Abstract
Background: Knowing the phenylalanine (Phe) content of foods is essential for managing the diet of patients with phenylketonuria. Data on the Phe content of foods are scarce and sometimes vary between different Food Composition Tables (FCT). Brazil created its own table of the Phe contents of fruits and vegetables based exclusively on the chemical analysis of protein content, considering that proteins contain 3–4% Phe (TCFA/ANVISA). This study compared the protein and Phe contents of vegetables and fruits provided by the TCFA/ANVISA with those listed in international food composition tables.
Methods: The Phe content of 71 fruits and vegetables listed in TCFA/ANVISA was classified into four subgroups, and the Wilcoxon nonparametric test compared the Phe and mean protein contents provided by the FCTs. All tests considered the bilateral hypothesis, and the level of significance was set at 5%. The Spearman’s correlation coefficient measured the statistical dependence between Phe and protein contents.
Results: The mean Phe content was <50 mg Phe/100 g for 15 fruits; >50 mg/100 g for 11 type-A vegetables; <50 mg/100 g for 8 type-B vegetables; ≤50 mg/100 g for 7 type-C vegetables. The percentage of Phe in protein varied from 3.13 ± 1.03% to 3.74 ± 2.55% in fruits; 3.33 ± 1.41 to 4.82 ± 1.17 in type-A vegetables; 3.46 ± 1.25% to 4.83 ± 2.46 in type-B vegetables; and 3.14% ± 1.49 to 4.62% ± 2.26 in type-C vegetables.
Conclusions: The Phe and protein contents provided by most FCTs were positively correlated, suggesting that it is possible to estimate the Phe content of fruits by multiplying its protein content by 3%. For type-A, -B, and -C vegetables, 4% may be used.
Keywords: Food composition tables, Phenylalanine, Phenylalanine restricted diet, Phenylketonuria
Introduction
Food Composition Tables (FCTs) and searchable databases provide information on nutrients, but data about the amino acid content of foods are frequently either unavailable, scarce, or outdated (Pennington 2008; Nalin et al. 2010; MacDonald et al. 2010; Demirkol et al. 2011). This is one of the various factors that may explain the absence of the amino acid profile of fresh fruits and vegetables in several FCTs (Charrondiere et al. 2013; Blau et al. 2010; Feillet et al. 2010a, b; Guimarães and Lanfer Marquez 2002, 2005; Greenfield and Southgate 2003; Osmo et al. 2008).
Phenylketonuria (PKU) is the most common inborn error of amino acid metabolism with a global prevalence ranging from 1:30,000 to 1:1,000 of live newborns, depending on country (Monteiro and Cândido 2006; Ahring et al. 2009; Martins et al. 2009; Blau et al. 2010). Due to persistently high plasma Phe level, untreated PKU causes neurological impairment, intellectual disability, speech delay, convulsions, skin hypopigmentation, and eczema, among others (Brandalize and Czeresnia 2004; Feillet et al. 2010a, b; De Groot et al. 2010; Camp et al. 2012).
Reliable information on the Phe content of foods is indispensable for patients with phenylketonuria, since they require a diet low in this essential amino acid. Fresh fruits and vegetables are poor protein sources that do not contribute significantly to protein requirement. Although no consensus has been reached, some studies suggest that patients with phenylketonuria should be allowed to consume low-Phe fruits and vegetables. No significant negative impact on short-term metabolic control has been observed, but the literature encourages more research to confirm this finding (Mac Donald et al. 2003; Weetch and MacDonald 2006; MacDonald et al. 2011; Rohde et al. 2012; Zimmermann et al. 2012).
Therefore, it is crucial to know the Phe content of fruits and vegetables and their variability in international food tables and searchable food composition databases to guarantee that fruits and vegetables indeed contribute little to Phe intake. The present study compared the Phe content of fresh fruits and vegetables listed in the Brazilian PKU table (TCFA/ANVISA), with those listed in eight international FCTs. Additionally, the possibility of using the mean Phe content of the FCTs for some fruits and vegetables was investigated.
Methods
The project was approved by the Research Ethics Committee of the School of Health Sciences of the University of Brasilia (N. 389.679/2013).
Food Composition Tables and Study Design
Table of Phenylalanine Content of Foods of the Brazilian National Sanitary Surveillance Agency (TCFA/ANVISA)
Table 1 of TCFA/ANVISA lists 71 fresh fruits and vegetables, which were grouped into subgroups according to their edible parts: fruits (n = 27); type-A vegetables (n = 18): the edible parts of these vegetables are the leaves, flowers, buds, or stems; type-B vegetables (n = 14): the edible parts of these vegetables are fruits, seeds, or parts that develop on the ground; and type-C vegetables (n = 12): the edible parts of these vegetables are those that grow underground and palm trees.
Table 1.
Mean phenylalanine content of fruits and vegetables (mg/100 g) in TCFA/ANVISA
| Fruits | Phe (mg/100 g) | Type-A vegetablesa | Phe (mg/100 g) | Type-B vegetablesb | Phe (mg/100 g) | Type-C vegetablesc | Phe (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Acai (Euterpe oleracea mart.) | 27 | Artichoke (globe) (Cynara scolimus L.) | 96 | Bur cucumber (Cucumis anguria L.) | 49 | Beets (Beta vulgaris) | 54 |
| Apple (Pyrus malus) | 10 | Arugula (Eruca sativa Mill) | 97 | Chayote (Sechium edule) | 31 | Carrot (Daucus carota) | 41 |
| Avocado (Persea americana mil.) | 45 | Butter green bean (Phaseolus vulgaris L.) | 75 | Cucumber (Cucumis sativus L.) | 29 | Cassava (Manihot esculenta crantz manihot utilissima) | 43 |
| Banana (Musa sp.) | 40 | Cabbage, white and red (Brassica oleracea L.) | 35 | Eggplant (Solanum melogena L.) | 34 | Garlic (Allium sativum L.) | 236 |
| Cashew (Anacardium occidentale L.) | 27 | Cauliflower (Brassica oleracea L.) | 62 | Okra (Abelmoschus esculentus) | 82 | Leeks (Allium porrum L.) | 58 |
| Fig (Ficus carica L.) | 26 | Celery (Apium graveolens L.) | 38 | Peas, green (Pisum sativum L.) | 120 | Onion (Allium cepa L.) | 68 |
| Grapes, red or green (Vitis sp.) | 24 | Chard, swiss (Beta vulgaris L.) | 36 | Peppers, sweet, yellow (Capsicum annuum) | 42 | Palm heart (Euterpe edulis) | 98 |
| Jackfruit (Arctocarpus heterophyllus) | 49 | Chicory (Chichorim endivia L.) | 42 | Peppers, sweet, green (Capsicum annuum) | 33 | Pupunha (palm heart) (Bactris gasipaes kunth.) | 88 |
| Kiwifruit (Actinidia chinensis plack.) | 44 | Chicory greens (Cichorium intybus) | 62 | Peppers, sweet, red (Capsicum annuum) | 36 | Potato (Solanum tuberosum) | 71 |
| Indian Cherry (Malgiphia emarginata) | 31 | Coriander (Coriandrum sativum L.) | 173 | Pumpkin (Cucurbita spp.) | 46 | Radish (Raphanus sativus) | 26 |
| Mango (Mangifera indica) | 25 | Dutch string bean (Phaseolus vulgaris L.) | 98 | Scarlet eggplant (Solanum gilo Raddi.) | 40 | Sweet Potato (Ipomoea batatas L.) | 58 |
| Melon (Cucumis melo) | 22 | Endive (Cichorium endivia L.) | 66 | Squash, summer (Cucurbita pepo) | 42 | Yam (Dioscorea spp.) | 54 |
| Papaya (Carica papaya L.) | 18 | Kale (Brassica oleracea) | 96 | Squash, winter (Cucurbita maxima) | 87 | Taro (Colocasia esculenta) | 62 |
| Passion fruit (granadilla) (Passiflora edulis) | 138 | Lettuce (Lactuca sativa L.) | 40 | Tomatoes (Solanum lycopersicon sp.) | 38 | Turnips (Brassica rapa L.) | 41 |
| Peaches (Prunus persica) | 33 | Onions, spring or scallions (Allium fistulosum L.) | 57 | ||||
| Pears (Pyrus communis L.) | 20 | Parsley, fresh (Petroselinum sativum) | 211 | ||||
| Pequi (Caryocar brasiliense camb.) | 82 | Spinach (Tetragonia expansa Murr.) | 65 | ||||
| Persimmon (Diospyros kaki L.) | 21 | String bean (Phaseolus vulgaris L.) | 66 | ||||
| Pineapple (Ananas comosus L. merril) | 29 | Watercress (Nasturtium officinale L.) | 126 | ||||
| Pomegranates (Punica granatum L.) | 21 | ||||||
| Plums (Prunus salicina Lindl.) | 43 | ||||||
| Soursop (Annona muricata) | 56 | ||||||
| Strawberry (Fragaria vesca L.) | 33 | ||||||
| Sugar apples (sweetsop) (Annona squamosa) | 92 | ||||||
| Surinam cherry (Eugenia uniflora L.) | 30 | ||||||
| Tamarinds (Tamarindus indica L.) | 105 | ||||||
| Tangerines, mandarin oranges (Citrus reticulata) | 29 |
aThe edible part of the vegetables consists of the leaves, flowers, buds, or stems
bThe edible part of the vegetables consists of fruits, seeds, or parts that develop on the ground
cConsists of the parts that grow underground and palm trees
The TCFA/ANVISA includes moisture, protein, and Phe contents. The protein content of all fruits and vegetables was given by their total N content, determined by Kjeldahl’s method, which was then multiplied by 5.7 to obtain the crude protein content. The Phe content was estimated by multiplying the protein content by 4% and expressed as mg Phe/100 g food. Therefore, 1 g of fruit or vegetable protein has about 40 mg of Phe. The value of 4% was taken from studies that reported that fruits and vegetables contain about 40 mg of Phe per gram of protein, which is lower than the Phe content of other types of natural proteins, such as animal proteins, which seem to have a Phe content closer to 5% (50 mg/g protein) (Ahring et al. 2009; Weetch and MacDonald 2006; MacDonald et al. 2011; AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA 2013). In most countries including the United States (USA), multiplying plant protein content by 5% would force patients to eat less for fear of reaching the actual daily Phe intake tolerance.
International Food Composition Tables
The protein and Phe contents of foods provided by the TCFA/ANVISA were compared with those furnished by eight international FCTs listed in the International Food Composition Tables Directory (Table 2). The directory is maintained by the International Network of Food Data Systems (INFOODS) (www.fao.org/infoods). In addition to the FCTs listed by INFOODS, the Low Protein Food List for PKU (LPFL-PKU) was also included, due to its relevance for patients with phenylketonuria (Schuett 2010).
Table 2.
Food composition tables included in the study
| Name | Organization | Source of Phe contents |
|---|---|---|
| Brazilian table for PKU, 2013 (TCFA/ANVISA) | National Sanitary Surveillance Agency (ANVISA) | Estimated by multiplying their protein content by 4% |
| Food Amino-Acid Content of Foods and Biological Data on Proteins, 1970 (FAO-AA/FAO) | Food and Agriculture Organization of the United Nations | Analytical data published in the scientific literature, databases or other FCTs |
| Danish Food Composition Databank, 2009 version 7 (DTU FOOD) | National Food Institute/Technical University of Denmark (DTU) | Laboratory analyses conducted specifically for the FCTs and analytical data published in the scientific literature or databases, including the USDA-SR database and other FCTs |
| Food Composition and Nutrition Tables, 2008 (FCNT) | German Research Centre for Food Chemistry | Laboratory analyses conducted specifically for the FCTs and analytical data published in the scientific literature, databases, or other FCTs |
| Nutrient Tables – Food Standards Australia New Zealand (FSANZ), 2010 (NUTTAB) | Food Standards Australia New Zealand | Laboratory analyses conducted specifically for the FCTs |
| Health Canada – Canadian Nutrient File, 2015 (HCNT) | Health Canada | Analytical data published in the USDA database, SR 23–27 |
| New Zealand Food Composition Database, FOODfiles 2014 Version 01 (NZFC) | The New Zealand Institute for Plant & Food Research Limited and the Ministry of Health (New Zealand) | Laboratory analyses conducted specifically for the FCTs and analytical data published in the scientific literature, databases, or other FCTs |
| Low Protein Food List for PKU, 2010 (LPFL-PKU) | Waisman Center in Madison, Wisconsin | Analytical data published in the USDA database (SR 22, 2009, SR 23, 2010, and SR 28, 2015) |
| USDA National Nutrient Database for Standard Reference, Release 28, 2015 (USDA-SR) | United States Department of Agriculture | Laboratory analyses conducted specifically for the FCTs |
The international tables were selected based on the following criteria: free internet access; available in electronic format: such as Excel, Access, or PDF files, or online database; available in English, Portuguese, or Spanish; and containing the Phe content of fruits and vegetables with a protein content of 5% or less. The exclusion criteria were: restricted access; information not available in English or Spanish; Phe content not available for some or all items, or available only for fresh vegetables and fruits that do not have similar counterparts in the TCFA/ANVISA Table.
Moisture, protein, and Phe contents provided by the eight international FCTs were determined by different analytical techniques used specifically to construct the FCTs. Sometimes data were also obtained from analytical data published in the literature or compiled from other databases and FCTs (Table 2).
Fresh vegetables listed in the TCFA/ANVISA Table were compared with their counterparts in the international FCTs and identified by their popular and scientific names, by the edible parts, and also by their taxonomic description, including genus, species, and variety. Different varieties of fruits and vegetables of the same species, and fruits and vegetables without variety information were grouped, and the mean Phe content was calculated. However, some fruits and vegetables, which are commonly found in Brazil, were not found in the international FCTs. Hence, they were maintained in the TCFA/ANVISA Table (Table 1), but were not compared.
The protein contents in the international FCTs were calculated using a conversion factor of 6.25 to transform total N into protein by considering that the protein fraction has a mean nitrogen content of 16%. However, all data from the TCFA/ANVISA Table used a conversion factor of 5.75, which seems to be closest to the actual protein content (Greenfield and Southgate 2003). Therefore, the protein level contents provided by the different tables could only be compared after recalculating all the data using the conversion factor of 5.75.
In addition to absolute Phe content, we calculated the percent contribution of Phe in the protein of each food to verify whether Phe content was relatively constant for each type of vegetable, therefore, possibly genetically determined. Then the percentage of Phe present in the proteins of the fruits and vegetables listed in the international FCTs were compared with the Phe content of 4% in vegetable proteins used by the TCFA/ANVISA Table.
Statistical Analysis
The Wilcoxon nonparametric test compared the protein and Phe contents provided by the TCFA/ANVISA Table and each of the eight international FCTs. All tests considered bilateral hypotheses and used a significance level of 5%. The correlation between Phe and protein contents was given by Spearman’s correlation coefficient (Conover and Conover 1980).
Results
From the total number of fruits listed in the TCFA/ANVISA, 11 could not be compared because they were either not present in the majority of the other tables, or the Phe content was not provided. Some of these fruits are tropical, so analytical information is not easily available. The excluded fruits are: acai, cashew, Indian cherry, soursop, jackfruit, sweet passion fruit, pequi, sugar apple, Surinam cherry, pomegranate, and tamarind. Four type-A vegetables (butter green bean, Dutch string bean, endive, and string bean), two type-B vegetables (scarlet eggplant and bur cucumber), and two type-C vegetables (palm heart and pupunha palm heart) also were not compared because they were not listed in the other tables.
Figure 1a–d shows the Phe content of fruits and vegetables listed in the nine FCTs, including the TCFA/ANVISA Table. Fruits have the lowest protein content and, as expected, the lowest Phe content. In Fig. 1a, 15 out of the 16 fruits had a mean Phe content smaller than 50 mg/100 g fruit, ranging from 8 to 49 mg/100 g. The only exception was avocado, which varied considerably, from 45 to 150 mg/100 g. The median Phe content of avocado, considering the eight FCTs and the TCFA/ANVISA Table, was 93 mg/100 g, which might be the most accurate Phe content of this fruit. In the TCFA/ANVISA Table, the Phe content of avocado is 45 mg/100 g, so this value should be reexamined to confirm the discrepancy in relation to the other FCTs.
Fig. 1.
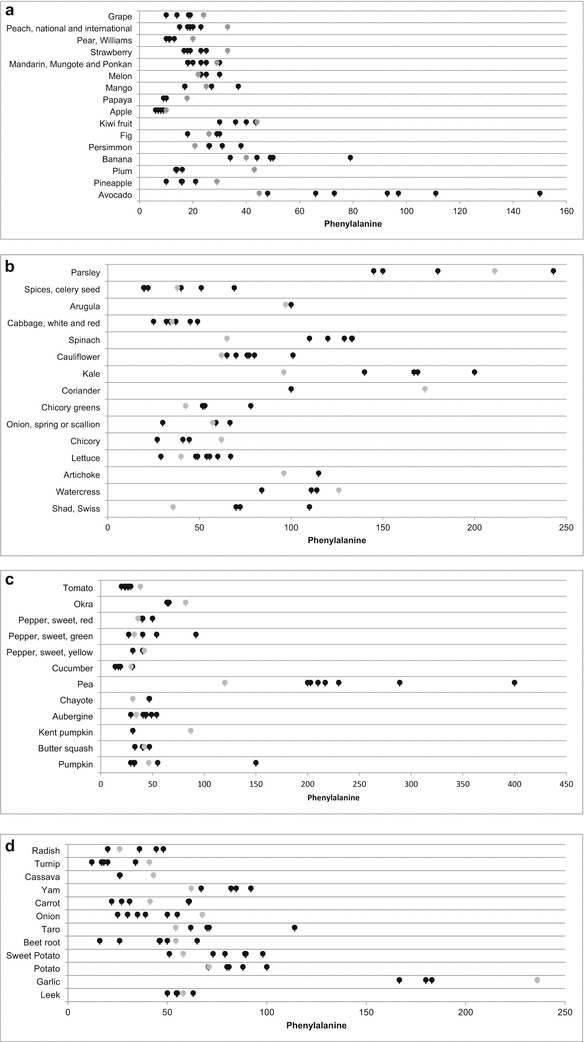
Phenylalanine content (mg/100 g) of fruits (a) and type-A, -B, and -C vegetables (b, c, d) provided by TCFA/ANVISA (gray dots) and eight international food composition tables (black dots): DTU FOOD/Denmark, FAO-AA, FCNT/Germany, HCNT/Canada, LPFL-PKU/USA, NUTTAB/Australia, NZFC/New Zealand, USDA-SR/USA. Legend for fruits: Grape, several varieties (Thompson, White, Niagara) (Vitis sp.); Peach, Brazilian and international (Prunus persica); Pear, Williams (Pyrus communis L.); Strawberry (Fragaria vesca L.); Mandarin, Mungote and Ponkan (Citrus reticulata “Murgote”); Melon (Cucumis melo); Mango (Mangifera indica L.); Papaya (Carica papaya L.), Apple (Pyrus malus); Kiwi fruit (Actinidia chinensis Plack.); Fig (Ficus carica L.); Persimmon (Diospyros kaki L.); Banana (several varieties) (Musa sp.); Plum (Prunus salicina Lindl.); Pineapple (Ananas comosus); Avocado (Persea americana mil.). Legend for type-A vegetables: Parsley (Petroselinum sativum), Spices, celery seed (Apium graveolens L.), Arugula (Erucata sativa), Cabbage, white and red (Brassica oleracea L.), Spinach (Tetragonia expansa Murr.), Cauliflower (Brassica oleracea L.), Kale (Brassica oleracea L.), Coriander (Coriandrum sativum L.), Chicory greens (Cichorium intybus L.), Onions spring or scallions (Allium fistulosum L.), Chicory (Chicorium endivia), Lettuce (Lactuca sativa L.), Artichokes, globe (Cynara scolimus L.), Watercress (Nasturtium officinale L.), Chard, Swiss (Beta vulgaris L. var. cicla). Legend for type-B vegetables: Tomatoes, several varieties (Solanum lycopersicon Mill., Lycopersicon sp.), Okra (Hibiscus esculentus), Pepper, sweet, red (Capsicum annuum), Pepper, sweet, green (Capsicum annuum), Pepper, sweet, yellow (Capsicum annuum), Cucumber (Cucumis sativus L.), Peas, green (Pisum sativum L.), Chayote (Sechium edule), Aubergine (Solanum melogena L.), Kent pumpkin (Cucurbita maxima), Butter, squash, (Cucurbita pepo), Pumpkin (Cucurbita spp.). Legend for type-C vegetables: Radish (Raphanus sativus), Turnip (Brassica rapa L.), Cassava (Manihot esculenta Crantz Manihot utilissima), Yam (Colocasia esculenta), Carrot (Daucus carota L.), Onion (Allium cepa L.), Taro (Dioscorea spp.), Beet root (Beta vulgaris), Sweet potato (Ipomoea batatas L.), Potato (Solanum tuberosum), Garlic (Allium sativum L.), Leek (Allium porrum L.)
In the international FCTs, the proportion of Phe in protein varied from 3.13 ± 1.03% to 3.74 ± 2.55% and was always lower than 4%, with significant differences between five international FCTs and the TCFA/ANVISA Table, which varied from 3.13% ± 1.03 to 3.28% ± 0.93 (Table 3a). Therefore, the results suggest that it is possible to estimate the Phe content of fruits by multiplying their protein content by 3%.
Table 3.
Mean, standard deviation, and comparative analysis of the percentage of phenylalanine in the protein portion of foods (a) and association between the Phe and protein contents (Spearman’s correlation) (b) of foods listed in the study food composition tables and the Table of Phenylalanine Content of Foods ofANVISA/Brazil
| (a) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruits | Type-A vegetables | Type-B vegetables | Type-C vegetables | |||||||||
| International FCT | Number of foods | FCTx | TCFA/ANVISAy | Number of foods | FCTx | TCFA/ANVISAy | Number of foods | FCTx | TCFA/ANVISAy | Number of foods | FCTx | TCFA/ANVISAy |
| DTU FOOD | 16 | 3.19 ± 0.9a | 3.97 ± 0.2b | 11 | 4.31 ± 1.31a | 4.01 ± 0.07a | 10 | 6.18 ± 7.47a | 4b | 11 | 3.14 ± 1.49a | 3.98 ± 0.04a |
| FAO-AA | 9 | 3.28 ± 0.93a | 4 ± 0.22b | 6 | 4.82 ± 1.17a | 3.98 ± 0.04a | 5 | 3.46 ± 1.25a | 4a | 9 | 4 ± 1.07a | 3.99 ± 0.03a |
| FCNT | 7 | 3.61 ± 1.29a | 3.96 ± 0.21a | 8 | 4.34 ± 1.33a | 4.01 ± 0.08a | 6 | 4.07 ± 1.64a | 4a | 7 | 3.41 ± 1.41a | 4a |
| HCNT | 16 | 3.74 ± 2.55a | 3.97 ± 0.2a | 10 | 4.3 ± 1.29a | 3.99 ± 0.03a | 11 | 4.83 ± 2.46a | 4b | 9 | 4.62 ± 2.26a | 3.98 ± 0.04a |
| LPFL-PKU | 15 | 3.16 ± 1.03a | 3.97 ± 0.2b | 15 | 4.46 ± 1.07a | 4.01 ± 0.06a | 11 | 4.28 ± 0.96a | 4b | 12 | 4.36 ± 1.57a | 3.98 ± 0.04a |
| NUTTAB | 7 | 3.56 ± 1.3a | 3.96 ± 0.21a | 4 | 3.33 ± 1.41a | 3.98 ± 0.05a | 4 | 3.53 ± 0.67a | 4a | 6 | 3.93 ± 0.99a | 4a |
| NZFC | 10 | 3.13 ± 1.03a | 3.94 ± 0.17b | 3 | 4.53 ± 1.17a | 4a | 1 | 4.9 | 4 | 5 | 3.62 ± 0.91a | 3.98 ± 0.04a |
| USDA-SR | 16 | 3.25 ± 1.04a | 3.97 ± 0.2b | 12 | 4.28 ± 1.19a | 4.01 ± 0.07a | 12 | 4.72 ± 2.38a | 4b | 12 | 4.28 ± 1.72a | 3.98 ± 0.04a |
| (b) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruits | Type-A vegetables | Type-B vegetables | Type-C vegetables | |||||||||
| International FCT | Number of foods | Spearman’s correlation | Number of foods | Spearman’s correlation | Number of foods | Spearman’s correlation | Number of foods | Spearman’s correlation | ||||
| DTU FOOD | 16 | 0.841* | 11 | 0.918* | 10 | 0.390** | 11 | 0.664* | ||||
| FAO-AA | 9 | 0.883* | 6 | 0.771** | 5 | 0.900* | 9 | 0.908* | ||||
| FCNT | 7 | 0.889* | 8 | 0.905* | 6 | 0.986* | 7 | 0.714** | ||||
| HCNT | 16 | 0.692* | 10 | 0.879* | 11 | 0.221** | 11 | 0.764* | ||||
| LPFL-PKU | 15 | 0.814* | 15 | 0.900* | 11 | 0.528** | 12 | 0.673* | ||||
| NUTTAB | 7 | 0.857* | 4 | 1.000* | 4 | 1.000* | 6 | 0.899* | ||||
| NZFC | 10 | 0.848* | 4 | 0.400** | 1 | – | 5 | 0.900* | ||||
| TCFA/ANVISA | 16 | 0.972* | 15 | 0.997* | 12 | 0.998* | 12 | 0.995* | ||||
| USDA-SR | 16 | 0.747* | 12 | 0.923* | 12 | 0.377** | 12 | 0.722* | ||||
Statistical analysis: different letters in lines between FCTs and TCFA/ANVISA indicate significant differences (p < 0.05)
*p < 0.05; **p > 0.05
xMean Phe content ± standard deviation in the protein fraction provided by international FCTs
yMean Phe content ± standard deviation in the protein fraction provided by TCFA/ANVISA
Vegetables have also very low Phe content, but in general and with some overlapping, they seem to have more Phe than fruits. Figure 1b shows the Phe content furnished by the FCTs for 15 study vegetables. On average, 11 out of the 15 type-A vegetables had a mean Phe content higher than 50 mg/100 g, ranging from 53 to 179 mg/100 g. The Phe content of proteins in these foods provided by the international FCTs ranged from 3.33 ± 1.41% to 4.82% ± 1.17, with no significant differences between the eight FCTs and the TCFA/ANVISA Table (Table 3a).
Not all 14 type-B vegetables listed in the TCFA/ANVISA Table were listed in the international FCTs, which resulted in a variable number of samples. Figure 1c shows the Phe content of 12 type-B vegetables. Of these, eight have a mean Phe content lower than 50 mg/100 g, ranging from 20 to 47 mg/100 g. The Phe content of peapods was variable and higher than 200 mg/100 g. Peapod is a legume with high protein content associated with degree of ripeness, as protein is synthesized in advanced maturation stages.
The mean Phe content in the protein fraction of type-B vegetables provided by the TCFA/ANVISA Table did not differ significantly from those provided by three of the eight international FCTs (FAO-AA, FCNT/Germany, and NUTTAB/Australia), whose percentages varied from 3.46 ± 1.25% (FAO-AA) to 4.07 ± 1.64% (FCNT/Germany). In the other FCTs, the mean percentages were significantly higher, varying from 4.28 ± 0.96% (LPFL-PKU/USA) to 4.83 ± 2.46% (HCNT/Canada) (Table 3a). However, the percentages of 27% Phe in the protein fraction of pumpkin provided by DTU FOOD (Denmark) and of roughly 11% in green pepper provided by HCNT (Canada) and USDA-SR (USA) may be incorrect because they differ greatly the percentages provided by the literature (3–5%) and do need confirmation (Greenfield and Southgate 2003; de Menezes et al. 2003).
Figure 1d shows the Phe content of 12 type-C vegetables even though not all of them were listed in the international FCTs, resulting in the comparison of a variable number of samples. On average, seven of these foods had a Phe content of 50 mg/100 g or less, ranging from 22 to 50 mg/100 g. The mean Phe content of the other four type-C vegetables varied from 73 to 88 mg/100 g, except for garlic, which had a mean Phe content of 190 mg/100 g. The Phe content in the protein of 50% of these foods varied significantly, from 3.14 ± 1.49% to 4.62 ± 2.26% in the international FCTs, but the contents were not significantly different from those provided by the TCFA/ANVISA Table (Table 3a).
Phe and protein contents were positively correlated in all FCTs (Table 3b). The correlation for fruits was statistically significant in all eight international FCTs, and the Spearman’s correlation coefficient varied from ρ = 0.692 to 0.972 (p < 0.05). For type-C vegetables the correlation was significant in seven of the eight FCTs (ρ = 0.664 to 0.995; p < 0.05). The correlation for type-A vegetables was significant in six FCTs (Spearman, ρ = 0.879 to 1.000; p < 0.05). The correlation for type-B vegetables was significant in three FCTs (ρ = 0.900 to 1.000; p < 0.05).
Discussion
The present study compared the Phe content of fresh fruits and vegetables listed in nine food composition tables. The Brazilian table (TCFA/ANVISA) was used as reference for the number of samples compared. The possibility of using a mean Phe content for each food group was also investigated.
Based on our results about 70% of the fresh fruits and vegetables listed in the FCTs have similar Phe content: apple, artichoke, arugula, aubergine, banana, cassava, cauliflower, chayote, chicory, cucumber, endive, fig, grape, kiwi, leek, lettuce, mandarin, mango, melon, okra, onion, papaya, peach, pear, persimmon, pineapple, plum, squash summer, strawberry, taro, turnip, yellow pepper, radish, red pepper, white and red cabbage, and tomato.
The Phe content of about 30% of the study fresh fruits and vegetables differ considerably between the nine FCTs: avocado, beet root, carrot, celery seed, coriander, garlic, green pepper, kale, onion spring or scallion, parsley, pea, potato, pumpkin, kent pumpkin, spinach, swiss chard, watercress, sweet potato, and yam.
The differences may stem from several factors, such as accuracy of protein and Phe estimates, origin of the food, genetic variability, climate, degree of maturation, time of harvest, and even a transcription error of raw data, which cannot be omitted. Therefore, an adequate number of samples of these vegetables should be reanalyzed to obtain more accurate Phe and protein estimates, and consequently explain or correct the different protein and Phe contents provided by the FCTs. Result dispersion may be minimized by analyzing a representative number of samples of the species and varieties of fruits and vegetables cultivated and consumed in a country, taking into account factors that may affect nutrient composition (de Menezes et al. 2003). The sampling design, analytical method, expression of the results, and data treatment should also be considered.
According to the nine FTCs, 58 and 84% of the 55 fresh study fruits and vegetables had a mean Phe content of 50 mg/100 g or less, and 100 mg/100 g or less, respectively. Since clinical trials suggest that fruits and vegetables with Phe content of 50 mg to 100 mg/100 g are safe for patients with phenylketonuria, these fruits and vegetables could be classified as unrestricted for these individuals (Mac Donald et al. 2003; Weetch and MacDonald 2006; MacDonald et al. 2011; Rohde et al. 2012; Zimmermann et al. 2012).
We understand that the data in the FCTs, some based on chemical analyses and some on the literature, and the small number of analyzed samples are study limitations. Therefore, caution is advised when using the Phe contents provided by the FCTs, since many factors contribute to different or incorrect results.
Although chemical analyses are recommended to obtain more accurate Phe content data, the positive correlation found between the protein and Phe contents of the study fruits and vegetables indicates that Phe content can be reliably estimated from protein content.
Pimentel et al. (2014) made a similar attempt by analyzing the protein and amino acid contents of 16 vegetable and fruit preparations usually included in the diet of Portuguese patients with phenylketonuria. The Phe and protein contents of these preparations were highly correlated. Lanfer Marquez et al. (1997) studied the chemical composition of cereal flakes and found a linear correlation between the samples’ total nitrogen and Phe contents.
Hence, given the scarcity of analytical Phe data and the present comparative analysis, the Phe content of fresh fruits and vegetables can be estimated from their protein content, despite the limitations associated with result accuracy. Comparative analyses indicated that 3% seems to be the most appropriate multiplier to calculate the Phe content in the fruit protein contents provided by most FCTs.
For type-A, type-B, and type-C vegetables, the results support the possibility of estimating the Phe content of leaf vegetables by multiplying their protein content by 4%, as performed by the TCFA/ANVISA Table (Brazil) and most FCTs. These results confirm the literature reports that Phe contributes with 3–5% of the total amino acid content of these foods (Weetch and MacDonald 2006; Bremer et al. 1996). However, when the percentage of 3 or 4% is used for estimating Phe content, the result is lower than 50 mg/g of protein. When the percentage of 5% is used, the result exceeds 50 mg/g of protein.
Processed plant-based foods, such as fruit and vegetable juices, and jams, should also be studied to determine whether the correlation between the Phe and protein contents of fresh vegetables and fruits also applies to these products. If so, the concentrations of 3–5% Phe in proteins could be used to estimate the Phe content of these products. Vegetable and fruit preparations should also be chemically analyzed because processing may change the Phe content of the food (Weetch and MacDonald 2006; Pimentel et al. 2014). Weetch and MacDonald (2006) found that the Phe content of different potato varieties prepared in different ways resulted in a mean Phe content of 28 mg/g of protein. For cooked potatoes, the Phe content varied from 44 mg to 109 mg/100 g. The vegetable preparations analyzed by Pimentel et al. (2014) had Phe contents of 12 mg to 33 mg/g of protein, and Phe and protein contents were highly correlated.
Conclusion
For fruits, 3% seems to be the best multiplier. For type-A, -B, and -C vegetables, 4% may be used. Analysis of the Phe and protein contents of the 55 fruits and vegetables listed in the nine FCTs indicated that it is possible to calculate mean Phe content from the amounts of Phe and protein provided by the various FCTs. In the absence of analytical Phe data, it is possible to estimate the Phe content of fresh fruits and vegetables from their protein content, despite the limitations associated with the accuracy of this method.
These findings may be useful for updating FCTs for patients with phenylketonuria, assisting dietitians in their practice and patients in estimating the Phe content of their diet. This information may increase the number of dietary options that best fit patients with phenylketonuria’s daily routine.
It is important to create national FCTs with Phe data in the local language, accessible to the population, and to include in local FCTs regional fruits, vegetables, and preparations that would not normally be found in international FCTs.
Knowing Phe content variability in fruits and vegetables may help to reduce uncertainty, provide more reliable Phe contents, and expand the dietary guidelines of foods for patients with phenylketonuria.
Take-Home Message
The Phe content of most fresh fruits and vegetables listed in Brazilian PKU table is similar to those listed in other food composition tables.
Compliance with Ethics Guidelines
Ana Claudia Marquim Araújo, Wilma M. C. Araújo, Ursula M. Lanfer Marquez, Rita Akutsu, and Eduardo Y. Nakano declare that they have no conflict of interest.
This chapter does not contain any studies with human or animal subjects performed by any of the authors.
Authors’ Contributions
A.C.M.A. designed and conducted the research, analyzed samples, performed the statistical analyses, and wrote the paper; W.M.C.A. designed the research and wrote the paper; U.M.L.M. designed the research and reviewed the manuscript; R.A. reviewed the manuscript; E.Y.N. performed the statistical analyses.
Contributor Information
Ana Claudia Marquim F. Araújo, Email: anamarquim@gmail.com
Wilma M. C. Araújo, Email: wilma.araujo@terra.com.br
Ursula M. Lanfer Marquez, Email: lanferum@usp.br.
Rita Akutsu, Email: rita.akutsu@gmail.com.
Eduardo Y. Nakano, Email: nakano@unb.br
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Ahring K, Bélanger-Quintana A, Dokoupil K, et al. Dietary management practices in phenylketonuria across European centres. Clin Nutr. 2009;28:231–236. doi: 10.1016/j.clnu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376:1417–1427. doi: 10.1016/S0140-6736(10)60961-0. [DOI] [PubMed] [Google Scholar]
- Brandalize SRC, Czeresnia D. Avaliação do programa de prevenção e promoção da saúde de fenilcetonúricos (Evaluation of the program for prevention and health promotion in phenylketonuria patients in Brazil) Rev Saude Publica. 2004;38:300–306. doi: 10.1590/S0034-89102004000200021. [DOI] [PubMed] [Google Scholar]
- Bremer HJ, Anninos A, Schulz B. Amino acid composition of food products used in the treatment of patients with disorders of the amino acid and protein metabolism. Eur J Pediatr. 1996;155:S108–S114. doi: 10.1007/PL00014223. [DOI] [PubMed] [Google Scholar]
- Camp KM, Lloyd-Puryear MA, Huntington KL. Nutritional treatment for inborn errors of metabolism: indications, regulations, and availability of medical foods and dietary supplements using phenylketonuria as an example. Mol Genet Metab. 2012;107:3–9. doi: 10.1016/j.ymgme.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrondiere UR, Stadlmayr B, Wijesinha-Bettoni R, et al. INFOODS contributions to fulfilling needs and meeting challenges concerning food composition databases. Procedia Food Sci. 2013;2:35–45. doi: 10.1016/j.profoo.2013.04.007. [DOI] [Google Scholar]
- Conover WJ, Conover W (1980) Practical nonparametric statistics
- de Groot MJ, Hoeksma M, Blau N, et al. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99:S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- de Menezes EW, Giuntini EB, Lajolo FM. Quality and variability of food composition data. Nutr Rev Soc Bras Aliment Nutr. 2003;26:63–76. [Google Scholar]
- Demirkol M, Giżewska M, Giovannini M, Walter J. Follow up of phenylketonuria patients. Mol Genet Metab. 2011;104:S31–S39. doi: 10.1016/j.ymgme.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Feillet F, MacDonald A, Hartung (Perron) D, Burton B (2010a) Outcomes beyond phenylalanine: an international perspective. Mol Genet Metab 99:S79–S85. doi:10.1016/j.ymgme.2009.09.015 [DOI] [PubMed]
- Feillet F, van Spronsen FJ, MacDonald A et al (2010b) Challenges and pitfalls in the management of phenylketonuria. Pediatrics 126:333–341. doi:10.1542/peds.2009-3584 [DOI] [PubMed]
- Greenfield H, Southgate DA (2003) Food composition data: production, management, and use: Food & Agriculture Organization
- Guimarães CP, Lanfer Marquez UM. Chemical composition of bouillon cubes protein nitrogen, non protein nitrogen and phenylalanine. Ciência e Tecnol Aliment. 2002;22:308–313. doi: 10.1590/S0101-20612002000300019. [DOI] [Google Scholar]
- Guimarães CP, Lanfer-Marquez UM. Estimation of phenylalanine (Phe) contents in dehydrated soups: significance of non-protein nitrogen. Brazilian J Pharm Sci. 2005;41:365–375. [Google Scholar]
- Lanfer Marquez UM, Nishi LE, Barros RMC, et al. Estudo da composição química de flocos de cereais com ênfase nos teores de fenilalanina. Food Sci Technol. 1997;17(3):314–319. [Google Scholar]
- Mac Donald A, Mac Donald A, Rylance G, et al. Free use of fruits and vegetables in phenylketonuria. J Inherit Metab Dis. 2003;26:327–338. doi: 10.1023/A:1025150901439. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Gokmen-Ozel H, van Rijn M, Burgard P. The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis. 2010;33:665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- Macdonald A, Rocha JC, Van Rijn M, Feillet F. Nutrition in phenylketonuria. Mol Genet Metab. 2011;104:S10–S18. doi: 10.1016/j.ymgme.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Martins FF, Mendes AB, Cruz WM de S, Boaventura GT (2009) Calcium metabolism in phenilketonuria. Rev Nutr 22:419–428. doi:10.1590/S1415-52732009000300012
- Monteiro LTB, Cândido LMB (2006) Phenylketonuria in Brazil: evolution and cases. Rev Nutr 19:381–387. doi:10.1590/S1415-52732006000300009
- Nalin T, Perry I, Refosco L, Netto C, Souza C, Vieira T, et al. Phenylketonuria in the Public Health System: assessment of adherence to treatment in a medical care center in Rio Grande do Sul. Clin Biomed Res. 2010;30(3):225–232. [Google Scholar]
- Osmo H, Silva I, Feferbaum R. Phenylketonuria: from the dietary restriction to social-economic inclusion. Rev Bras Nutr Clin. 2008;23:104–110. [Google Scholar]
- Pennington JA. Applications of food composition data: data sources and considerations for use. J Food Compos Anal. 2008;21:S3–S12. doi: 10.1016/j.jfca.2007.02.005. [DOI] [Google Scholar]
- Pimentel FB, Alves RC, Costa ASG, et al. Phenylketonuria: protein content and amino acids profile of dishes for phenylketonuric patients. The relevance of phenylalanine. Food Chem. 2014;149:144–150. doi: 10.1016/j.foodchem.2013.10.099. [DOI] [PubMed] [Google Scholar]
- Rohde C, Mütze U, Weigel JFW, et al. Unrestricted consumption of fruits and vegetables in phenylketonuria: no major impact on metabolic control. Eur J Clin Nutr. 2012;66:633–638. doi: 10.1038/ejcn.2011.205. [DOI] [PubMed] [Google Scholar]
- AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. Tabela de Conteúdo de Fenilalanina em Alimentos. 2013
- Schuett VE (2010) Low protein food list for PKU: National PKU News
- Weetch E, Macdonald A. The determination of phenylalanine content of foods suitable for phenylketonuria. J Hum Nutr Diet. 2006;19:229–236. doi: 10.1111/j.1365-277X.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Jacobs P, Fingerhut R, et al. Positive effect of a simplified diet on blood phenylalanine control in different phenylketonuria variants, characterized by newborn BH4 loading test and PAH analysis. Mol Genet Metab. 2012;106:264–268. doi: 10.1016/j.ymgme.2012.04.016. [DOI] [PubMed] [Google Scholar]


