the National Institutes of Health, U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence. In addition, Dr. Garavan is supported by a Tobacco Centers of Regulatory Science award (P50DA036114).
Dr. Banaschewski has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. Dr. Gallinat has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen-Cilag, and Bristol-Myers Squibb; he has received speaking fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb. Dr Barker has received honoraria from General Electric for teaching on scanner programming courses.
Footnotes
Disclosures
The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.APA. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 3.McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1948–1956. doi: 10.1176/appi.ajp.161.11.1948. [DOI] [PubMed] [Google Scholar]
- 4.Almeida Montes LG, Ricardo-Garcell J, Barajas De La Torre LB, Prado Alcantara H, Martinez Garcia RB, Fernandez-Bouzas A, et al. Clinical correlations of grey matter reductions in the caudate nucleus of adults with attention deficit hyperactivity disorder. J Psychiatry Neurosci. 2010;35:238–246. doi: 10.1503/jpn.090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry. 2011;68:1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A, et al. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psychiatry. 2011;69:857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Kan CC, Buitelaar J, et al. Brain alterations in adult ADHD: effects of gender, treatment and comorbid depression. Eur Neuropsychopharmacol. 2014;24:397–409. doi: 10.1016/j.euroneuro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 10.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res. 2010;182:231–237. doi: 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pironti VA, Lai MC, Muller U, Dodds CM, Suckling J, Bullmore ET, et al. Neuroanatomical abnormalities and cognitive impairments are shared by adults with attention-deficit/hyperactivity disorder and their unaffected first-degree relatives. Biol Psychiatry. 2014;76:639–647. doi: 10.1016/j.biopsych.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montes LG, Ricardo-Garcell J, De la Torre LB, Alcantara HP, Garcia RB, Acosta DA, et al. Cerebellar gray matter density in females with ADHD combined type: a cross-sectional voxel-based morphometry study. J Atten Disord. 2011;15:368–381. doi: 10.1177/1087054710366421. [DOI] [PubMed] [Google Scholar]
- 14.Makris N, Liang L, Biederman J, Valera EM, Brown AB, Petty C, et al. Toward Defining the Neural Substrates of ADHD: A Controlled Structural MRI Study in Medication-Naive Adults. J Atten Disord. 2015;19:944–953. doi: 10.1177/1087054713506041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amico F, Stauber J, Koutsouleris N, Frodl T. Anterior cingulate cortex gray matter abnormalities in adults with attention deficit hyperactivity disorder: a voxel-based morphometry study. Psychiatry Res. 2011;191:31–35. doi: 10.1016/j.pscychresns.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Bonath B, Tegelbeckers J, Wilke M, Flechtner HH, Krauel K. Regional Gray Matter Volume Differences Between Adolescents With ADHD and Typically Developing Controls: Further Evidence for Anterior Cingulate Involvement. J Atten Disord. 2016 doi: 10.1177/1087054715619682. [DOI] [PubMed] [Google Scholar]
- 17.Batty MJ, Palaniyappan L, Scerif G, Groom MJ, Liddle EB, Liddle PF, et al. Morphological abnormalities in prefrontal surface area and thalamic volume in attention deficit/hyperactivity disorder. Psychiatry Res. 2015;233:225–232. doi: 10.1016/j.pscychresns.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. Int J Methods Psychiatr Res. 2007;16(Suppl 1):S16–23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mous SE, White T, Muetzel RL, El Marroun H, Rijlaarsdam J, Polderman TJ, et al. Cortical morphology as a shared neurobiological substrate of attention-deficit/hyperactivity symptoms and executive functioning: a population-based pediatric neuroimaging study. J Psychiatry Neurosci. 2016;41:150371. doi: 10.1503/jpn.150371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen TV, Karama S, et al. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry. 2012;51:18–27 e12. doi: 10.1016/j.jaac.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 23.Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, et al. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. The American journal of psychiatry. 2011;168:143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris S, Cuthbert B. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martel MM, Schimmack U, Nikolas M, Nigg JT. Integration of symptom ratings from multiple informants in ADHD diagnosis: a psychometric model with clinical utility. Psychol Assess. 2015;27:1060–1071. doi: 10.1037/pas0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 27.Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, et al. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- 29.Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 32.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- 33.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 34.Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 36.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misus. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horton NJ, Laird NM, Zahner GEP. Use of Multiple Informant Data as a Predictor in Psychiatric Epidemiology. International Journal of Methods in Psychiatric Research. 1999;8:6–18. [Google Scholar]
- 38.Rosseel Y. lavaan: An R Package for Structural Equation Modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 39.Gorgolewski KJ, Fox AS, Chang L, Schafer A, Arelin K, Burmann I, et al. Organization for Human Brain Mapping. Hamburg, Germany: 2014. Tight fitting genes: Finding relations between statistical maps and gene expression patterns. [Google Scholar]
- 40.Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging. 1994;13:716–724. doi: 10.1109/42.363096. [DOI] [PubMed] [Google Scholar]
- 41.Maier S, Perlov E, Graf E, Dieter E, Sobanski E, Rump M, et al. Discrete Global but No Focal Gray Matter Volume Reductions in Unmedicated Adult Patients with Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Roffman JL, Tanner AS, Eryilmaz H, Rodriguez-Thompson A, Silverstein NJ, Ho NF, et al. Dopamine D1 signaling organizes network dynamics underlying working memory. Sci Adv. 2016;2:e1501672. doi: 10.1126/sciadv.1501672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luca P, Laurin N, Misener VL, Wigg KG, Anderson B, Cate-Carter T, et al. Association of the dopamine receptor D1 gene, DRD1, with inattention symptoms in families selected for reading problems. Mol Psychiatry. 2007;12:776–785. doi: 10.1038/sj.mp.4001972. [DOI] [PubMed] [Google Scholar]
- 45.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 46.Lichter DG, Cummings JL. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: Guilford Press; 2001. [Google Scholar]
- 47.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 48.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


