Abstract
Background
Previous literature documents associations between low socioeconomic status (SES) and poor health outcomes, including asthma. However, this literature has largely focused on the effects of current family circumstances.
Objective
To test an intergenerational hypothesis, that the childhood SES that parents experience will be associated with asthma outcomes in their children, independent of effects of current family SES. Secondly, to test whether this association is in part due to difficulties in current parent-child relationships.
Methods
Observational study, whereby 150 parents were interviewed about their childhood SES, and their children (physician-diagnosed with asthma, ages 9–17) were interviewed about current family stress. Asthma control was assessed by parent- and child-report (primary outcome), and blood was collected from children to measure cytokine production relevant to asthma (secondary outcomes).
Results
To the degree that parents had lower childhood SES, their offspring showed worse asthma outcomes across multiple indicators. This included lower asthma control scores (parent and child-report, p’s<.05), and greater stimulated production of Th-2 and Th-1 cytokines by peripheral blood mononuclear cells (PBMC) (p’s<.05). These associations were independent of current family SES. Mediation analyses were consistent with a scenario wherein parents with low childhood SES had current family relationships that were more stressful, and these difficulties in turn related to worse asthma control and greater cytokine production in children.
Conclusions
These results suggest the potential ‘long reach’ of low socioeconomic status across generations, and the importance of expanding theories of how the social environment can affect childhood asthma to include characteristics of earlier generations.
Keywords: socioeconomic status, family stress, asthma, childhood
INTRODUCTION
Low socioeconomic status (SES) has robust associations with a number of adverse health outcomes1,2, including asthma3,4. For example, children with asthma who come from low SES backgrounds are more likely to visit the emergency department, to be hospitalized for asthma, and to experience greater functional impairment (e.g., greater activity limitations) because of asthma5–10. In children with asthma, lower SES also is associated with a tendency to express inflammatory profiles indicative of poorly controlled asthma, including higher eosinophil counts and larger Th-2 cytokine responses following in vitro stimulation of peripheral blood mononuclear cells (PBMC)11,12.
The majority of the research in this area has focused on the effects of current SES on health. However, in more recent years there has been growing interest in the idea of intergenerational effects – that is, the idea that environments experienced in one generation could have effects on the health of subsequent generations13,14. These effects have been primarily demonstrated using experimental manipulations in animal models with outcomes such as birthweight, obesity, diabetes, cardiovascular risk, and behaviors, whereby the effects of an environmental condition (e.g., changes to diet) performed on the grandparent generation are evident in the grandoffspring generation (even when the grandoffspring do not experience the same environment as their grandparents)15–18. These types of findings raise the intriguing question of whether it is possible for parents’ own childhood SES environments to affect asthma outcomes in their children, above and beyond the effects of current SES.
In human populations, some preliminary evidence exists in support of the idea of intergenerational transmission of environmental effects. For example, women who were pregnant during the Dutch Hunger Winter of World War II had grandchildren with greater adiposity and poorer health compared to women whose pregnancy occurred outside the window of the Dutch famine19. Other evidence includes the finding that parents who grew up with low childhood SES were more likely to have children with higher blood pressure and higher levels of an inflammatory biomarker that predicts cardiovascular disease, C reactive protein, even after controlling for current SES20, as well as to have children with lower birthweights21. In addition, grandmother smoking during pregnancy has been found to predict an increased risk of asthma in grandchildren, independent of maternal smoking during pregnancy22. In the present study, we tested whether the childhood SES that parents grew up with would forecast their children’s level of asthma control (primary outcome), independent of the effects of current family SES. Secondarily, we investigated whether parents’ childhood SES would be linked to immune responses relevant to asthma. Asthma is an inflammatory disease, characterized by T-helper cell release of cytokines in response to allergens, infections, and other triggers. A key role has been proposed for the release of Th-2 cytokines in airway inflammation23,24. In addition, the release of Th-1 cytokines linked to anti-viral cellular responses may also be relevant to asthma25. Alternatively, front-line immune defenses involved in innate immunity, such as Toll-like receptors that recognize pathogens and produce pro-inflammatory cytokines, are also thought to contribute to asthma exacerbations26,27. Because each of these represent a different immunologic pathway to asthma, we investigated separately the PBMC production of Th-2, Th-1, and pro-inflammatory cytokines in response to in vitro stimulation, in order to document plausible biological correlates of intergenerational SES effects.
Why might parents’ early life circumstances predict asthma outcomes in their children? One psychosocial explanation may be related to stress in the parent-child relationship. If parents grow up in low SES environments, they are on average more likely to have been exposed to conflictual, harsh, and unsupportive environments28–30. Parents who grow up in these environments may then be more likely to engage in punitive and inconsistent parenting behaviors as adults31. In turn, factors such as dysfunctional family interactions, parenting difficulties, family conflict, and lack of parent support have been associated with atopic illness, asthma diagnosis, asthma symptoms and mortality, and poorer pulmonary function in children32–35. The idea that stressful environments experienced early in a parent’s life can impact offspring behavior is also seen in animal models (mice) whereby manipulations of childhood stress (e.g., social instability) in a parent generation produce behavioral changes (e.g., anxiety) in offspring mice36,37.
Thus the goals of the present study were three-fold. We first tested whether parents who experienced lower childhood SES environments were more likely to have children with worse asthma outcomes, as reflected in parent- and child-reports of asthma control (primary outcome) and secondarily, to have children with greater stimulated production of cytokines implicated in asthma. Second, to determine whether these associations simply reflect the inter-generational continuity of poverty, we examined whether they persisted following statistical adjustment for families’ current SES. Lastly, we used statistical modeling to examine the plausibility of a mediational scenario, wherein parents with low childhood SES have more stressful current family relationships, with these difficulties in turn fostering worse child asthma outcomes.
METHOD
Participants
One hundred and fifty children ages 9–17, physician-diagnosed with asthma, were recruited through one health care system, NorthShore University Health System, and one federally-qualified health center, Erie Family Health Center (hence all families in this study had access to health care). See Online Supplement. Children came to the research center with one parent between July 2013 and December 2014 to complete the assessments below. Families were required to be fluent in English, and children had to be free of acute respiratory illness at the time of the visit and have no other chronic physical illnesses other than asthma. Participants had current asthma, with 96% having a current beta agonist prescription, 71% having a current inhaled corticosteroid prescription, and all had a recent office visit for asthma. Children gave written assent and parents provided written consent. This study was approved by the Northwestern, NorthShore, and Erie IRBs.
Measures
Socioeconomic status (SES)
SES was measured by interviewing parents about family resources. Parents’ childhood SES was assessed via early childhood home ownership (home ownership is often used as a measure childhood SES, given that other types of assets can be difficult for adults to retrospectively recall). Parents were asked whether their family owned or rented their home during their first 3 years of life. Thus renting a home constituted the lower SES group, and owning a home constituted the higher SES group. The accuracy and predictive validity of this question has been previously established, as well as the importance of SES during the early childhood years38,39. Current family SES was measured by asking parents about the amount of assets (family savings, investments, etc.) that their family could easily convert to liquid cash in an emergency. This measure is consistent with previous approaches to measuring family resources11,40 (also see www.macses.ucsf.edu).
Family relationship stress
Family relationship stress was determined by interviewing children using the University of California Los Angeles Life Stress Interview41,42. This interview probes chronic stress within the family over the past 6 months, focusing on conflict between family members, trust, and support. Interviewers rate the extent of a participant’s family relationship stress on a 1–5 scale (including .5 ratings), with higher numbers reflecting more severe and persistent family relationship difficulties (e.g., greater conflict, less trust, and lower support). Reliability and validity for this interview have been demonstrated in children as young as 842,43. Interrater reliability (intraclass correlation coefficient) across interviewers was .93.
Asthma control
The Asthma Control Test44,45 is a 5-item questionnaire that assesses asthma symptoms, use of rescue medications, and the effects of asthma on daily functioning over the previous 4 weeks. Reliability for this questionnaire is high (.84), and validity has been established through asthma specialists’ ratings of control44. This measure is commonly used in clinical settings, and has parent- and child-report versions. Higher numbers indicate more well-controlled asthma (possible range: 5–25).
Immunologic measures - cytokine production
We measured stimulated cytokine secretion by PBMCs. Although airway cells would better reflect activity at the site of disease, obtaining them requires an invasive procedure difficult for children without a clinical indication. For that reason, pediatric asthma studies often rely on assessments of PBMC activity, which correlate with data obtained via bronchoalveolar lavage specimens, and also with eosinophil counts and disease severity46,47. Antecubital blood was drawn into BD Cell Preparation Tubes (Becton Dickinson, Franklin Lakes, NJ) containing sodium heparin, and PBMCs were isolated by density-gradient centrifugation according to manufacturer instructions, and dispensed into the wells of culture plates in the presence of different mitogens. We focused on one common mitogen known to generate Th-1 and Th-2 cytokine release: here we incubated 0.5×106 PBMCs with 25ng/mL of phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St. Louis, MO) + 1ug/mL of ionomycin (INO; Sigma-Aldrich, St. Louis, MO) for 24 hours at 37°C in 5% CO2, similar to previous studies11,12,48. An unstimulated well was prepared containing the same number of PBMCs but no mitogen, and cultured under the same conditions. At the end of the incubation, supernatants were harvested by centrifugation, and frozen at −80°C until assayed in batch via electrochemiluminescence on a SECTOR Imager 2400A (Meso Scale Discovery, MSD). This instrument gives accurate, sensitive multiplex readouts across a wide dynamic range49. We made use of MSD’s Human Th-1/Th-2 7-Plex Tissue Culture Kit, which measures both Th-2 (IL-2, IL-4, IL-5, and IL-13) and Th-1 (IFN-γ, IL-10) cytokines in parallel. Mean inter-assay coefficients of variation ranged from 1.50–3.64%. Cytokine responses were quantified by subtracting values in the unstimulated wells from those in the PMA/INO wells.
To measure pro-inflammatory cytokine production in response to TLR stimulation, we utilized one microbial and one viral analogue ligand. 0.5×106 PBMCs were dispensed into plates containing either 0.1ng/mL of lipopolysaccharide (LPS, a molecule found on Gram-negative bacteria that stimulates the Toll Like Receptor (TLR)-4 pathway; Invivogen, San Diego, CA) or 100ug/mL of Poly I:C (double stranded RNA, which stimulates the TLR-3 pathway; Invivogen, San Diego, CA) and incubated for 24 hours at 37°C in 5% CO2, similar to previous studies50,51. An unstimulated well was also included on the plate. Supernatants were assayed in batch for cytokine production using the Sector Imager and a custom MSD Human Pro-Inflammatory Tissue Culture kit, which measured IL-1β, IL-6, and TNF-α in parallel. Interassay coefficients of variation were 3.49–8.68%, and as per above, unstimulated values were subtracted out prior to analysis.
Statistical Analyses
Statistical analyses involved 4 sets of tests: (1) associations of parents’ childhood SES with children’s asthma control and immune outcomes; (2) associations of parents’ childhood SES with current family relationship stress; (3) associations of current family relationship stress with child asthma control and immune outcomes; and (4) statistical mediation analyses of the pathway: parents’ childhood SES → current family relationship stress → child asthma outcomes. For (1) and (2), analyses of covariance (ANCOVAs) were conducted, given the dichotomous nature of the parent childhood SES variable. For (3), multiple regression analyses were conducted, given the continuous nature of the family stress variable. Child age, gender, ethnicity (White vs. other), use of beta agonists, and use of inhaled corticosteroids (number of days in the past week) were included as covariates. We note that rather than including whether the child has a current prescription for medication as a covariate (given that 96% were on a beta agonist so there would be little variability in this measure), we included instead the number of days each medication was used in the past week as a proxy for current medication adherence/usage. In a second set of analyses, current SES was added to the above models to test whether the effects of parent childhood SES persisted over and above the effects of current SES. For (4), we tested the significance of the indirect effect using statistical mediation analyses with nonparametric bootstrapping to obtain the bias corrected and accelerated confidence intervals of indirect effects, as recommended by Preacher and colleagues52. Confidence intervals that do not include 0 indicate statistically significant indirect pathways. Although the study was observational, and hence cannot determine causality, this statistic tells us whether the data are consistent with a mediation explanation that parents’ childhood SES operates through current family relationship stress to affect children’s asthma.
RESULTS
See Table 1 for descriptive information about the sample. Note that parents’ childhood SES and current family SES were correlated, r=.23, p=.006. However, 37% of the sample moved in SES grouping over time (i.e., parents living in a rented home during childhood but then parents being above the median in current SES for the sample, or parents living in a home their family owned during childhood but then parents being below the median in current SES for the sample). These patterns suggest that SES is not entirely stable across generations, and hence that the findings below are not solely a function of remaining persistently low in SES over time.
Table 1.
Descriptive information about sample
| M | SD | % | |
|---|---|---|---|
| Child age | 14.12 | 2.07 | |
| Gender – male | 57 | ||
| Ethnicity | |||
| Caucasian | 49 | ||
| African American | 25 | ||
| Asian | 13 | ||
| Hispanic | 11 | ||
| Other | 2 | ||
| Beta agonist | 96 | ||
| Inhaled corticosteroid | 71 | ||
| Parent childhood SES – rent | 46 | ||
| Current family SES | 5.00 | 2.74 | |
| Family stress | 2.19 | 0.76 | |
| Asthma control (Child report) | 20.84 | 3.54 | |
| Asthma control (Parent report) | 19.85 | 3.34 | |
| Poly I:C | −0.01 | 0.94 | |
| LPS | 0.00 | 0.91 | |
| PMA/INO (Th-1) | 0.00 | 0.94 | |
| PMA/INO (Th-2) | 0.00 | 0.90 | |
Note: Beta agonist and inhaled corticosteroid use refers to the % who have a current prescription for that medication. Current family SES ranges from 1–9, with a 5 corresponding to $20,000-$49,999. Family stress ranges from 1–5. Asthma control ranges from 5–25. LPS=lipopolysaccharide. PMA/INO = phorbol myristate acetate/ionomycin. Cytokine production is represented by composite indicators derived from factor analyses. They include a Th-2 factor (IL-2, 4, 5, and 13), a Th-1 factor (IFN-γ, IL-10), and a pro-inflammatory factor (IL-1β, IL-6, TNF-α). In all cases, values are corrected for non-specific production of each cytokine, then standardized and aggregated into composites.
Parents’ Childhood SES → Current Family Stress → Child Asthma Control
Parent Childhood SES and Child Asthma Control
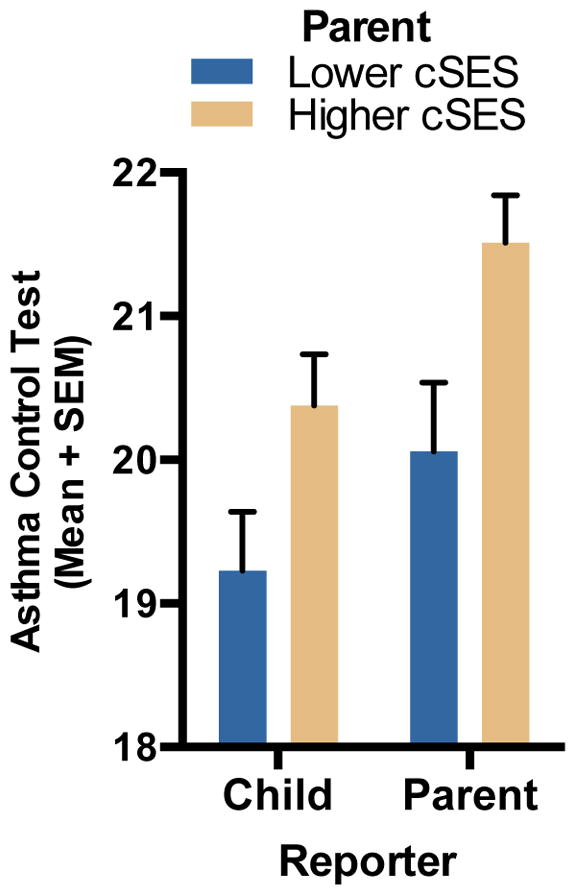
ANCOVA analyses revealed significant differences in children’s asthma control by parents’ childhood SES. These disparities were evident in both parents’ [F(1, 142)=6.65, p=.011], and children’s reports [F(1,143)=3.927, p=.037]. As Table 2 and Figure 1 reveal, parents who grew up in lower SES circumstances [parent: M=20.11, 95% CI: 19.34–20.87; child: M=19.31, CI: 18.58–20.04] had children with poorer asthma control compared to those who grew up under higher SES circumstances [parent: M=21.47, CI: 20.76–22.17; child: M=20.31, CI:19.64–20.99]. Cohen’s d statistic is often used to express the magnitude of an effect, that is, how large group differences are in standard deviation units. The d values for the above effects range from .33–.43.
Table 2.
ANCOVA analyses of parents’ childhood SES predicting child asthma clinical and immune outcomes.
| Low Parent SES (n=69) | High Parent SES (n=81) | F | p | |||
|---|---|---|---|---|---|---|
| M | SE | M | SE | |||
| Asthma control | ||||||
| Parent report | 20.11 | .38 | 21.47 | .36 | 6.66 | .011 |
| Child report | 19.31 | .37 | 20.31 | .34 | 3.93 | .049 |
| Cytokine production | ||||||
| Poly I:C (innate) | 0.14 | .12 | −0.13 | .11 | 2.81 | .096 |
| LPS (innate) | 0.08 | .12 | −0.07 | .11 | 0.92 | .339 |
| PMA/INO (Th-1) | 0.27 | .12 | −0.23 | .10 | 10.19 | .002 |
| PMA/INO (Th-2) | 0.18 | .11 | −0.15 | .10 | 4.77 | .031 |
Note: LPS=lipopolysaccharide. PMA/INO = phorbol myristate acetate/ionomycin. Cytokine production is represented by composite indicators derived from factor analyses. They include a Th-2 factor (IL-2, 4, 5, and 13), a Th-1 factor (IFN-γ, IL-10), and a pro-inflammatory factor (IL-1β, IL-6, TNF-α). In all cases, values are corrected for non-specific production of each cytokine, then standardized and aggregated into composites. Models include the covariates child age, gender, ethnicity, and usage of beta agonists and inhaled corticosteroids, with M and SE representing adjusted values.
Figure 1.
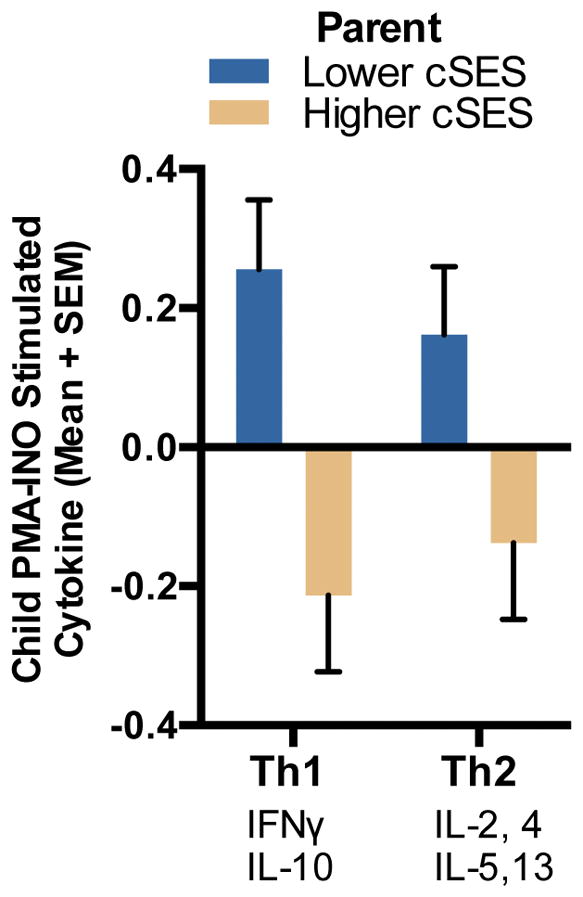
Children’s asthma control by parents’ childhood SES (cSES). Bars are shown by both child report on the Asthma Control Test, as well as parent report on the Asthma Control Test.
When current SES was added to these models, the group differences noted above remained significant [parent report: F(1,136)=6.16, p=.014. Low SES M=20.06, CI:19.27–20.84. High SES M=21.40, CI:20.68–22.13; child report: F(1,137)=4.44, p=.037. Low SES M=19.27, CI:18.53–20.00. High SES M=20.34, CI:19.66–21.02]. These results indicate that associations of parents’ childhood SES with offspring asthma control are independent of families’ current SES circumstances.
Parent Childhood SES and Current Family Relationship Stress
ANCOVA analyses revealed a significant difference in current family relationship stress by parents’ childhood SES [F(1,144)=7.75, p=.006. Low SES M=2.37, CI:2.20–2.54. High SES M=2.04, CI:1.89–2.20]. Cohen’s d for the effect size was .45. If parents grew up in lower SES circumstances, their current family relationships were rated as more stressful. When current SES was added into the model, this association remained significant [F(1,138)=7.68, p=.006. Low SES M=2.39, CI:2.21–2.56. High SES M=2.06, CI:1.90–2.22], indicating that the effect of parents’ childhood SES on current family stress was independent of current family SES.
Current Family Relationship Stress and Child Asthma Control
Regression analyses revealed significant associations of current family relationship stress with asthma control as reported by parents (standardized β=−.17, p=.048) and children (β= −.22, p=.006). These patterns indicated that higher levels of family relationship stress were associated with poorer asthma control in children. See Table 3. When including current SES as a covariate, the association with parent report ACT was: β= −.16, p=.07, and the association with child report ACT was: β= −.23, p=.005.
Table 3.
Regression analyses of current family relationship stress predicting child asthma immune and clinical outcomes.
| B | Standard Error | ΔR2 | p | |
|---|---|---|---|---|
| Asthma control | ||||
| Parent report | −.81 | .40 | .026 | .048 |
| Child report | −.96 | .35 | .043 | .006 |
| Cytokine production | ||||
| Poly I:C (innate) | .23 | .11 | .033 | .035 |
| LPS (innate) | .12 | .11 | .008 | .298 |
| PMA/INO (Th-1) | .15 | .11 | .013 | .186 |
| PMA/INO (Th-2) | .09 | .11 | .005 | .434 |
Note: Current family relationship stress is coded on a 1–5 scale, with higher numbers indicating greater family stress. B=unstandardized b coefficient. LPS = lipopolysaccharide. PMA/INO = phorbol myristate acetate/ionomycin. Cytokine production is represented by composite indicators derived from factor analyses. They include a Th-2 factor (IL-2, 4, 5, and 13), a Th-1 factor (IFN-γ, IL-10), and a pro-inflammatory factor (IL-1β, IL-6, TNF-α). In all cases, values are corrected for non-specific production of each cytokine, then standardized and aggregated into composites. Models include the covariates child age, gender, ethnicity, and usage of beta agonists and inhaled corticosteroids.
Family Relationship Stress as a Pathway?
We conducted statistical mediation analyses to test whether current family relationship stress mediated the relationship between low parent childhood SES and child asthma control. Analyses revealed a significant mediated, or indirect, effect of −.20 for parent-report of asthma control [confidence interval, CI: −.60, −.01]. There was also a significant indirect effect of −.34 for child-report of asthma control [CI: −.88, −.07]. These findings are consistent with a scenario wherein the quality of current family relationships serves as one pathway linking parents’ early life circumstances to their children’s current asthma control.
Parent Childhood SES → Current Family Stress → Child Cytokine Production
For cytokine data, distributions of each cytokine were reviewed, and those that were not normally distributed were log transformed. Factor analyses revealed that cytokine responses could be aggregated into a Th-2 factor (IL-2, 4, 5, and 13), a Th-1 factor (IFN-γ, IL-10) for PMA/INO stimulation, and into a single pro-inflammatory factor (IL-1β, IL-6, TNF-α) for LPS and Poly I:C53. Results of these factor analyses were used to reduce the number of dependent variables by combining conceptually and empirically related cytokines. Composite indicators were created by standardizing and averaging individual cytokine values (unweighted). The analyses below utilize these cytokine composites.
Parent Childhood SES and Child Cytokine Production
ANCOVA analyses revealed significant associations between parents’ childhood SES and children’s PMBC production of both Th-2 [F(1,134)=4.77, p=.031, Cohen’s d=.38. Low SES M=.18, CI: −.04 – .40. High SES M=−.15, CI: −.36 – .05] and Th-1 cytokines [F(1,134)=10.19, p=.002, Cohen’s d=.54. Low SES M=.27, CI:.04–.50. High SES M=−.23, CI: −.44 to −.02] following stimulation with PMA/INO. For pro-inflammatory cytokine responses to Poly I:C, the association was [F(1,130)=2.81, p=.096, Cohen’s d=.29. Low SES M=.14, CI: −.10 – .37. High SES M=−.13, CI: −.35 – .08]. There were no differences in cytokine response to LPS [F(1,130)=0.92, p=.339, d=.16. Low SES M=.08, CI: −.15 – .31. High SES M=−.07, CI: −.28 – .14]. These patterns indicated that parents who grew up in lower SES circumstances had children whose PBMCs exhibited larger Th-2 and Th-1 cytokine responses. See Table 2 and Figure 2.
Figure 2.
Parents’ childhood SES (cSES) and children’s cytokine production in response to stimulation with PMA/INO (25ng/mL/1ug/mL). Bars are shown for both Th1 and Th2 cytokine responses. Cytokine values are standardized and aggregated into composites.
When current SES was added to these models, the above patterns remained the same [Th-2: F(1,129)=5.72, p=.018. Low SES M=.21, CI:.00–.44. High SES M=−.15, CI: −.35 – .05; Th-1: F(1,129)=11.25, p=.001. Low SES M=.31, CI:.08–.54. High SES M=−.22, CI: −.44 to −.01; Poly I:C pro-inflammatory cytokines: F(1,125)=2.56, p=.11. Low SES M=.13, CI: −.11 – .37. High SES M=−.14, CI: −.36 – .08].
Parent Childhood SES and Current Family Relationship Stress
As reported above, there was a significant association of parents’ childhood SES with current family relationship stress [F(1,144)=7.75, p=.006], that persisted after controlling for current SES [F(1,138)=7.68, p=.006].
Current Family Relationship Stress and Child Cytokine Production
Regression analyses revealed significant associations between current family relationship stress and pro-inflammatory cytokine responses to Poly I:C stimulation (β=.19, p=.035). Higher levels of family relationship stress were associated with larger pro-inflammatory cytokine responses in the PBMCs of children. Even after current SES was added as a covariate, the above finding remained significant (β=.21, p=.028). No associations were found with Th-1 or Th-2 cytokines. See Table 3.
Family Relationship Stress as a Pathway?
Statistical mediation analyses revealed a significant mediated, or indirect, effect of .05 for pro-inflammatory cytokine responses to Poly I:C with a bootstrapped 95% CI of [.0037, .1789]. These findings are consistent with the explanation that the quality of current family relationships serves as one pathway linking parents’ early life circumstances to their children’s pro-inflammatory cytokine responses.
Sensitivity Analyses
To test the robustness of associations of parents’ childhood SES with child asthma outcomes, we repeated the above analyses controlling for a different current family SES variable (parent education instead of family assets). When years of parent education was added to the models, the results were as follows: [parent ACT: F(1,141)=5.85, p=.02; child ACT: F(1,142)=3.60, p=.06; PIC: F(1,129)=2.71, p=.10; PMA/INO Th-2: F(1,133)=4.60, p=.03; PMA/INO Th-1: F(1,133)=9.96, p=.002]. These results indicate that, with the exception of child ACT (which went from p=.037 to p=.06), associations of parents’ childhood SES with offspring asthma outcomes are robust to type of current SES measure used as a covariate.
DISCUSSION
The results of this study provide early evidence that parents’ socioeconomic conditions when they were children are related to the health of the next generation: their own children’s asthma outcomes. Specifically, as compared to parents who grew up under better socioeconomic conditions, parents who grew up in lower SES households were more likely to have children with poorer asthma control. Secondarily, as we explored asthma-relevant immunologic measures, we found that when parents grew up in lower SES households, their children were more likely to have PBMCs that exhibited larger Th-2 and Th-1 cytokine responses to PMA/INO stimulation in vitro. Moreover, these findings were independent of families’ current SES circumstances.
These findings add to a growing body of literature on SES across the lifecourse, which has demonstrated that not only is current family SES related to numerous health outcomes including asthma3,8,54,55, but also that childhood SES predicts health outcomes later in adulthood, independent of current SES56–59. In the present study, we demonstrate the value of expanding lifecourse models60,61 to also include a consideration of environments from previous generations.
The present study’s findings also extend previous human studies on the intergenerational effects of poverty to a clinical disease context. Previous work has documented intergenerational effects of individuals experiencing poverty or associated conditions during early childhood or in utero on future generations’ (these individuals’ children) risk factor profiles, including adiposity and blood pressure19,20. In addition, intervention work has demonstrated that providing nutrition supplements to individuals during their early childhood or in utero years results in these individuals later in life having children with greater birthweights and who are taller62,63. To our knowledge, however, no research has yet documented intergenerational effects of low SES on clinical outcomes in populations with chronic diseases.
Why would the childhood socioeconomic circumstances of parents be associated with asthma outcomes in their children? While there may be numerous explanations (e.g., epigenetic modifications13,14), in this study, we focused on one psychosocial possibility related to family stress. Parents who grow up in low SES households are more likely to experience frequent family conflict, harsh parenting, and poorer quality family interactions as children28,29,64. These stressful childhood experiences are believed to engender behavioral tendencies that persist across the lifecourse, including subsequently being more likely to develop abrasive social relationships as adults (involving greater conflict and rejection, less support and warmth)59. Thus parents who grow up under low SES circumstances may be more likely to have conflictual and less supportive and trusting relationships with their children. The present study’s findings support this notion, documenting associations of low parent childhood SES with greater current family relationship stress, above and beyond effects of current family SES.
In turn, among children with asthma, experiencing family relationships that are more conflictual and less supportive is associated with poorer asthma outcomes. For example, previous research has shown that parenting difficulties when children were young predicted a subsequent diagnosis of childhood asthma34. In addition, family dysfunction and parent-child conflict discriminated children with severe asthma who died from asthma from children with severe asthma who did not35. Family relationships also have been associated with immune markers in children with asthma. For example, in another sample of children with asthma, greater family relationship stress was associated with greater Th-2 cytokine responses to PMA/INO stimulation in vitro11. In addition, lower levels of parent support have been associated with higher levels of eosinophil cationic protein (a cytotoxic protein released from activated eosinophils, considered a marker of airway inflammation) and with reduced PBMC sensitivity to glucocorticoid inhibition in children with asthma65. The present study’s findings add to this literature in documenting that greater family stress was related to both poorer child asthma control and greater pro-inflammatory cytokine responses to PBMC stimulation in children with asthma. We note that although both parent childhood SES and current family stress were related to cytokine responses, they were associated with different markers (Th-1 and Th-2 responses for the former, and pro-inflammatory responses for the latter), suggesting that different social exposures may affect different immunologic processes that have implications for asthma control. In addition, because these measures are taken in peripheral blood, local immune processes in the airways may serve as more proximal mediators to clinical outcomes.
In addition, statistical mediation analyses documented support for the indirect pathway–that is, the pathways from low parent childhood SES to greater current family relationship stress to poorer child asthma control and to larger pro-inflammatory cytokine responses were significant. Although this study cannot make claims about causality because it was not experimental, these findings are nonetheless consistent with an explanation that one reason why parents’ childhood SES is associated with their child’s asthma outcomes is because of the difficulties in current family relationships that parents and children in these households are experiencing.
Limitations to the present study include the design being a cross-sectional observational study. As such, we are unable to determine causality or directionality. In addition, the sample size is small by epidemiological standards, though it is comparable to other studies that have investigated links between psychosocial factors and inflammatory processes in clinical populations66–68. Future studies that are able to recruit larger cohorts and incorporate longitudinal designs that follow children’s trajectories over time, and as well, across multiple generations, would be ideal for testing intergenerational hypotheses and for obtaining more reliable estimates of effect sizes. In addition, the assessment of parents’ childhood SES was retrospective and relied on parent-report and hence could be subject to recall biases. For example, it is possible that those parents with children whose asthma is more severe are more likely to recall more difficult childhoods, and that this accounts for the associations we see in these analyses. While this is entirely possible and hence a limitation of the present study, we also note that: 1) our measure of childhood SES was a single dichotomous variable that is concrete and easy for many to recall (home ownership). Previous research has documented that subjective perceptions of socioeconomic conditions are more prone to recall biases than objective questions, and as well, that objective childhood socioeconomic status questions can be recalled with a relatively high degree of accuracy69,70; 2) previous research has demonstrated that personality characteristics that are known to bias memories do not affect associations between retrospective childhood SES (as measured by home ownership) and adult health38, suggesting that childhood home ownership may not be as susceptible to such recall biases; and 3) in order to avoid retrospective reporting for a study with a design such as this one, we would have needed an approximately 40-year longitudinal follow-up (given that the average age of parents was 45). While we strongly advocate the investigation of clinical cohorts with this length of follow-up, until these can be funded, we may need to rely on retrospective reports of childhood SES for intergenerational studies of asthma such as this one. In addition, having greater details about parents’ childhood psychosocial environment in future studies would be informative. Finally, studies that are able to test additional pathways related to intergenerational transmission, including epigenetics and physical environment exposures, would help with the development of conceptual models of how parent early life environments can get transmitted to children.
In sum, these findings suggest that efforts to understand how the social environment can affect childhood asthma may need to expand to a consideration of periods before the child was born. Over and above a family’s current SES, the childhood SES environments that parents grew up in predict their children’s asthma control and secondarily, cytokine responses. These findings suggest the potential ‘long reach’ of low socioeconomic status across generations, and imply that efforts to ameliorate asthma disparities in our society may have to go beyond improving the current socioeconomic circumstances of families to addressing the social circumstances that children grow up in and the potential effects of these environments across generations.
Supplementary Material
Key messages.
Parents who grew up in lower socioeconomic status (SES) backgrounds were more likely to have children with worse asthma outcomes, independent of current family SES.
These effects are partially explained by more stressful current family relationships.
These findings suggest that theories of how the social environment can affect childhood asthma may need to be expanded to include influences from earlier generations.
Acknowledgments
Source of Funding: This work was supported by NIH grant R01 HL108723.
Abbreviations
- SES
socioeconomic status
- PBMC
peripheral blood mononuclear cells
- IL
interleukin
- IFN
interferon
- PMA/INO
phorbol 12-myristate 13-acetate/ionomycin
- LPS
lipopolysaccharide
- ACT
Asthma Control Test
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 2.Marmot M, Wilkinson RG. Social determinants of health. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 3.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 4.Schreier HM, Chen E. Socioeconomic status and the health of youth: a multilevel, multidomain approach to conceptualizing pathways. Psychol Bull. 2013;139(3):606–654. doi: 10.1037/a0029416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maziak W, von ME, Keil U, et al. Predictors of health care utilization of children with asthma in the community. Pediatric Allergy and Immunology. 2004;15(2):166–171. doi: 10.1046/j.1399-3038.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 6.Amre DK, Infante-Rivard C, Gautrin D, Malo JL. Socioeconomic status and utilization of health care services among asthmatic children. Journal of Asthma. 2002;39:625–631. doi: 10.1081/jas-120014927. [DOI] [PubMed] [Google Scholar]
- 7.Simon PA, Zeng ZW, Wold CM, Haddock W, Fielding JE. Prevalence of childhood asthma and associated morbidity in Los Angeles County: Impacts of race/ethnicity and income. Journal of Asthma. 2003;40(5):535–543. doi: 10.1081/jas-120018788. [DOI] [PubMed] [Google Scholar]
- 8.Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. American Journal of Public Health. 2000;90:428–430. doi: 10.2105/ajph.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst P, Demissie K, Joseph L, Locher U, Becklake MR. Socioeconomic status and indicators of asthma in children. American Journal of Respiratory and Critical Care Medicine. 1995;152:570–575. doi: 10.1164/ajrccm.152.2.7633709. [DOI] [PubMed] [Google Scholar]
- 10.Mielck A, Reitmeir P, Wjst M. Severity of childhood asthma by socioeconomic status. International Journal of Epidemiology. 1996;25:388–393. doi: 10.1093/ije/25.2.388. [DOI] [PubMed] [Google Scholar]
- 11.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117(5):1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Chen E, Fisher EB, Jr, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosomatic Medicine. 2003;65:984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- 13.Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update. 2014;20(1):63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- 14.Burton T, Metcalfe NB. Can environmental conditions experienced in early life influence future generations. Proc Biol Sci. 2014;281(1785):20140311. doi: 10.1098/rspb.2014.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180(1):1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 16.Susser E, Kirkbride JB, Heijmans BT, Kresovich JK, Lumey LH, Stein AD. Maternal prenatal nutrition and health in grandchildren and subsequent generations. Annual Review of Anthropology. 2012;41:577–610. [Google Scholar]
- 17.Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patti ME. Intergenerational programming of metabolic disease: evidence from human populations and experimental animal models. Cell Mol Life Sci. 2013;70(9):1597–1608. doi: 10.1007/s00018-013-1298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115(10):1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 20.Schreier HM, Chen E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain Behav Immun. 2010;24(8):1324–1331. doi: 10.1016/j.bbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Astone NM, Misra D, Lynch C. The effect of maternal socio-economic status throughout the lifespan on infant birthweight. Paediatr Perinat Epidemiol. 2007;21(4):310–318. doi: 10.1111/j.1365-3016.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 22.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127(4):1232–1241. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 23.Busse WW, Lemanske RF. Advances in immunology: Asthma. New England Journal of Medicine. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 24.Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–857. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtzman MJ, Morton JD, Shornick LP, et al. Immunity, inflammation, and remodeling in the airway epithelial barrier: Epithelial-viral-allergic paradigm. Physiological Reviews. 2002;82(1):19–46. doi: 10.1152/physrev.00020.2001. [DOI] [PubMed] [Google Scholar]
- 26.Simpson JL, Brooks C, Douwes J. Innate immunity in asthma. Paediatric Respiratory Reviews. 2008;9(4):263–270. doi: 10.1016/j.prrv.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Finn PW, Bigby TD. Innate immunity and asthma. Proc Am Thorac Soc. 2009;6(3):260–265. doi: 10.1513/pats.200807-064RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley RH, Corwyn RF, Mcadoo HP, Coll CG. The home environments of children in the United States part I: Variations by age, ethnicity, and poverty status. Child Development. 2001;72(6):1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- 29.Repetti RL, Taylor SE, Seeman T. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- 30.Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 31.Wahler RG. Some perceptual functions of social networks in coercive mother–child interactions. Journal of Social and Clinical Psychology. 1990;9:43–53. [Google Scholar]
- 32.Gustafsson PA, Kjellman NIM, Bjorksten B. Family interaction and a supportive social network as salutogenic factors in childhood atopic illness. Pediatric Allergy and Immunology. 2002;13(1):51–57. doi: 10.1034/j.1399-3038.2002.00086.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma_. American Journal of Respiratory and Critical Care Medicine. 2007;176:644–649. doi: 10.1164/rccm.200610-1473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: Predictors from infancy. Pediatrics. 2001;108:e69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- 35.Strunk RC, Mrazek DA, Fuhrmann GS, LaBrecque JF. Physiologic and psychological characteristics associated with deaths due to asthma in childhood. Journal of the American Medical Association. 1985;254:1193–1198. [PubMed] [Google Scholar]
- 36.Franklin TB, Russig H, Weiss IC, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68(5):408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Saavedra-Rodríguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biol Psychiatry. 2013;73(1):44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine. 2004;66(4):553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 39.Miller GE, Chen E. Unfavorable socioeconomic conditions in early life presage expression of pro-inflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 40.Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21(1):31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- 41.Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 42.Adrian C, Hammen C. Stress Exposure and Stress Generation in Children of Depressed Mothers. Journal of Consulting and Clinical Psychology. 1993;61(2):354–359. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- 44.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Corrigan CJ, Kay AB. CD4 T-lymphocyte activation in acute severe asthma: Relationship to disease severity and atopic status. American Review of Respiratory Disease. 1990;141:970–977. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- 47.Gemou-Engesëth V, Kay AB, Bush A, Corrigan CJ. Activated peripheral blood CD4 and CD8 T-lymphocytes in child asthma: Correlation with eosinophilia and disease severity. Pediatric Allergy and Immunology. 1994;5:170–177. doi: 10.1111/j.1399-3038.1994.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 48.Rosenblum Lichtenstein JH, Hsu YH, Gavin IM, et al. Environmental mold and mycotoxin exposures elicit specific cytokine and chemokine responses. PLoS One. 2015;10(5):e0126926. doi: 10.1371/journal.pone.0126926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods. 2009;340(1):55–64. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Wright RJ, Visness CM, Calatroni A, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182(1):25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 53.Chen E, Shalowitz MU, Story RE, et al. Dimensions of socioeconomic status and childhood asthma outcomes: Evidence for distinct behavioral and biological associations. doi: 10.1097/PSY.0000000000000392. under submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marmot M. The Health Gap: The Challenge of an Unequal World. New York, NY: Bloomsbury Press; 2015. [Google Scholar]
- 55.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Journal of the American Medical Association. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 56.Kittleson MM, Meoni LA, Wang NY, Chu AY, Ford DE, Klag MJ. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Archives of Internal Medicine. 2006;166(21):2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- 57.Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: Systematic review and interpretation. Epidemiologic Reviews. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 58.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 59.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben SY, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 61.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 62.Stein AD, Barnhart HX, Hickey M, Ramakrishnan U, Schroeder DG, Martorell R. Prospective study of protein-energy supplementation early in life and of growth in the subsequent generation in Guatemala. Am J Clin Nutr. 2003;78(1):162–167. doi: 10.1093/ajcn/78.1.162. [DOI] [PubMed] [Google Scholar]
- 63.Behrman JR, Calderon MC, Preston SH, Hoddinott J, Martorell R, Stein AD. Nutritional supplementation in girls influences the growth of their children: prospective study in Guatemala. Am J Clin Nutr. 2009;90(5):1372–1379. doi: 10.3945/ajcn.2009.27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conger RD, Elder GH. Families in troubled times. New York, NY: Aldine de Gruyter; 1994. [Google Scholar]
- 65.Miller GE, Gaudin A, Zysk E, Chen E. Parental support and cytokine activity in childhood asthma: the role of glucocorticoid sensitivity. J Allergy Clin Immunol. 2009;128(5):970–976. doi: 10.1016/j.jaci.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Lu Y, Ho R, Lim TK, et al. Neuropeptide Y may mediate psychological stress and enhance TH2 inflammatory response in asthma. J Allergy Clin Immunol. 2015;135(4):1061–1063. doi: 10.1016/j.jaci.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 67.Chen E, Strunk RC, Trethewey A, Schreier HM, Maharaj N, Miller GE. Resilience in low-socioeconomic-status children with asthma: Adaptations to stress. J Allergy Clin Immunol. 2011;128(5):970–976. doi: 10.1016/j.jaci.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism. J Clin Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward MM. Concordance of sibling’s recall of measures of childhood socioeconomic position. BMC Med Res Methodol. 2011;11:147. doi: 10.1186/1471-2288-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krieger N, Okamoto A, Selby JV. Adult female twins’ recall of childhood social class and father’s education: a validation study for public health research. Am J Epidemiol. 1998;147(7):704–708. doi: 10.1093/oxfordjournals.aje.a009512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


