Abstract
Background: There are currently ten intravenous enzyme replacement therapy (ERT) products available for the treatment of eight different lysosomal diseases (LD) in the USA. Additional ERT products are in clinical trials. The most common ERT adverse events are infusion reactions (IR). While IR are often defined as hypersensitivity or anaphylactoid reactions occurring concurrently with (i.e., during) infusion administration (CIR), there exists the potential for delayed infusion reactions (DIR), which present after completion of infusion administration.
Hypothesis: Concurrent infusion reactions (CIR) are not the only infusion reactions associated with enzyme therapy.
Methods: This study evaluated the occurrence of infusion reactions in 46 patients with LD who had received ERT for a minimum of 2 years. Infusion reactions were evaluated according to symptoms, time of onset, and duration of reactions. The frequency of infusion reactions with each ERT product was compared to that reported in the FDA-approved product package insert.
Results and Conclusions: In this study, DIR were observed and occurred as often as CIR in the study population, despite not being characterized or reported in most ERT product package inserts. Effective methods for managing DIR and CIR differed, thus emphasizing the importance of monitoring for both types of infusion reactions in order to optimize outcomes for patients using ERT.
Introduction
Intravenous (IV) enzyme replacement therapy (ERT) is FDA approved for the treatment of eight different lysosomal diseases (LD): Gaucher disease (OMIM catalogue number 230800), Fabry disease (OMIM catalogue number 301500), Pompe disease (OMIM catalogue number 232300), mucopolysaccharidosis type I (MPS I) (OMIM catalogue number 607014), mucopolysaccharidosis type II (MPS II) (OMIM catalogue number 309900), mucopolysaccharidosis type IV A (MPS IVA) (OMIM catalogue number 253000), mucopolysaccharidosis type VI (MPS VI) (OMIM catalogue number 253200), and lysosomal acid lipase deficiency (LAL-D) (OMIM catalogue number 278000). There are currently ten FDA-approved ERT products (Table 1) and additional ERT products are in development or in clinical trials OMIM 2016.
Table 1.
FDA-approved enzyme replacement therapies currently available for lysosomal diseases
| Lysosomal disease | Enzyme Replacement Therapy (ERT) | FDA-approved dosing | Dosing frequency | |
|---|---|---|---|---|
| Generic (trade) names | Manufacturer (IBD)a | |||
| Fabry disease | Agalsidase beta (Fabrazyme® (2003)) EC 3.2.1.22 | Genzyme (1994) | 1 mg/kg | Biweekly |
| Gaucher disease type I | Imiglucerase (Cerezyme® (2014)) EC 3.2.1.45 | Genzyme (1994) | 2.5–60 units | Three times weekly–biweekly |
| Velaglucerase alfa (VPRIV® (2010)) EC 3.2.1.45 | Shire (2010) | 60 units/kg | Biweekly | |
| Taliglucerase (Elelyso® (2012)) EC 3.2.1.45 | Pfizer (2012) | 60 units/kg | Biweekly | |
| Pompe disease – infantile and late onset | Alglucosidase alfa (Lumizyme®) EC 3.2.1.3 | Genzyme (2010) | 20 mg/kg | Biweekly |
| MPS I | Laronidase (Aldurazyme® (2002)) EC 3.2.1.76 | BioMarin (2003) | 0.58 mg/kg | Weekly |
| MPS II | Idursulfase (Elaprase®) EC 3.1.6.13 | BioMarin (2006) | 0.5 mg/kg | Weekly |
| MPS IV type A | Elosulfase alfa (Vimizim® (2013)) EC 3.1.6.4 | BioMarin (2014) | 2 mg/kg | Weekly |
| MPS VI | Galsulfase (Naglazyme® (2013)) EC 3.1.6.12 | BioMarin (2005) | 1 mg/kg | Weekly |
| Lysosomal acid lipase deficiency (LAL-D) | Sebelipase alpha (Kanuma™ (2015)) EC 3.1.1.13 | Alexion (2015) | 1 mg/kg | Weekly |
aIBD: International Birth Date
Note: Biweekly denotes ERT infusions once every 2 weeks (14 days)
The most common adverse reactions associated with ERT are infusion reactions (IR). IR symptoms are most frequently defined as those of an allergic or type I hypersensitivity reaction, occurring during or shortly after the infusion administration. Commonly reported IR symptoms include flushing, hives, fever, chills, hypertension/hypotension, arrhythmias, rigors, dyspnea, bronchospasm, and wheezing. These symptoms are usually readily ameliorated by reducing the rate of the infusion or temporarily discontinuing the infusion, administration of antipyretics (e.g., acetaminophen), and/or anti-inflammatory agents (e.g., ibuprofen), and/or a steroid (e.g., hydrocortisone), and/or antihistamines (e.g., diphenhydramine).
Delayed infusion reactions (DIR) to ERT were first described in 2008 (Utz et al. 2008). An 8-week study of 23 patients receiving ERT noted two distinct types of IR: (1) concurrent infusion reactions (CIR), occurring during or within 4 h after completion of ERT infusion with symptoms corresponding to those described in the product package inserts (PI), resembling a type I hypersensitivity reaction, (2) DIR, with a late onset and symptoms that begin within 4–24 h after completion of the infusion and continuing for a duration of 24–72 h. DIR often presented as a combination of flu-like symptoms that included fatigue/lethargy, body aches (e.g., leg pain), headache, abdominal pain, nausea, vomiting, and diarrhea.
A number of case reports and articles have described infusion reactions to ERT and its management (Miebach 2009; Nicholls et al. 2012; Kim et al. 2008). Some studies have focused on the mechanism and physiology of CIR (Haller et al. 2016). Studies to identify the incidence, associated symptoms, and management of DIR have not been conducted. Of the ten ERT products that are FDA approved for use, only the PI for alglucosidase alfa (Lumizyme® 2014) and idursulfase (Elaprase® 2014) mention the possibility of an infusion reaction occurring after completion of the infusion.
Hypothesis
This study hypothesized that CIR are not the only drug reactions associated with ERT, and that DIR may be associated with all ERT drugs.
Methods
This study was conducted under University of Minnesota Institutional Review Board approval.
Retrospective and prospective chart reviews were performed at the Advanced Therapies Clinic of the University of Minnesota Medical Center (UMMC 2016) to identify patients with LD treated with ERT between 2008 and 2015. All sexes and age groups were included in this study. Patients must have received ERT infusions for at least 2 consecutive years. This allowed reasonable comparison of IR incidence in the study population to that reported in product package inserts, as PI data was from clinical trials taking place over 1–2 years.
Patients were counseled about CIR and DIR prior to starting treatment with ERT. Any CIR was documented by the infusion nurse during ERT administration, and DIR were reported by the patient or caregiver voluntarily, or were discovered when they were questioned about DIR during pharmacotherapy consultation, followed by documentation in the patient electronic medical record (EMR).
IR to ERT were evaluated according to symptom presentation, time of onset, and duration. A subanalysis was performed to evaluate the association of sex, age, and the presence or absence of antidrug antibodies (ADA) with IR. ADA were measured through processes provided by individual ERT manufacturers for determining and interpreting presence or absence of ADA. The percentage of patients who had experienced any type of IR to a given ERT were compared to that reported in the corresponding PI. Preventative and treatment interventions employed for the management of IR in the study population were also noted. When EMR did not include specific information required for this study (i.e., history of presence or absence of IR symptoms), the patients and/or their representatives were contacted for that information. Subject characteristics were summarized with mean and standard deviation for continuous variables, and frequencies and percentages for categorical variables. Differences between groups in proportions and corresponding confidence intervals and P-values were based on a two-sided chi-squared test. Confidence intervals and two-sided P-values for individual proportions were based on the exact binomial distribution. All analyses were performed using R v3.1.1 (R Core Team 2014).
Results
Patients
Between 2008 and 2015, 46 patients with an LD were identified who had received ERT for a minimum of 2 years: 27 (59%) male and 33 (73%) adult patients. The mean age was 40.2 ± 20.5 years (range: 9–85 years). The patient demographics based on sex, age, and LD are presented in Table 2.
Table 2.
Demographics of subjects and their lysosomal diseases
| Total patients (N = 46) | Fabry disease (n = 17) | Gaucher disease (n = 7) | Pompe disease (n = 9) | MPS I (n = 4) | MPS II (n = 4) | MPS IV (n = 1) | MPS VI (n = 3) | LAL-D (n = 1) |
|---|---|---|---|---|---|---|---|---|
|
Sex
Male (n = 27) Female (n = 19) |
11 (64.7%) | 3 (43%) | 5 (56%) | 1 (25%) | 4 (100%) | 0 (0%) | 2 (67%) | 1 (100%) |
| 6 (35.3%) | 4 (57%) | 4 (44%) | 3 (75%) | 0 (0%) | 1 (100%) | 1 (33%) | 0 (0%) | |
|
Age
Adult (n = 33) (≥18 years old) Pediatric (n = 13) (<18 years old) |
14 (82.4%) | 7 (100%) | 8 (89%) | 0 (0%) | 1 (25%) | 1 (100%) | 1 (33%) | 1 (100%) |
| 3 (17.6%) | 0 (0%) | 1 (11%) | 4 (100%) | 3 (75%) | 0 (0%) | 2 (67%) | 0 (0%) |
Infusion Reaction Analyses
Of the 46 patients evaluated, 25 patients (54.3%) reported at least one IR to ERT. Of these 25 patients, 16 patients (34.8%) experienced a DIR at least once, 16 patients (34.8%) experienced a CIR at least once, and 7 patients (15.2%) experienced a CIR and DIR at least once during their history of ERT. Three patients (6.5%) reported having, at one or more times, CIR during the infusion administration followed by a DIR occurring after completion of the very same infusion.
The percentage of patients experiencing specific symptoms of CIR and DIR are shown in Table 3. The most common CIR symptom was dyspnea/shortness of breath (69%), while the most common DIR symptom was fatigue/lethargy (88%). DIR symptoms such as fatigue/lethargy were distinguished from the sedative side effects of antihistamine and antiemetic premedications since only 3 (22%) out of 14 patients who experienced fatigue/lethargy had received sedative antihistamines, and no patients were premedicated with sedating antiemetics prior to ERT infusions.
Table 3.
Frequency of infusion-associated symptoms in CIR (during infusion) and DIR (post-infusion)
| Infusion reaction symptoms | Subjects with symptoms during infusion (n = 16) | Subjects with symptoms post-infusion (n = 16) |
|---|---|---|
| Chills | 1 (6%) | 0 |
| Fever/Pyrexia | 0 | 0 |
| Rigors | 0 | 0 |
| Dyspnea/Shortness of breath | 11 (69%) | 0 |
| Chest pain/Tightness | 4 (25%) | 0 |
| Throat tightness/Bronchospasm/Wheezing | 1 (6%) | 0 |
| Dizziness/Lightheadedness | 3 (19%) | 0 |
| Blood pressure changes (hypertension or hypotension) | 1 (6%) | 0 |
| Arrhythmias (tachycardia or bradycardia) | 0 | 0 |
| Flushing | 0 | 0 |
| Rash | 1 (6%) | 0 |
| Urticaria/Hives | 0 | 0 |
| Pruritus/Itching | 0 | 0 |
| Nasal congestion | 0 | 0 |
| Edema (peripheral, facial, etc.) | 0 | 0 |
| Somnolence/Drowsiness/Sleepiness | 0 | 0 |
| Headache | 0 | 2 (13%) |
| Gastrointestinal side effects (nausea, vomiting, diarrhea, abdominal pain) | 0 | 4 (25%) |
| Paresthesia | 0 | 0 |
| Pain in extremities (leg pain) | 0 | 1 (6%) |
| Back pain | 0 | 0 |
| Myalgia | 0 | 0 |
| Fatigue/Lethargy | 0 | 14 (88%) |
| Flu-like body ache | 0 | 2 (13%) |
CIR concurrent infusion reaction
DIR delayed infusion reaction
Subanalysis of the percentage of patients experiencing CIR and DIR for sexes and age groups showed that in patients with Fabry disease, DIR was higher in females compared to males (67% versus 36%, P = 0.492). Statistical subanalyses of other groups were not feasible due to small sample sizes.
When data were available, the presence (positive) or absence (negative) of ADA was documented for patients in each LD group. The ADA measured by the manufacturers were IgG. No IgE levels were reported. It was found that ADA values were not consistently available for patients at times that corresponded with infusion reactions. Furthermore, some ERT (i.e., taliglucerase and elosulfase alfa) do not have ADA measurement services provided by the manufacturers. Due to these limitations and the small sample sizes, calculation of odds ratios and confidence intervals for ADA was not possible.
The percentage of patients who experienced IR was compared to that reported by the PI for the ERT they were receiving (Table 4). The incidence of any type of IR was found to be highest among patients with Fabry disease (71%, P = 0.152), followed by Pompe disease (44%, P = 0.001) and Gaucher disease (33%, P = 0.623 and 29%, P = 0.098 for velaglucerase alfa and imiglucerase, respectively). The small number of patients with MPS disorders (I, II, IV A, and VI) and LAL-D prevented reporting a meaningful IR incidence, precluding formal comparisons to their respective PI.
Table 4.
Comparison of the incidence of infusion reactions observed in the study population and FDA-approved package inserts
| Lysosomal disease | ERT generic (trade) names | Infusion reactions in FDA-approved PI | CIR (95% CI) | DIR (95% CI) | IR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Fabry disease | Agalsidase beta (Fabrazyme® (2003)) | 50–55% | 41% (18%, 67%) | 47% (23%, 72%) | 71% (44%, 90%) | 0.152 |
| Gaucher disease type I | Imiglucerase (Cerezyme® (2014)) | 6.6–9% | 29% (4%, 71%) | 29% (4%, 71%) | 29% (4%, 71%) | 0.098 |
| Velaglucerase alfa (VPRIV® (2010)) | 22.5% C | 33% (4%, 78%) | 33% (4%, 78%) | 33% (4%, 78%) C | 0.623 | |
| 51.9% N | – | – | – | – | ||
| Taliglucerase (Elelyso® (2012)) | 44–46% | 0 | 0 | 0 | – | |
| Pompe disease – infantile and late onset | Alglucosidase alfa (Lumizyme® (2014))) | ≥3% | 22% (3%, 60%) | 33% (7%, 70%) | 44% (14%, 79%) | 0.001 |
| MPS I | Laronidase (Aldurazyme® (2002)) | 32–49% | 50% | 25% | 75% | – |
| MPS II | Idursulfase (Elaprase®) | 68.8% | 25% | 25% | 50% | – |
| MPS IV type A | Elosulfase alfa (Vimizim® (2013)) | 71.2% | 0% | 0% | 0% | – |
| MPS VI | Galsulfase (Naglazyme® (2013)) | 56–70% | 67% | 33% | 67% | – |
| Lysosomal acid lipase deficiency (LAL-D) | Sebelipase alpha (Kanuma™ (2015)) | 20% | 0% | 0% | 0% | – |
CIR concurrent infusion reactions in study population
DIR delayed infusion reactions in study population
IR infusion reactions (CIR or DIR) in study population
PI package insert
C patients who switched from Cerezyme to VPRIV, N Naïve patients who initially received VPRIV as ERT
A adults, P pediatrics
Dashes (–) insufficient data available
Discussion
This is the first study to characterize DIR to ERT and compare infusion reaction occurrence to that reported in the FDA-approved package inserts. The symptom profile of CIR was clearly distinguished from DIR in this study population. Dyspnea, chest tightness, and dizziness were the most common symptoms noted in patients with CIR, while fatigue, gastrointestinal side effects (nausea, vomiting, and diarrhea), flu-like body aches, and pain in the extremities were the most common symptoms reported by patients with DIR.
Alglucosidase alfa (2010) is the only ERT product whose PI mentions the possibility of a “delayed-onset reaction”; however, the PI does not clarify when the symptoms of “delayed-onset reaction” occur in relation to infusion administration (Lumizyme® 2014). Moreover, the reported incidence of IR in the PI is stated as “≥3%,” leaving questions about the actual overall IR risk for patients. The percentage of patients with Pompe disease at the University of Minnesota who experienced any IR to alglucosidase alfa was higher than that reported by the PI, and the difference was statistically significant (44% versus 3%, P = 0.001). The PI for idursulfase described a “late-emergent or biphasic anaphylactoid/anaphylactic reaction” (Elaprase® 2014). It is important to note that the biphasic reaction is distinguished from the DIR observed in the University of Minnesota study population. Specifically, the “biphasic reaction” to idursulfase had CIR symptoms occurring during the infusion, followed by an identical set of CIR symptoms recurring 24 h later.
The overall percentage of Fabry disease patients in this study who had ever experienced any type of IR to agalsidase beta was greater than that reported in the PI (71% versus 50–55%, P = 0.152). Although the study population was small, a similar trend was seen among patients with Gaucher disease using imiglucerase (29% versus 6.6%, P = 0.098), as well as those who switched to velaglucerase alfa (33% versus 22.5%, P = 0.623). Even though these differences were not statistically significant, one might question if the discrepancies were due to underreporting of DIR in the clinical trials.
The subanalysis demonstrated higher incidence of DIR among females compared to males in patients with Fabry disease (67% versus 36%, P = 0.492). This difference is not statistically significant, but the trend is notable (DIR was observed almost twice as frequently in female patients compared to males).
A clear association was not observed between the presence of ADA and the occurrence of IR in this study, consistent with information reported by most PI. Unfortunately, the sample size and limited available laboratory data prevented a more comprehensive analysis of ADA. Additionally, the incidence of ADA positivity may be influenced by a number of factors related to assay methodology, sample handling, timing of sample collection relative to dosing, concomitant medications, and underlying disease, and due to this, direct comparisons between different ERT are not recommended (Kishnani et al. 2016).
Measures taken to prevent and treat IR to ERT at the University of Minnesota are presented in Fig. 1. Strategies to prevent CIR included slowing the infusion rate, and administration of premedications that include oral acetaminophen, intravenous (IV) diphenhydramine antihistamine and IV corticosteroid. If a DIR was persistent and causing a significant impact on quality of life (e.g., patient missing days at work or school for 2–3 days after each ERT infusion due to DIR), the use of a longer-acting steroid premedication (e.g., IV methylprednisolone) was often effective in preventing DIR. Whenever possible, premedications were gradually tapered off or reduced to the minimum effective dose with which IR could be prevented.
Fig. 1.
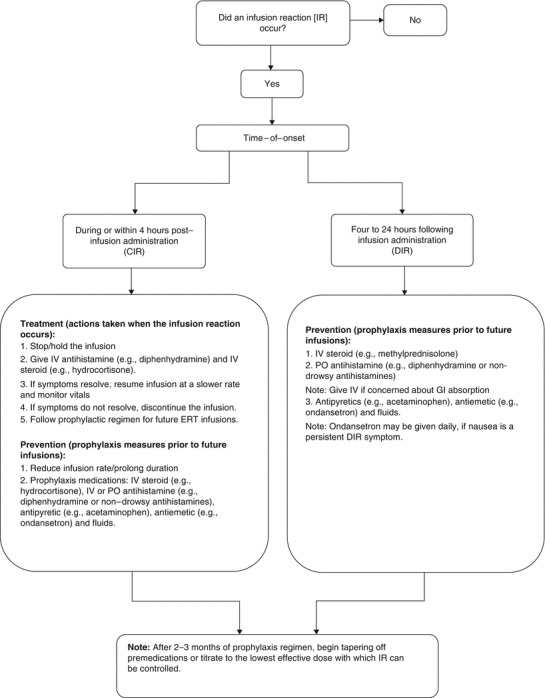
Prevention and treatment strategies for CIR and DIR (guidelines from University of Minnesota Medical Center (UMMC 2016) Advanced Therapies Program). CIR Concurrent infusion reactions, DIR Delayed infusion reactions
Of the 25 patients who experienced any type of IR in this study, 17 patients received prophylactic therapy to prevent IR with subsequent infusions: eight with history of CIR, three with history of DIR, and five with history of both CIR and DIR. Prophylactic measures were successful in preventing all CIR from reoccurring. In contrast, successful prevention of DIR was achieved in only 50% of the patients. Patients who did not receive prophylactic therapy included six patients with DIR symptoms consisting of mild-moderate fatigue for 24 h after completion of infusion, who chose not to receive prophylactic therapy. One patient was lost to follow-up due to relocation to a different state.
It is notable that two patients chose to discontinue ERT due to infusion reactions. One of these patients experienced both CIR and DIR, and had partial improvement in the severity and duration of symptoms following prophylactic management. Despite some improvement, the patient chose to discontinue ERT due to the impact of IR on their quality of life. The other patient experienced a CIR and opted for discontinuing ERT without trying premedications. These two cases, in particular, underscore the importance of proper identification and management of infusion reactions to improve patient adherence to therapy.
Limitations of This Study
An important limitation of this study is the sample size of certain LD groups, which prevented statistical analyses of IR incidence by sex, age, ADA response, as well as the comparison of IR incidence to that of their respective PI. Future studies that include larger patient sample sizes and ADA values (e.g., IgG, IgE, and neutralizing ADA) ordered consistently and in relation to infusion reactions would improve analysis and understanding of infusion reactions (CIR and DIR) to ERT.
Conclusions
The recognition and management of infusion reactions to ERT are critical to ensure patient safety during and after ERT administration. In this study, a distinct form of IR to ERT was observed, which can be distinguished by symptoms, time of onset, and duration. Pharmacovigilance and adverse event reporting systems for ERT have previously characterized IR as occurring during the infusion administration, but have rarely captured information on reactions occurring after completion of the infusion. Inadequate management of IR has resulted in patients discontinuing ERT, and this has occurred in a setting in which ERT has been the only treatment option for debilitating, and often catastrophic diseases. Recognizing DIR will lead to safer and more effective administration of ERT, while enhancing the quality of life for patients who are receiving potentially lifelong therapy with ERT.
Acknowledgements
This research was partially funded by the Lysosomal Disease Network. The Lysosomal Disease Network (U54NS065768) is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Zahra Karimian conducted this study during a fellowship training program, the Pharmacotherapy of Inherited Metabolic Diseases Post-Doctoral PharmD Fellowship (PIMD) which received funding from an unrestricted educational grant by Genzyme-Sanofi. The authors would like to thank Dr. Barry Finzel, Dr. Tim Stratton, Dr. Li Ou, and Ms. Evelyn Redtree for their thoughtful comments, insight, and assistance in reviewing and editing this manuscript. Last but not least, the authors are grateful to the reviewers at the Journal of Inherited Metabolic Disease (JIMD) for their insightful remarks.
One Sentence Synopsis of Article
Although not reported in most product package inserts, delayed infusion reactions to enzyme replacement therapy must be recognized, and their successful prevention and treatment require distinction from concurrent infusion reactions.
Contributions of Individual Authors
Dr. Jeanine R. Utz is the Principal Investigator and Director of the Pharmacotherapy of Inherited Metabolic Diseases (PIMD) fellowship program. She provides ongoing clinical care for the patients in the study. Dr. Utz conceived, designed, and conducted the study, orchestrated funding, coordinated efforts of collaborators, and participated in the interpretation of data. She is the guarantor for this article, accepts full responsibility for the work and the conduct of this study, and provided final approval of the version to publish.
Dr. Chester B. Whitley is the Co-Investigator for this study, Director of the Advanced Therapies Program and Gene Therapy Center. He provides continuous medical care for the patients in the study, has made significant contributions to formulating the hypothesis and study design, and offered critical revisions of the article.
Dr. Kyle D. Rudser is a senior biostatistician at the Clinical and Translational Science Institute. He offered statistical expertise in the study design, data monitoring, and analyses for this project, conducted the statistical analysis and interpretation of the results, and wrote relevant portions of the paper.
Dr. Zahra Karimian is a postdoctoral research associate and fellow in the PIMD program. Contributed substantially to the project through acquisition, reporting, analysis, and interpretation of the data, and wrote major portions of the paper.
All authors are responsible for the validity of the entire work and manuscript submitted for publication.
Funding and Conflict of Interest
Dr. Jeanine Utz is the recipient of an unrestricted educational grant from Genzyme-Sanofi which helps fund the PIMD fellowship in addition to the NIH LDN grant (U54NS065768). She provides consultation for the scientific content of the annual WORLD Symposium meeting and is on the Speakers bureau for Genzyme, Shire, and Pfizer.
Dr. Chester Whitley is the Principal Investigator for the NIH Lysosomal Disease Network (LDN) grant (U54NS065768). He provides consultation for gene therapies being developed by Sangamo and the scientific content of the annual WORLD Symposium meeting.
Dr. Kyle D. Rudser is a recipient of the NCATS award (UL1TR000114) and the NIH LDN grant (U54NS065768).
Dr. Zahra Karimian is a postdoctoral research fellow in the Pharmacotherapy of Inherited Metabolic Diseases (PIMD) program, which is supported by an unrestricted educational grant from Genzyme-Sanofi in addition to the NIH LDN grant (U54NS065768).
The contents of this article have not been influenced by the financial resources disclosed above and the authors declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This study was conducted under University of Minnesota Institutional Review Board (IRB) approval. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in this study. Patients were made aware that participation in the study was voluntary, that they had the right to withdraw from the study at any time, and would continue to have access to unrestricted health care despite withdrawal.
Contributor Information
Zahra Karimian, Email: karimian@umn.edu.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Aldurazyme® [package insert] (2002) BioMarin, San Rafael, CA. Downloadable full U.S. prescribing information is available at: https://www.genzyme.com/Products/Product-Information.aspx (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Cerezyme® [package insert] (2014) Genzyme, Cambridge, MA. Downloadable full U.S. prescribing information is available at: https://www.genzyme.com/Products/Product-Information.aspx (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Elaprase® [package insert] (2014) BioMarin, San Rafael, CA. Downloadable full U.S. prescribing information is available at: http://elaprase.com/ (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Elelyso® [package insert] (2012) Pfizer, New York, NY. Downloadable full U.S. prescribing information is available at: http://www.elelyso.com/ (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Fabrazyme® [package insert] (2003) Genzyme, Cambridge, MA. Downloadable full U.S. prescribing information is available at: https://www.genzyme.com/Products/Product-Information.aspx (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Haller C, Agarwal S, Kakkis E. A novel approach to characterization and categorization of infusion reactions associated with ERT using adverse physiology related groups. Mol Genet Metab. 2016;117(2):S54. doi: 10.1016/j.ymgme.2015.12.282. [DOI] [Google Scholar]
- Kanuma™ [package insert] (2015) BioMarin, San Rafael, CA. Downloadable full U.S. prescribing information is available at: http://www.kanuma.com/ (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Kim KH, Decker C, Burton BK. Successful management of difficult infusion-associated reactions in a young patient with mucopolysaccharidosis type VI receiving recombinant human arylsulfatase B (galsulfase [Naglazyme]) Pediatrics. 2008;121(3):e714–e717. doi: 10.1542/peds.2007-0665. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Dickson PI, Muldowney L, et al. Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction. Mol Genet Metab. 2016;117(2):66–83. doi: 10.1016/j.ymgme.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Lumizyme® [package insert] (2014) Genzyme, Cambridge, MA. Downloadable full U.S. prescribing information is available at: https://www.genzyme.com/Products/Product-Information.aspx (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Miebach E. Management of infusion-related reactions to enzyme replacement therapy in a cohort of patients with mucopolysaccharidosis disorders. Int J Clin Pharmacol Ther. 2009;47(Suppl 1):S100–S106. doi: 10.5414/cpp47100. [DOI] [PubMed] [Google Scholar]
- Naglazyme® [package insert] (2013) BioMarin, San Rafael, CA. Downloadable full U.S. prescribing information is available at: http://www.naglazyme.com/#indication_read (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- Nicholls K, Bleasel K, Becker G. Severe infusion reactions to Fabry enzyme replacement therapy: rechallenge after tracheostomy. JIMD Rep. 2012;5:109–112. doi: 10.1007/8904_2011_106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man (OMIM) (2016). http://www.omim.org/ (Retrieved from 4-20-2016)
- R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (Retrieved from January 2016)
- University of Minnesota Medical Center (UMMC) Advanced Therapies Clinic (2016) Protocol for the management of infusion reactions to ERT in lysosomal diseases: treatment and prevention strategies. Accessed Jan 2016
- Utz JR, Doyen C, Whitley CB. Delayed infusion reactions to enzyme replacement therapy. Mol Genet Metab. 2008;93:S40. doi: 10.1016/j.ymgme.2007.10.113. [DOI] [Google Scholar]
- Vimizim® [package insert] (2013) BioMarin, San Rafael, CA. Downloadable full U.S. prescribing information is available at: http://www.vimizim.com/ (Retrieved from 4-21-2016). Accessed 14 Sept 2015
- VPRIV® [package insert] (2010) Shire, Lexington, MA. Downloadable full U.S. prescribing information is available at: http://www.vpriv.com/general/important-safety-info.php#forhcps (Retrieved from 4-21-2016). Accessed 14 Sept 2015


