Abstract
Study Design
Regional measurements of fixed charge densities (FCDs) of healthy human cartilage endplate (CEP) using a two-point electrical conductivity approach.
Objective
To determine the FCDs at four different regions (central, lateral, anterior, and posterior) of human CEP, and correlate the FCDs with tissue biochemical composition.
Summary of Background Data
The CEP, a thin layer of hyaline cartilage on the cranial and caudal surfaces of the intervertebral disc, plays an irreplaceable role in maintaining the unique physiological mechano-electrochemical environment inside the disc. FCD, arising from the carboxyl and sulfate groups of the glycosaminoglycans (GAG) in the extracellular matrix of the disc, is a key regulator of the disc ionic and osmotic environment through physicochemical and electrokinetic effects. While FCD in the annulus fibrosus (AF) and nucleus pulposus (NP) have been reported, quantitative baseline FCD in healthy human CEP has not been reported.
Methods
CEP specimens were regionally isolated from human lumbar spines. FCD and ion diffusivity were concurrently investigated using a two-point electrical conductivity method. Biochemical assays were used to quantify regional glycosaminoglycan (GAG) and water content.
Results
FCD in healthy human CEP was region-dependent, with FCD lowest in the lateral region (p=0.044). Cross-region FCD was 30–60% smaller than FCD in NP, but similar to the AF and articular cartilage (AC). CEP FCD (average: 0.12±0.03 mEq/g wet tissue) was correlated with GAG content (average: 31.24±5.06 μg/mg wet tissue) (p=0.005). Additionally, the cross-region ion diffusivity in healthy CEP (2.97±1.00×10−6 cm2/s) was much smaller compared to the AF and NP.
Conclusions
Healthy human CEP acts as a biomechanical interface, distributing loads between the bony vertebral body and soft disc tissues and as a gateway impeding rapid solute diffusion through the disc.
Keywords: intervertebral disc, cartilage endplate, fixed charge density, electrical conductivity, ion diffusivity
INTRODUCTION
Comprising the largest avascular tissue in the human body, the intervertebral disc (IVD) includes three components: cartilage endplate (CEP), annulus fibrosus (AF), and nucleus pulposus (NP). The CEP, a thin layer of hyaline cartilage on the cranial and caudal surfaces of the disc, is the main pathway for solute exchange (e.g. ions and nutrient solutes) through the IVD 1–4. Pathological changes in CEPs (e.g. calcification or degeneration) significantly alter the extracellular mechano-electrochemical environment inside the disc by changing mechanical and transport functions of the CEP 5,6, which may initiate and accelerate disc degeneration by triggering a cascade of biological sequelae at cellular or molecular levels 7–10. Healthy CEPs play a critical role in maintaining the physiological extracellular mechano-electrochemical environment inside the disc.
All three IVD components are negatively charged, due to the carboxyl and sulfate groups on the glycosaminoglycan (GAG) in the extracellular matrix 11. Fixed charge density (FCD), representing the negative charges attached to the matrix in each tissue unit (per weight or volume), is a key factor regulating the unique physiological extracellular mechano-electrochemical environment of healthy IVD. Electrostatic interactions between FCD and mobile free ions results in physicochemical and electrokinetic effects, including high cation and low anion concentrations, Donnan osmotic pressure and swelling, and streaming potential/current 12–14. Studies have shown marked effects of changes to the ionic/osmotic environment on isolated or in situ disc cell metabolism 15,16. Additionally, FCD significantly impacts the mechanical (e.g. swelling pressure and compressive modulus) and transport properties (e.g. hydraulic permeability) of IVD tissues 17,18. GAG loss, which reduces FCD, is correlated to human IVD degeneration 19. Therefore, FCD may indicate the degree of disc degeneration. Although FCDs in AF and NP have been measured 20,21, to our knowledge, no quantitative assessment of FCD in healthy human CEP has been reported.
Previous FCD measurements have used the traditional tracer cation method (radio-labeled ions), imaging techniques (e.g. MRI), and mechanical indentation testing 11,22,23. Recently, an electrical conductivity approach has proved to be a simple and reliable FCD measurement in bovine NP and AF 21. Human CEP is a thin hyaline cartilage layer with an average thickness of ~0.7 mm 24, which could limit imaging techniques. Consequently, the electrical conductivity approach is more appropriate for FCD measurements in thin CEP specimens in vitro. Previous studies have revealed regional differences in morphology, biochemical composition, biphasic mechanical properties, and glucose/lactate diffusion rates between the central and peripheral regions of the CEP 4,24–26. Therefore, we hypothesized that FCD in healthy human CEP was region-dependent and correlated with biochemical composition (i.e., GAG content). The objective of this study was to measure FCD at four regions (central, lateral, anterior, and posterior) in healthy human CEP using a two-point electrical conductivity method 21. At the same time, ion diffusivity was determined. Regional biochemical composition was characterized, including GAG content and porosity, to determine potential correlations with FCD and ion diffusivity in the CEP 21,27, respectively. By determining baseline measurements of FCD and ion diffusivity in healthy human CEPs, this study may establish a role of the CEP in maintaining a healthy mechano-electrochemical environment inside human IVD, and a potential mechanism of disc degeneration.
MATERIALS AND METHODS
Specimen preparation
Twelve human lumbar spines (aged 33–65 years) obtained from an Organ Procurement Organization (LifePoint Inc., Charleston, SC) under institutional approval were screened based on the Thompson grading system 28. Spines with degenerated discs (Grade III–V) were not used for baseline measurements. After dissection, it was confirmed that the CEPs from the spines with healthy discs (Grade I & II) had no signs of the artifacts (e.g. fissures and calcification). To limit tissue variation, only L4–L5 motion segments were used. Considering these criteria, four L4–L5 motion segments were harvested from four lumbar spines within 24 hours post-mortem (33 year old male, 42 year old male, 54 year old female, and 58 year old female).
Motion segments were immediately opened through the median plane of the IVD with a scalpel. Disc-shaped CEP specimens with a 5 mm diameter were prepared from superior and inferior surfaces at four disc regions (central, lateral, anterior, and posterior) (detailed in Figure 1). Due to disc symmetry, specimens harvested from left and right lateral regions were included in the same group. Prepared CEP specimens had an average height of 0.685±0.094 mm, similar to previous studies 4,24. Four groups of CEP specimens harvested from four L4–L5 discs were tested in this study: central (n=8), lateral (n=9), anterior (n=8), and posterior (n=6). No posterior specimen was harvested from one of the discs due to its small size. Seven specimens harvested from lateral regions were used for testing protocol development, not data collection.
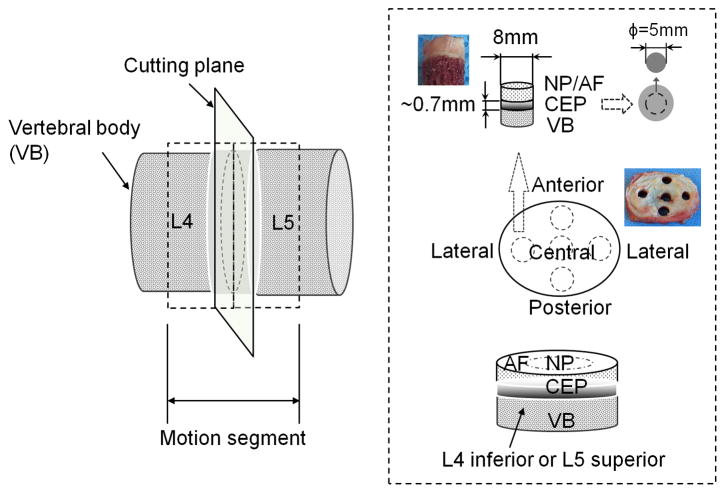
Figure 1.
Schematic view of specimen preparation. Motion segments were opened through the median plane of the IVD with a scalpel. A cylindrical plug (8 mm diameter) of NP or AF/endplate/bone was extracted from superior and inferior surfaces at four regions of the disc (center, lateral, anterior, and posterior) with a serrated-edge cutter. Each plug was then mounted onto the freezing stage of a sledge microtome (Leica SM2400, Leica Biosystem, Chicago, IL) to remove the overlying NP/AF (relatively more transparent than CEP tissue) and vertebral bone. The final disc-shaped CEP specimens were punched with a 5 mm corneal trephine for electrical conductivity measurements. Specimen preparation was performed in a humidified hood to prevent tissue dehydration.
Electrical conductivity measurements
FCD for each specimen was determined using a previously established two-point electrical conductivity method 21. Following preparation, specimens were first immersed in 0.15 M KCl solution (isotonic) for a 12 hour equilibration period at 4°C before conductivity measurements. During incubation, specimens were axially confined between two hydrophilic polyethylene porous platens (50–90 μm, Small Parts, Inc., Miami Lakes, FL), and radially confined within an acrylic chamber with an inner diameter of 5 mm to minimize swelling and proteoglycan (PG) leaching (Figure 2). Electrical conductivity was measured with a previously developed conductivity apparatus (Figure 2) 29,30. Using a four-wire method, the resistance across the CEP specimen was measured at a low and constant current density of 0.015 mA/cm2 supplied by a Keithley Sourcemeter (Model 2400, Keithley Instruments, Inc., Cleveland, OH). CEP electrical conductivity (χ) was calculated by:
| (1) |
where R, h, and A are the resistance, height, and cross sectional area of the specimen, respectively. Specimen height was measured with a current sensing micrometer. After the initial electrical conductivity measurement, each specimen was re-equilibrated in 0.03 M KCl solution (hypotonic) for 12 hours and electrical conductivity measurements were retested. All conductivity measurements were performed at room temperature (22°C).
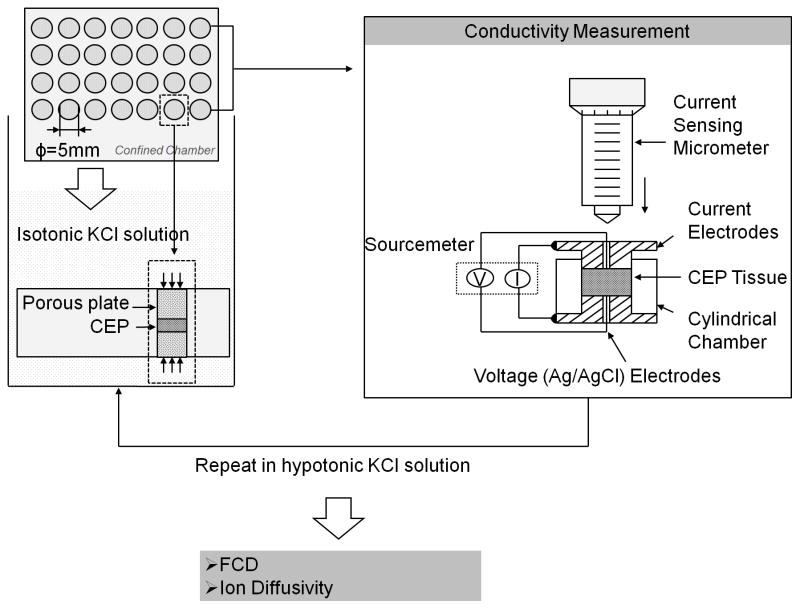
Figure 2.
Experimental flow of the two-point conductivity approach for FCD and ion diffusivity measurements in healthy human CEPs. Electrical conductivity was measured with a previously developed conductivity apparatus 29,30, consisting of two stainless steel current electrodes coaxial to two Teflon-coated Ag/AgCl voltage electrodes placed on the top and bottom of a cylindrical nonconductive Plexiglass chamber (5mm diameter). Measurements were achieved by sequentially equilibrating tissue specimens in two concentrations (i.e., isotonic and hypotonic) of potassium chloride (KCl) solution and measuring the corresponding electrical conductivity of the tissue.
FCD and ion diffusivity calculations
Specimen FCD was determined by measuring the electrical conductivities of the specimen in two KCl solutions (isotonic condition: 0.15 M KCl; hypotonic condition: 0.03 M KCl). According to the two point electrical conductivity method 21, tissue FCD (cF) was calculated by:
| (2) |
where χi and (i = Iso or Hypo) are the electrical conductivity in the CEP and the concentration of the KCl solution under isotonic or hypotonic conditions. This method also determined the K+ or Cl− ion diffusivity in CEP tissue. The diffusivity of cations (D+) was assumed to be the same as anions (D−) due to similar Stokes’ radii (K+: rs=0.137 nm; Cl−: rs=0.142 nm) 27. Thus, ion diffusivity (D=D+=D−) was determined by 21:
| (3) |
where Fc is the Faraday constant, ϕw is porosity, and R and T are the gas constant and absolute temperature.
Porosity and GAG measurements
The porosity (water volume fraction) of the CEP specimen was determined using a buoyancy method 31. The specimen weight in air (Wwet) and in phosphate buffered saline (PBS) solution (WPBS) were measured using the density-determination kit of a Sartorius analytical balance (Sartorius YDK01, Germany). Then, specimens were lyophilized and the dry weight (Wdry) recorded. Porosity was calculated by 31:
| (4) |
where ρPBS and ρw are the mass densities of the PBS solution and water, respectively. Lyophilized tissues were then assayed for GAG content by Blyscan Glycosaminoglycan Assay kit (Biocolor Ltd., Newtonabbey, Northern Ireland) based on 1,9-dimethylmethylene blue dye binding 32, with standards provided by the manufacturer.
Statistical analysis
All measurements were reported using the means and standard deviations (SD). Significant differences by disc region were determined by one-way ANOVA for isotonic and hypotonic electrical conductivities, FCD, ion diffusivity, GAG content, and porosity. Correlations between FCD and GAG content, and between ion diffusivity and porosity were determined 33 by linear mixed effects model with a random effect for spine. Due to the limited sample size, sex and age effects were not considered. The statistical analysis was conducted in R (R Core Team, 2015) and statistical significance was determined at p-values < 0.05.
RESULTS
Electrical conductivity
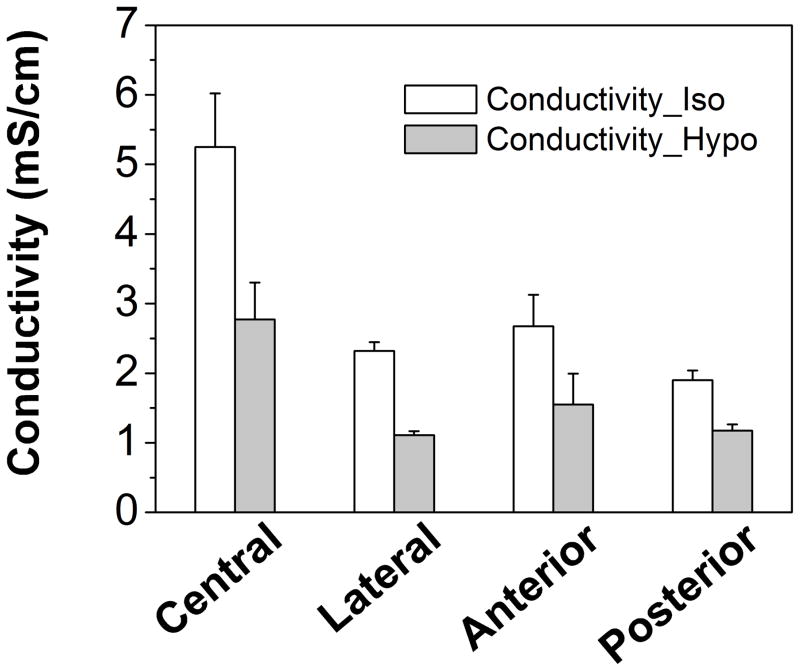
The cross-region electrical conductivities of CEP were 3.04±1.37 mS/cm and 1.65±0.75 mS/cm under isotonic and hypotonic conditions, respectively (Table 1). Significant regional variation was detected at both isotonic and hypotonic conditions in four regions (p<0.0001; Figure 3). The electrical conductivities in the central region were significantly higher (p<0.0001) than all other regions (Table 1).
Figure 3.
Region-dependent electrical conductivities of healthy human CEPs under isotonic and hypotonic conditions. Electrical conductivity in the central region was significantly higher than in other regions under both isotonic (p<0.0001) and hypotonic conditions (p<0.0001).
FCD and Ion diffusivity
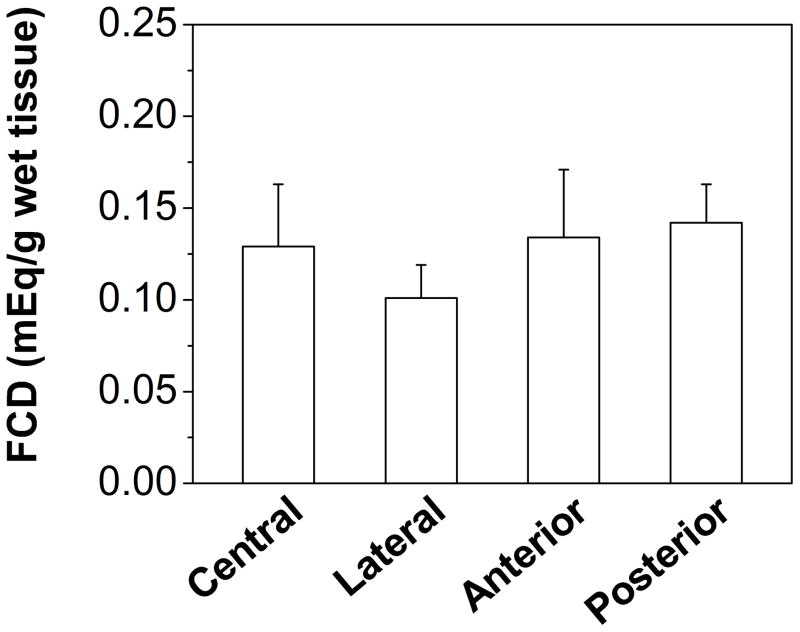
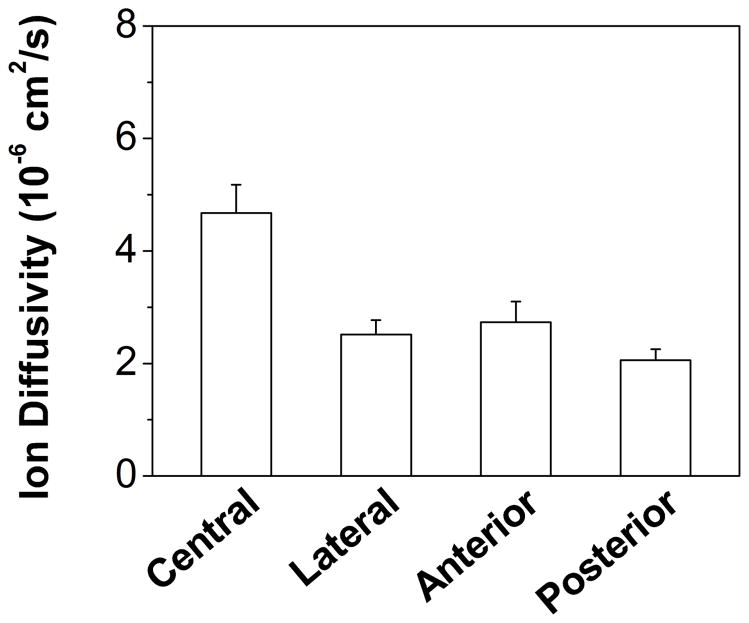
FCD was reported as milliequivalent moles (mEq) per gram wet or dry tissue. The cross-region FCD of CEP was 0.124±0.032 mEq/g wet tissue or 0.363±0.118 mEq/g dry tissue (Table 1). Significant regional variation, with the lowest value in lateral region (Lateral: 0.101±0.018 mEq/g wet tissue), was found for FCD (p=0.044, Figure 4A). By contrast, no significant regional variation was found for FCD per gram dry tissue (p=0.254, Table 1), although the lowest mean value was found in lateral region (Lateral: 0.314±0.128 mEq/g dry tissue). Cross-region ion (K+/Cl−) diffusivity in CEP was 2.97±1.00×10−6 cm2/s (Table 1). Significant regional variation, with the highest value in the central region (Central: 4.67±0.50×10−6 cm2/s), was found for ion diffusivity (p<0.0001; Figure 5A).
Figure 4.
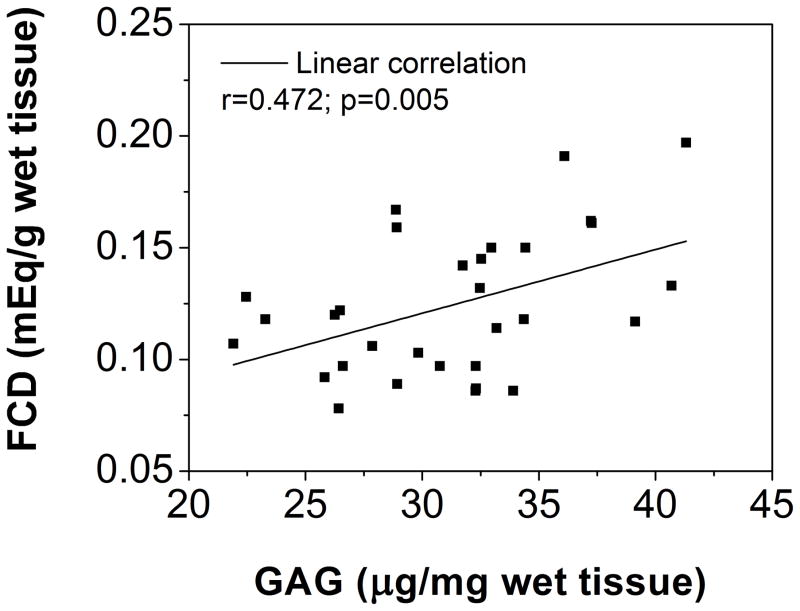
(A) Region-dependent FCDs from electrical conductivity measurements in healthy human CEPs. FCD in the lateral region was significantly lower (p=0.04) than in other regions. (B) The linear correlation between FCD and GAG content in healthy human CEPs was significant (r=0.472, p=0.005).
Figure 5.
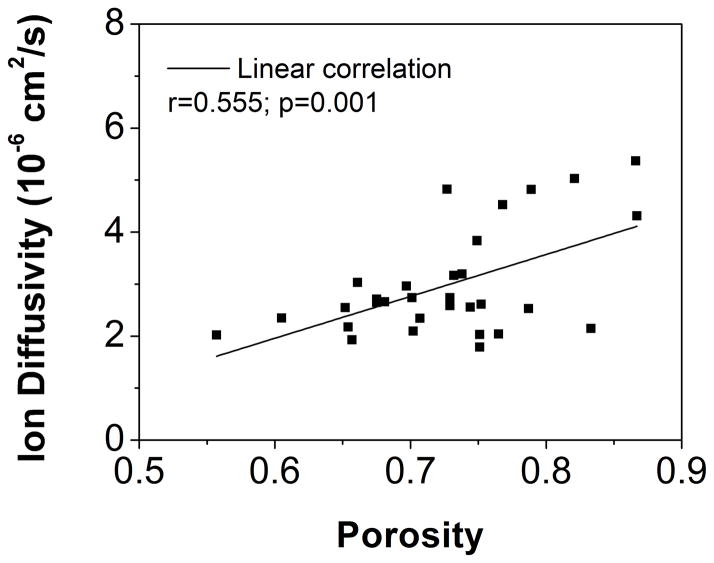
(A) Region-dependent relative ion diffusivities of healthy human CEPs. Ion diffusivity in the central region was significantly higher (p<0.0001) than in other regions. (B) The linear correlation between ion diffusivities and porosities in healthy human CEPs was significant (r=0.555, p=0.001).
GAG content and Porosity
Cross-region GAG content in CEP was 31.24±5.06 μg/mg wet tissue or 93.64±18.43 μg/mg dry tissue, with no significant regional variations detected (p=0.121 for wet tissue, p=0.063 for dry tissue; Table 1). Cross-region CEP porosity was 0.726±0.069. Significant regional variation in CEP porosity was detected, with the central region (Central: 0.798±0.055) having higher values than other regions (p=0.0003; Table 1).
Correlation between FCD/Ion diffusivities and tissue biochemical composition
The correlation between FCD and GAG content was statistically significant (r=0.472, p=0.005; Figure 4B), while there was no statistical significant correlation between FCD and porosity (r=0.135; p=0.470; not shown). The correlation between ion diffusivity and porosity was also statistically significant (r=0.555, p=0.001; Figure 5B).
DISCUSSION
This study determined baseline FCD in healthy human CEP and correlated results with relevant biochemical composition (GAG content). The amount of FCD in human CEP was significant and similar to human AF and articular cartilage (AC) (Table 2), however 30–60% smaller than human NP 14,20,34. Significant FCD creates a unique extracellular physicochemical environment inside the CEP, elevating cation and reducing anion concentrations, and raising osmotic pressure surrounding CEP cells. Changes to the ionic/osmotic environment have shown significant effects on the behavior of AF, NP, and AC cells, such as energy metabolism and cell volume regulation 7,35. While CEP cells have rarely been studied, it can be reasonably expected that maintaining the unique ionic/osmotic environment is critical for CEP cell homeostasis. Additionally, as in the NP and AF, osmotic pressure due to high FCD significantly contributes to the CEP compressive modulus. Based on the Donnan equation, electroneutrality condition, and van’t Hoff’s equation for osmotic pressure 12, the average measured FCD (0.124±0.032 mEq/g wet tissue) can generate 0.081±0.039 MPa osmotic pressure in healthy human CEP. It has been shown that decreases in FCD due to PG depletion reduces osmotic/swelling pressure, decreasing equilibrium and dynamic compressive moduli of AF tissues 18. Together with the larger tensile modulus and lower hydraulic permeability found in the CEP compared to other disc components (i.e., NP and AF) 26,36, this study demonstrated that healthy human CEP acts as a unique biomechanical interface distributing loads between the bony vertebral body and soft disc tissues. Under pathological conditions (e.g. calcification or degeneration), the FCD of disc tissues decreases significantly 19. Therefore, the FCD of healthy CEP measured in this study, as well as in other disc components (i.e., NP and AF) 20, provides baseline information for the evaluation of the mechano-electrochemical conditions (e.g. ionic concentrations, osmotic/swelling pressure, streaming potential and current) inside human IVD under physiological and pathological conditions 13,14,37.
FCD in healthy human CEP was significantly correlated with its GAG content, not porosity, which is similar to other IVD and cartilage tissues 21,23. This observation validated that the negatively charged nature of the CEP results from charged groups on the glycosaminoglycan (GAG) molecules on the proteoglycans (PGs) in the extracellular matrix. Moreover, there are two primary types of disaccharides in IVD tissues contributing to FCD, including chondroitin sulfate (CS), which has two negative charges per disaccharide, and keratin sulfate (KS), which carries one charged per disaccharide 21,38,39. Previous studies have shown that the ratio of KS to CS varies regionally through the human IVD 38,40, however the GAG content essay in this study wasn’t able to determine the ratio of KS to CS. It may explain the regional effect discrepancies between FCD and GAG content in the human CEP: significant regional FCD variations were revealed using the electrical conductivity method, with the lowest value in the lateral region, while no significant regional variation was found for GAG content measured using the biochemical assay. This difference further demonstrates that the electrical conductivity approach is a sensitive method to detect the physicochemical composition of the CEP 21,27,29,34.
The two-point conductivity method was also able to estimate the diffusivity of Cl− (or K+) ions in human CEPs, which were much smaller than in human NP, AF, and articular cartilage tissues (Table 2). Previous studies have found a lower glucose/lactate diffusivity and hydraulic permeability in the CEP compared to other disc and cartilaginous tissues 25,26. The lower diffusivity values for CEP tissues is likely due to the lower porosity, as diffusivity has been shown to be dependent upon tissue hydration. Ion diffusivity was significantly correlated with porosity in the CEP. Region-dependent ion diffusivity in healthy CEP was highest in the central region, corresponding to the highest porosity in the central CEP, supporting the hypothesis that water content is a dominant determinant of solute diffusion properties in IVD tissues 29,41. Due to the avascular nature of the human IVD, the healthy CEP acts as a gateway by blocking the rapid transport of ions, nutrient solutes, and angiogenic molecules, such as vascular endothelial growth factor (VEGF), through the disc and maintaining a stable physiological physicochemical environment inside the IVD 1,6,42–44. As such, a delicate extracellular environment may be vulnerable to any CEP pathological changes, such as calcification 5,6.
A few limitations should be noted for this study. A screening criteria (e.g. disc level: L4–L5; degeneration level: Grade I–II) was used to establish baseline characteristics of the healthy human CEP. As a result, 31 human CEP samples were harvested from 4 fresh, healthy and mature lumbar spines. In future work, more CEP specimens from a larger number of human lumbar spines will be collected to systematically characterize the effect of other factors (e.g. disc level, age, sex, diffusion direction, calcification, and degeneration) 19. To uncouple the effects of FCD and ion diffusivity on electrical conductivity (Equations 2 & 3), the diffusivities of cation and anion were assumed to be the same. Since the Stokes’ radii (rs) of K+ and Cl− are approximately equal (K+: rs=0.137 nm; Cl−: rs=0.142 nm), the value of their diffusivities can also be assumed to be the same (D=D+=D−). By contrast, the diffusivity of Na+ can be smaller than Cl− due to its larger Stokes’ radii (rs) (Na+: rs=0.197 nm) 21,27. Therefore, KCl instead of NaCl solution was used during the conductivity measurements to satisfy the assumption of equal diffusivities of cation and anion for calculating the FCD and ion diffusivity values 21.
In summary, this study measured baseline FCDs and ion (Cl−/K+) diffusivities in healthy human CEP, and further studied the effect of disc region on CEP properties. FCD and ion diffusivity in healthy human CEP were region-dependent due to its unique tissue biochemical composition (i.e., GAG content and porosity). FCD in healthy human CEP was similar to human AF and articular cartilage, while smaller than human NP. Significant FCD creates a unique extracellular ionic/osmotic environment critical for disc cell homeostasis. Osmotic pressure due to high FCD also contributes significantly to CEP mechanical function. Additionally, the ion diffusivity in human CEP were much smaller than in human NP an AF. Together, these results suggest that the healthy human CEP acts as a unique biomechanical interface distributing loads between the bony vertebral body and soft disc tissues, and acts as a gateway blocking the rapid diffusion of solutes through the IVD. These results further develop our understanding of the role of the human CEP in IVD biomechanics and physiology, and provide new insights into the mechanisms of disc degeneration and regeneration.
Supplementary Material
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). NIH grants (AR055775, DE018741 and DE021134) a grant (SCIRF0307) from the South Carolina Spinal Cord Injury Research Fund, a NSF RII grant fellowship (EPS-00903795), and a NIH T32 predoctoral fellowship (DE017551) funds were received in support of this work. Relevant financial activities outside the submitted work: consultancy, grants, royalties.
The authors thank Dr. Simona Baicu at LifePoint for assisting in human lumbar spine harvest.
Footnotes
Level of Evidence: N/A
References
- 1.Nachemson A, Lewin T, Maroudas A, et al. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41:589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- 2.Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–19. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 3.Ogata K, Whiteside LA. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine. 1981;6:211–6. [PubMed] [Google Scholar]
- 4.Wu Y, Cisewski SE, Wegner N, et al. Region and strain-dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J Biomech. 2016;49:2756–62. doi: 10.1016/j.jbiomech.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson AR, Huang CY, Gu WY. Effect of endplate calcification and mechanical deformation on the distribution of glucose in intervertebral disc: a 3D finite element study. Comput Methods Biomech Biomed Engin. 2011;14:195–204. doi: 10.1080/10255842.2010.535815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts S, Urban JP, Evans H, et al. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21:415–20. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Pritchard S, Guilak F. The role of F-actin in hypo-osmotically induced cell volume change and calcium signaling in anulus fibrosus cells. Ann Biomed Eng. 2004;32:103–11. doi: 10.1023/b:abme.0000007795.69001.35. [DOI] [PubMed] [Google Scholar]
- 8.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–30. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Urban JP, Tirlapur UK, et al. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthritis Cartilage. 2010;18:433–9. doi: 10.1016/j.joca.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard S, Erickson GR, Guilak F. Hyperosmotically induced volume change and calcium signaling in intervertebral disk cells: the role of the actin cytoskeleton. Biophys J. 2002;83:2502–10. doi: 10.1016/S0006-3495(02)75261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maroudas A, Thomas H. A simple physicochemical micromethod for determining fixed anionic groups in connective tissue. Biochimica et Biophysica Acta. 1970;215:214–6. [PubMed] [Google Scholar]
- 12.Gu WY, Lai WM, Mow VC. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. J Biomech Eng. 1998;120:169–80. doi: 10.1115/1.2798299. [DOI] [PubMed] [Google Scholar]
- 13.Gu WY, Mao XG, Rawlins BA, et al. Streaming potential of human lumbar anulus fibrosus is anisotropic and affected by disc degeneration. J Biomech. 1999;32:1177–82. doi: 10.1016/s0021-9290(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 14.Maroudas A. Physicochemical properties of cartilage in the light of ion exchange theory. Biophys J. 1968;8:575–95. doi: 10.1016/S0006-3495(68)86509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neidlinger-Wilke C, Mietsch A, Rinkler C, et al. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J Orthop Res. 2012;30:112–21. doi: 10.1002/jor.21481. [DOI] [PubMed] [Google Scholar]
- 16.Wuertz K, Urban JP, Klasen J, et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res. 2007;25:1513–22. doi: 10.1002/jor.20436. [DOI] [PubMed] [Google Scholar]
- 17.Gu WY, Yao H. Effects of hydration and fixed charge density on fluid transport in charged hydrated soft tissues. Ann Biomed Eng. 2003;31:1162–70. doi: 10.1114/1.1615576. [DOI] [PubMed] [Google Scholar]
- 18.Yao H, Justiz MA, Flagler D, et al. Effects of swelling pressure and hydraulic permeability on dynamic compressive behavior of lumbar annulus fibrosus. Ann Biomed Eng. 2002;30:1234–41. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]
- 19.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179–87. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Urban JP, Maroudas A. The measurement of fixed charged density in the intervertebral disc. Biochimica et Biophysica Acta. 1979;586:166–78. [Google Scholar]
- 21.Jackson AR, Yuan TY, Huang CY, et al. A conductivity approach to measuring fixed charge density in intervertebral disc tissue. Ann Biomed Eng. 2009;37:2566–73. doi: 10.1007/s10439-009-9792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu XL, Sun DD, Guo XE, et al. Indentation determined mechanoelectrochemical properties and fixed charge density of articular cartilage. Ann Biomed Eng. 2004;32:370–9. doi: 10.1023/b:abme.0000017534.06921.24. [DOI] [PubMed] [Google Scholar]
- 23.Chen CT, Fishbein KW, Torzilli PA, et al. Matrix fixed-charge density as determined by magnetic resonance microscopy of bioreactor-derived hyaline cartilage correlates with biochemical and biomechanical properties. Arthritis Rheum. 2003;48:1047–56. doi: 10.1002/art.10991. [DOI] [PubMed] [Google Scholar]
- 24.Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine. 1989;14:166–74. doi: 10.1097/00007632-198902000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Cisewski SE, Sachs BL, et al. The region-dependent biomechanical and biochemical properties of bovine cartilaginous endplate. J Biomech. 2015;48:3185–91. doi: 10.1016/j.jbiomech.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu WY, Yao H, Vega AL, et al. Diffusivity of ions in agarose gels and intervertebral disc: effect of porosity. Ann Biomed Eng. 2004;32:1710–7. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 28.Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–5. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Gu WY, Justiz MA, Yao H. Electrical conductivity of lumbar anulus fibrosis: effects of porosity and fixed charge density. Spine. 2002;27:2390–5. doi: 10.1097/00007632-200211010-00014. [DOI] [PubMed] [Google Scholar]
- 30.Jackson A, Yao H, Brown MD, et al. Anisotropic ion diffusivity in intervertebral disc: an electrical conductivity approach. Spine. 2006;31:2783–9. doi: 10.1097/01.brs.0000245842.02717.1b. [DOI] [PubMed] [Google Scholar]
- 31.Gu W, Lewis B, Lai WM, et al. A Technique for Measuring Volume and True Density of the Solid Matrix of Cartilaginous Tissues. Advances in Bioengineering, ASME BED. 1996:89–90. [Google Scholar]
- 32.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from Generalized Linear Mixed-effects Models. Methods Ecol Evol. 2013;4:133–42. [Google Scholar]
- 34.Hasegawa I, Kuriki S, Matsuno S, et al. Dependence of electrical conductivity on fixed charge density in articular cartilage. Clin Orthop Relat Res. 1983:283–8. [PubMed] [Google Scholar]
- 35.Bibby SR, Jones DA, Ripley RM, et al. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 36.Fields AJ, Rodriguez D, Gary KN, et al. Influence of biochemical composition on endplate cartilage tensile properties in the human lumbar spine. J Orthop Res. 2014;32:245–52. doi: 10.1002/jor.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urban JP, Maroudas A. Swelling of the intervertebral disc in vitro. Connect Tissue Res. 1981;9:1–10. doi: 10.3109/03008208109160234. [DOI] [PubMed] [Google Scholar]
- 38.Stevens RL, Ewins RJ, Revell PA, et al. Proteoglycans of the intervertebral disc. Homology of structure with laryngeal proteoglycans. Biochem J. 1979;179:561–72. doi: 10.1042/bj1790561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts S, Caterson B, Evans H, et al. Proteoglycan components of the intervertebral disc and cartilage endplate: an immunolocalization study of animal and human tissues. Histochem J. 1994;26:402–11. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- 40.Urban JP, Maroudas A. The chemistry of the intervertebral disc in relation to its physiological function and requirements. Clin Rheum Dis. 1980;6:51–77. [Google Scholar]
- 41.Perie DS, Maclean JJ, Owen JP, et al. Correlating material properties with tissue composition in enzymatically digested bovine annulus fibrosus and nucleus pulposus tissue. Ann Biomed Eng. 2006;34:769–77. doi: 10.1007/s10439-006-9091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koike Y, Uzuki M, Kokubun S, et al. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003;28:1928–33. doi: 10.1097/01.BRS.0000083324.65405.AE. [DOI] [PubMed] [Google Scholar]
- 43.Lama P, Zehra U, Balkovec C, et al. Significance of cartilage endplate within herniated disc tissue. Eur Spine J. 2014;23:1869–77. doi: 10.1007/s00586-014-3399-3. [DOI] [PubMed] [Google Scholar]
- 44.Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14:513–21. doi: 10.1016/j.spinee.2013.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson AR, Travascio F, Gu WY. Effect of mechanical loading on electrical conductivity in human intervertebral disk. J Biomech Eng. 2009;131:054505. doi: 10.1115/1.3116152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.