Abstract
The present review focuses on the cultivation of algal biomass for generating value-added products (VAP) and to assess their economic benefits and harmful environmental impact. Additionally, the impact of bioreactor designs on the yield of microalgal biomass for VAP is also considered. All these factors are discussed in relation to the impact of microalgae production on the bio-economy sector of commercial biotechnology.
Keywords: Value-added products (VAPs), Microalgae, Enviro-economical assessment, Photobioreactor
Introduction
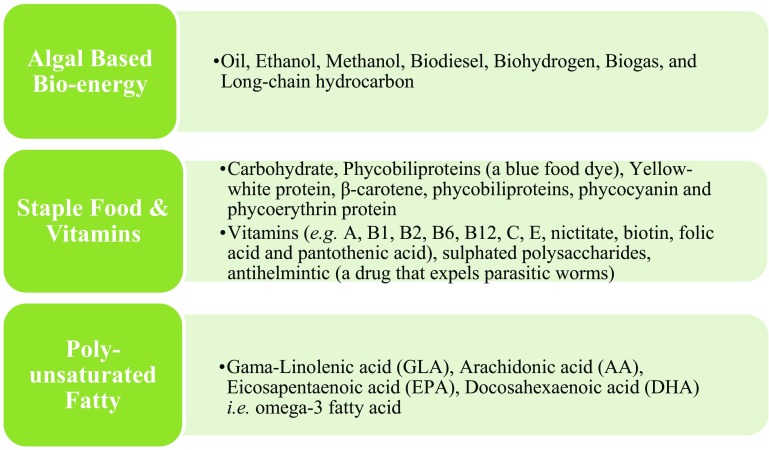
Microalgae, characterized by production of significant amounts of biomass and oil content can be used as feedstock for biodiesel production and has been proposed as a potential source of renewal energy. Additionally, residual microalgal biomass can also be utilized to generate biohydrogen using anaerobic digestion, biogas, bio-ethanol, bio-methanol, bio-plastics, bio-fertilizer, medicinal value products, and animal food (Tong et al. 2014; Gebreslassie et al. 2013; Gallezot 2012). However, the most common fuel generated from microalgae is biodiesel which is produced by transesterification of algal lipid (Zhu 2015; Huang et al. 2014; Gonçalves et al. 2013; Zhu and Ketola 2012). The potential benefit of microalgae for biodiesel production is relatively high in compared to crop plants as its’ growth requires less land space and can also be easily grown in wastewater (Bhatt et al. 2014; Chisti 2012; Pittman et al. 2011; Wijffels 2008; Brennan and Owende 2010). Previously published works have shown the benefits as well as weakness for the production of microalgal-based VAP, especially in the extraction and purification of VAPs. The improvement of these extraction and purification techniques to produce VAP at the commercial level has not yet been realized due to lack of research, high costs and unavailability of necessary facilities (Oswald et al. 1988). A variety of by-products along with biofuel is being produced in pilot scale by microalgal biomass. To increase biodiesel production, two reactor systems, namely, the open pond system and the close type photobioreactor, have been used to generate a large amount of microalgal biomass (Richardson et al. 2012). Designing and fabrication of a bioreactor (BR) is very important, and BR must be designed with different process options, and in accordance with the desired products such as medicinal, cosmetics, fertilizer, biofuel, bio-plastics, food supplements, etc. (Kumar et al. 2015; Richardson et al. 2012; Dasgupta et al. 2010). Though microalgal-based biofuel generation has advantageous over fossil fuel, it is also important to evaluate its economic feasibility and its environmental impacts prior to mass scale cultivation. Figure 1 shows the range of applications of microalgal biomass which includes biofuels as well as different types of high-value-added products (VAP).
Fig. 1.
Different possible applications of algal biomass
Greater significance should be given to the adaptation of eco friendly and low-cost approaches for the production of VAP. The research gaps present between the processing units (cultivation, harvesting, extraction, marketing, etc.) should act as a bridge to magnify the microalgal-based products at a pilot scale. The main benefit of microalgae-based VAP, over plants, lies in their metabolic flexibility which will allow the possibility of modification to their biochemical pathways. Microalgal-based VAP has an enormous market value and potential to reduce the dependency on fossil fuel as well as to produce different high-value chemical products. The objective of this article is to explore the various applications of microalgal biomass, different extraction methods of VAP, their market potential, and assess their enviro-economical impact.
Future bio-products from algae
Biofuels
The extensive use of fossil fuel for energy generation has increased CO2 levels in the atmosphere and is thought to be potential contributors to global warming. To combat global warming, alternative sources for energy production have been investigated, of which microalgae-based biofuel/bio-power production (3rd generation fuel) is being considered as one of the viable option (Chisti 2012). Photosynthetic efficiency of microalgae as well as the rate of microalgal-based bio-oil production is many folds higher over plants (Richardson et al. 2012) (Table 1). There are several high energy fuel types (biodiesel, biohydrogen, bio-ethanol, bio-oil, biogas) produced by microalgae. Usher et al. (2014) have reported that microalgal-based biohydrogen has higher energy content compared to gasoline and petro-diesel and that there is very little difference in energy content of algal-based biodiesel and bio-oil when compared to gasoline. Hence, biomasses from different microalgal species (Table 2) have been tested for their potential to produce biofuels. Microalgal-based bioenergy options are carbon neutral or carbon balanced process, as the lag period in carbon uptake by algae and carbon released from algal biofuel is shorter than any other known biofuel feedstocks. Therefore, cumulative application of microalgae for CO2 bio-fixation as well as in bioenergy generation is highly lucrative from environmental and economic view point.
Table 1.
Different crop plants and rate of oil production (Richardson et al. 2012)
| S. no. | Crop | Oil yield (L/acre) |
|---|---|---|
| 1 | Corn | 68.13 |
| 2 | Soybean | 181.68 |
| 3 | Sunflower | 386.07 |
| 4 | Rapeseed | 480.69 |
| 5 | Canola | 495.83 |
| 6 | Jatropha | 788.33 |
| 8 | Oil palm | 2403.47 |
| 9 | Microalgae | 19,000–57,000 |
Table 2.
Biofuel derived from different algal species
| Algae | Biodiesel (%) | Biohydrogen (mmol/L/h) | Bio-ethanol (%) | Bio-oil (%DW) | Biogas (m3/m3 days) | References |
|---|---|---|---|---|---|---|
| Cladophora fracta | 14.2–0.8 | Demirbas (2008) | ||||
| Chlorella protothecoides | 29.4–1.5 | Demirbas (2008) | ||||
| Botryococcus braunii | 17.85 | Órpez et al. (2009) | ||||
| Sydney et al. (2011) | ||||||
| Chlorella protothecoides | 55.2 | Xu et al. (2006) | ||||
| Gloeocapsa alpicola | 1.6 | Troshina et al. (2002) | ||||
| Spirulina platensis | 0.18 | Aoyama et al. (1997) | ||||
| Chlamydomonas reinhardtii | 0.13 | Gfeller and Gibbs (1984) | ||||
| Chlamydomona s reinhardtii cc124 | 0.094 | Kosourov et al. (2002) | ||||
| Platymonas subcordiformis | 0.002 | Guan et al. (2004) | ||||
| Chlamydomona s reinhardtii cc1036 | 0.48 | Laurinavichene et al. (2006) | ||||
| Palmaria | 38–74 | Ross et al. (2008) | ||||
| Porphyra | 40–76 | Jensen (1993) | ||||
| Ascophyllum | 42–70 | Becker (1994) | ||||
| Ulva lactuca | 55–60 | Inan (2014) | ||||
| Tetraselmis sp. CS-362 | 26.0 | Brown et al. (1998) | ||||
| Chlorococum sp. | 32.5 | Ike et al. (1997) | ||||
| Chlamydomonas reinhardtii UTEX 90 | 60.0 | Hirano et al. (1997) | ||||
| Botryococcus braunii | 29–75 | http://www.oilgae.com/algae/oil/yield/yield.html | ||||
| Hantzschia DI-160 | 66 | |||||
| Scenedesmus TR-84 | 45 | |||||
| Neochloris oleoabundans | 35–54 | |||||
| Schizochytrium | 50–77 | |||||
| Phaeodactylum tricornutum | 20–30 | Chisti (2007) | ||||
| Schizochytrium sp. | 50–77 | |||||
| Nitzschia sp. | 45–47 | |||||
| Chlorella sp. | 28–32 | |||||
| Botryococcus braunii | 25–75 | |||||
| Chlamydomonas reinhardtii | 587 ± 9 | Mussgnug et al. (2010) | ||||
| Chlorella kessleri | 335 ± 8 | Mussgnug et al. (2010) | ||||
| Spirogyra neglecta | 0.23 | Baltrėnas and Misevičius (2015) |
Value-added products (VAP)
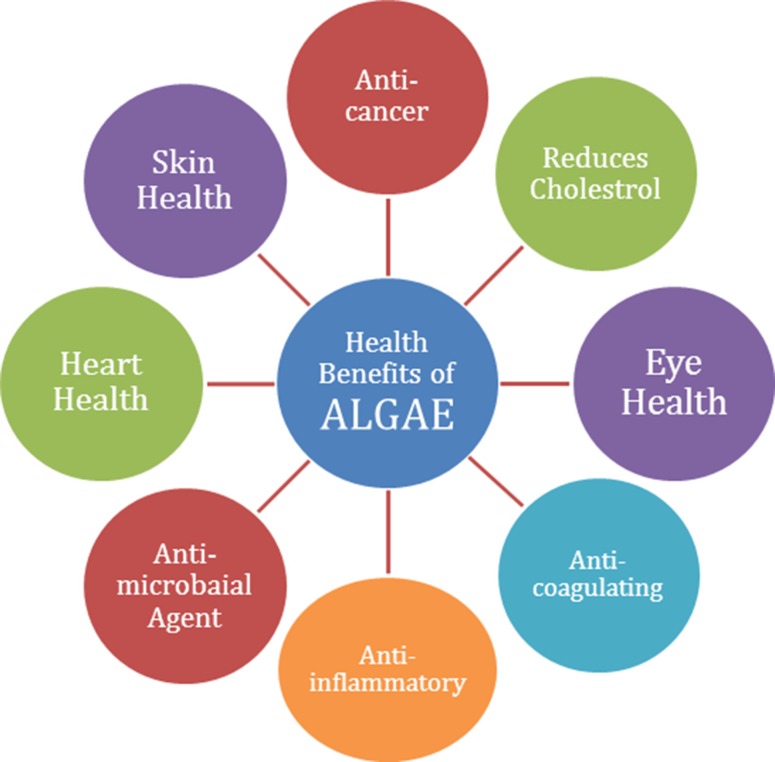
Microalgal species are potential source of various products associated with health sector such as anti-cancerous product, reduces cholesterol, improves skin health and anti-microbial agents, etc. Its use is not only restricted to medicine; it is being used in cosmetics to protect skin from sunburn and other skin beauty products. Microalgae are rich source of biologically active metabolites, which is widely used in pharmaceutics, and have nutritional importance (Zhang et al. 2014; Sydney et al. 2010). Figure 2 illustrates the different benefits of microalgae-based products to cure diseases.
Fig. 2.
Multiple health benefits of algal biomass
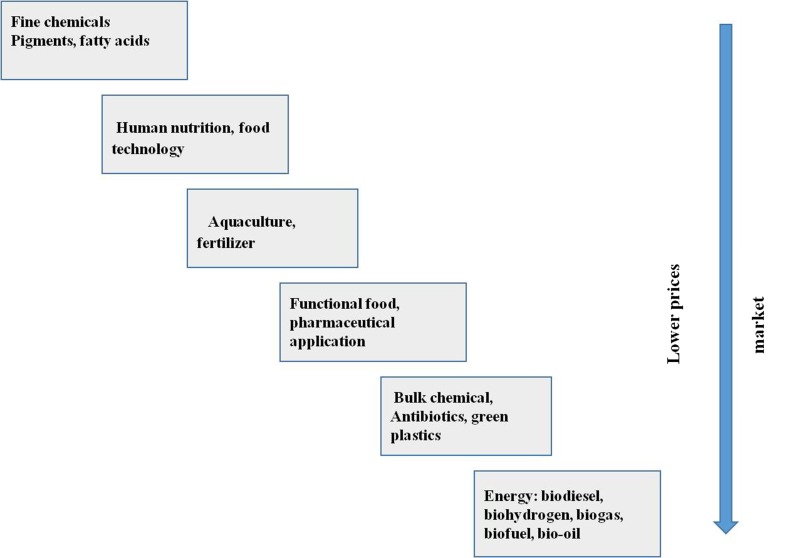
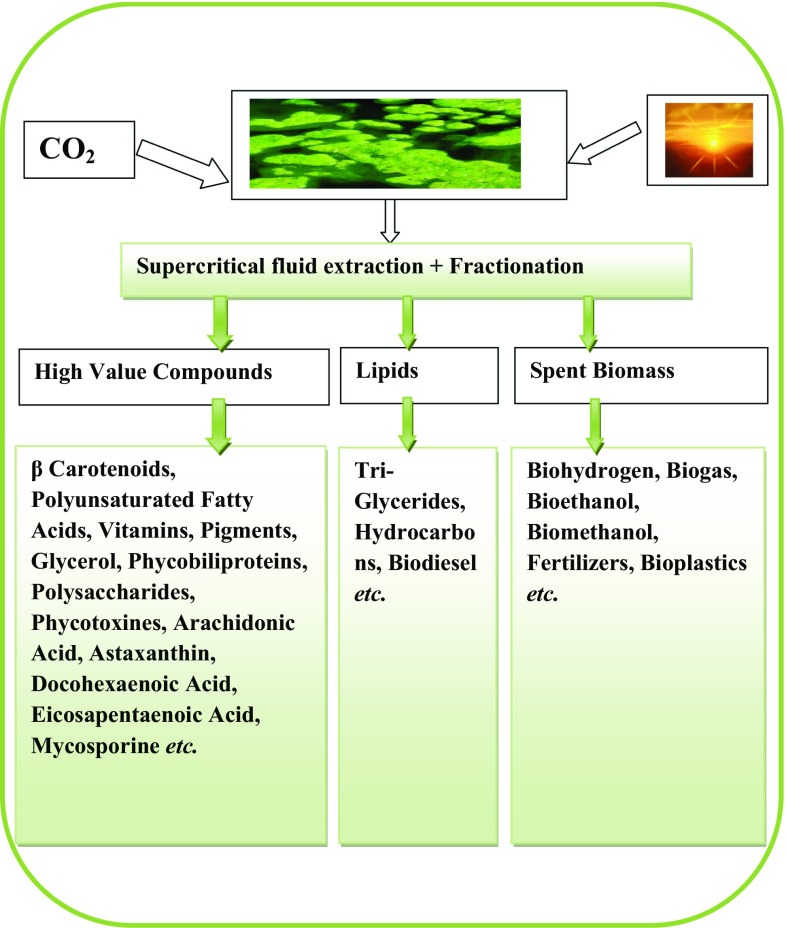
Microalgae synthesize pigments and biochemical compounds such as protein, carbohydrate, lipid and carotenoids. The biochemical composition of microalgae is used as an indirect measurement of algal cell metabolic rate. Microalgal biomass is being used as thickening agents, water-binding agents and antioxidants in the cosmetic industries such as Arthrospira and Chlorella are widely used in products related to skin care (Usher et al. 2014). VAPs such as face and skin care products, anti-aging cream, refreshing care products, emollient, anti-irritants, sunscreen, and hair care products, are widely prepared using extracts (Colla et al. 2007; Pulz and Gross 2004) of different microalgal species such as Chondrus crispus, Mastocarpus stellatus, Ascophyllum nodosum, Alaria esculenta, Spirulina platensis, Nannochloropsis oculata, Chlorella vulgaris and Dunaliella salina (Priyadarshani and Rath 2012). Microalgae produce several organic metabolites, such as sporopollenin, scytonemin and mycosporine-like amino acids to protect from UV radiation. These VAPs have wide application in different fields such as pharmaceutical, therapeutics, human nutrition, food technology, functional food, antibiotics and green plastics, etc. Figure 3, shows the multifarious applications of microalgal biomass after harvesting in different categories at major scale. Microalgae are an appropriate feedstock because they do not compete for land to produce different valuable compounds. Figure 4 illustrates the different possible VAPs produced by microalgal biomass. There is a range of high-value chemicals compounds such as pigments, antioxidants, β-carotenes, etc, which are largely being used as bulk commodities in different industrial and commercial sectors (Usher et al. 2014). These products are considered as potential alternative to replace chemical based commercialized products consumed by various industries as a raw material.
Fig. 3.
Milestones for microalgae industry
Fig. 4.
Value-added products (VAPs) from algal biomass
Some microalgal-based by-products such as lipids, α-linolenic acid, docosahexaenoic acid, astaxanthin are being extracted at commercial level due to their great importance as bioactive compounds (Usher et al. 2014). There is a wide range of extraction/purification methods which are used to extract the particular component. These extraction methods include organic solvents, breakdown, pretreatment process of encysted cells (cryogenic grinding and acid/base treatment), enzyme lysis (kitalase, cellulose, and abalone acetone powder mainly β-glucuronidase), mechanical disruption and spray drying, etc. Table 3 enlists some microalgae species with their main products, yields, and their extraction methods.
Table 3.
Extraction methods used for high-value chemicals from different microalgal species
| Species | Product | Yield (%) | Extraction/purification method | References |
|---|---|---|---|---|
| Arthrospira platensis | Lipids | 13.2 | Chloroform–methanol 1:1 (volume/volume) | Ryckebosch et al. (2011) |
| Chlorella vulgaris | α-Linolenic acid | 0.661 | Acid digestion of biomass with 4 normality of HCl | Batista et al. (2013) |
| Chlorella vulgaris | docosahexaenoic acid | 0.16 | Soxhlet method with petroleum ether for 6 h | Batista et al. (2013) |
| Crypthecodinium cohnii | docosahexaenoic acid | 99.2 | Purification by saponification, winterization and urea complexation | Mendes et al. (2007) |
| Diacronema vlkianum | α-Linolenic acid | 0.16 | Acid digestion of biomass with 4 normality of HCl | Batista et al. (2013) |
| Scenedesmus obliquus | Total lipids | 29.7 (dry weight) | Chloroform–methanol 1:1 (volume/volume) | Ryckebosch et al. (2011) |
| Spirulina maxima | α-Linolenic acid | 0.40% | Acid digestion of biomass with 4 normality of HCl | Batista et al. (2013) |
| Nannochloropsis gaditana | Eicosapentaenoic acid | 3.7 (dry weight) | Dichloromethane-ethanol (1:1) (volume/volume) | Ryckebosch et al. (2011) |
| Tetraselmis sp. | Eicosapentaenoic acid | 10.41% of phospholipids fraction | Chloroform–methanol 2:1 (volume/volume). Extract was washed with 0.88% (weight/volume) KCl to remove non-lipids | Makri et al. (2011) |
| Haematococcus pluvialis | Astaxanthin | 83 | SC-CO2 at 20 MPa, 55 °C and13% (weight/weight) ethanol for 120 min of extraction time | Reyes et al. (2014) |
| Limnothrix sp. | C-phycocyanin | 18 (dry weight) | Distilled water, activated carbon (1% weight/volume) and chitosan (0.01 g/L) for extraction. Ammonium sulfate (25%) was used for purification at 4 °C, overnight. The precipitate was re-suspended in 0.1 Mole PBS (pH 7.0), and tangential flow filtration system (30 kDa membrane pore) was used for pigment concentration | Gantar et al. (2012) |
| Phormidium sp. A27DM | Phycoerythrin | 62.6 | Freeze–thaw cycles (−30 and 4 °C) in 1 M Tris Cl buffer, two-step ammonium sulfate precipitation at 20 and 70% saturation and purification by gel permeation chromatography with a Sephadex G-150 matrix | Parmar et al. (2011) |
The current scenario of microalgal-based products is well established in the international market. The multiple applications and residue utilization at every step of processing unit have made the microalgae feasible and economically viable to enhance the market potential. It is widely used for human consumption and has highest market value (Table 4).
Table 4.
Market potential of value-added products based on algal biomass as suggested by Wijffels (2008)
| Algal biomass applications | Cost/kg biomass (€) | Market volume (€) |
|---|---|---|
| Nutraceuticals applications with human consumption | 100 | 60 million |
| Nutraceuticals applications with animal and fish feed | 5–20 | 3–4 billion |
| Produced bulk chemicals from algae | −5 | >50 billion |
| Biofuels | <0.40 | >1 trillion |
The current status of these products (microalgal-based VAPs) is not only restricted to research level, but it has expanded up to the industrial and commercial level (Table 5). The current status (commercial and lab scale) of microalgal-derived products with respect to their cost is important to determine as it indicates the market potential of the concerned product. The main drawbacks associated with microalgae products is that they produce less isotopic compounds, like phycobiliproteins and beta-carotene, which are in high demand. Microalgal applications for VAPs present a most recent development and newsworthy research trends that emphasize on scientific and technological related uses of microalgae. Therefore, economic feasible VAPs can be developed by concentrated efforts in research and development to maximize the product output at lower input cost.
Table 5.
| Products | Uses | Cost | Market value | Algal sp. | Product content (%) | Reactor | Current status |
|---|---|---|---|---|---|---|---|
| Isotopic compounds | Medicine | >$1000/kg | Small | Many | >5 | Tubular, indoors | Commercial |
| Phycobiliproteins | Research, food color | >$10,000/kg | Small | Red | 1–5 | Tubular, indoors | Commercial |
| Pharmaceutical | Antibiotics | (very high) | Unknown | Other | Tubular, fermentor | Research | |
| Beta-Carotene | Food suppl. | >$500/kg | Small | Dunaliella | 5 | Lined pond | Commercial |
| Xanthophylls | Chicken feed | $200–500/kg | Medium | Greens, Diatoms | 0.5 | Unlined pond | Research |
| Vitamins C&E | Vitamins | C: >$10/kg | Medium | Greens | <1 | Fermentor | Research |
| Health foods | Supplements | $10–20/kg | Medium to large | Chlorella, Spirulina | 100 | Lined pond | Commercial |
| Polysaccharide | Viscosities gums | $5–10/kg | Medium to large | Porphyridium, others | 50 | Lined pond | Research |
| Bivalves feeds | Seed raising | $20–100/kg | Small | Diatoms | 100 | Lined pond | Commercial Research |
| Soil inoculum | Fertilizers | >$100/kg | Unknown | Chlamydomonas N-fixing species | 100 | Indoor lined pond | Commercial Research |
| Amino acids | Proline | $5–50/kg | Small | Chlorella | 10 | Lined pond | Research |
| Single-cell protein | Animal feeds | $0.3–0.5/kg | Very large | Green algae, others | 100 | Unlined pond | Research |
| Vegetable oils | Food, feed | $0.4–0.6/kg | Very large | Greens | 30 | Unlined | Research |
Cultivating systems
Photobioreactor (PBR) system is mainly categorized into open-air pond system and closed system. A number of researches have explored the design specification and economic evaluation of these PBRs for lab as well as commercial scale algal cultivation to derive various bioenergy products. Apart from the bioenergy products researchers have also evaluated the viability of cultivation systems for production of various by-products such as nutraceuticals, pharmaceutical green plastic, polymers, etc.
Open-air/pond system (OPS)
Different forms of open-air/pond systems (OPS) have been used previously, e.g., open raceway pond, shallow pond, and circular pond. In OPS, size is restricted to 10,000 m2 because the mixing of the pond system by the rotating arm is not possible in larger ponds. Open raceway pond is widely used for microalgal biomass production at commercial level (Kumar et al. 2015). These ponds are generally constructed using a closed loop and re-circulation channel with a depth ranging from 0.2 to 0.5 m. It is mixed by paddled-wheel, to provide the homogeneous distribution of nutrient in pond. OPS requires low power, hence it is economically viable and easy to maintain, easily handled, and clean (Yoo et al. 2013). Therefore, it is the cheapest method of producing a large amount of microalgal biomass at commercial scale. Therefore, many advantages are associated with OPS, as it has potential to produce the bulk of algal biomass which can be utilized for various product formation, i.e., algal biomass-based bio-power and bio-products, whereas, disadvantages associated with OPS involve contaminations, environmental variation which imparts a direct effect on culture condition, optimization of pH, temperature, light intensity, etc. (Kumar et al. 2014; Brennan and Owende 2010).
Closed photobioreactor (CPBR)
CPBR has the potential to achieve tremendous microalgal biomass over OPSs as it overcomes the shortcomings associated with it. There are three basic principles that govern the designing and fabrication of closed photobioreactor, i.e., utilization of appropriate light sources (intensity and wavelength), increasing photon conversion efficiency, and maintenance of an appropriate microalgae biomass culture condition (Dasgupta et al. 2010). The close term of PBR, i.e., free from contamination inside the culture condition provides no direct exchange of gas between ambient environment and system. Table 6 gives an idea about different close type photobioreactor and its uses in various end-product generations. Such type of PBR is highly efficient regarding biomass productivity as it is fabricated to achieve maximum solar radiation with appropriate tilt angle. CPBR is an effective technology but it is being demonstrated at lab scale, and requires its application at commercial scale. Besides, open and closed types bioreactors, dark fermentation based bioreactors are also been studied to analyze its potential for microalgal-based bio-products (Zhang et al. 2013; Rittmann and Herwig 2012). Furthermore, Table 7 clearly shows product formations from the different dark fermentation-based reactors with algal biomass.
Table 6.
Specifications for different photobioreactors
| Types of bioreactors | Specifications | References |
|---|---|---|
| Tubular airlift and bubble column bioreactor |
Working It is characterised by the presence of vertical transparent tubes using glass or polyethylene so that maximum available sunlight can be achieved and CO2 supply is allowed through bubbling. Fabrication of vertical tubular bioreactor is inexpensive Drawbacks It does not offer elevated culture volume. Due to the lack of high area and volume ratio in such bioreactor efficient gas transfer cannot take place, which intern decreases photosynthesis efficiency. An additional downside is that it is having large angle size in comparison to sunlight consequently most of the solar radiation would go back as a consequence decreases biomass |
Mortuza et al. (2011); Akhtar et al. (2007); Trujilio et al. (2007) |
| Horizontal tubular bioreactor |
Working In such type of bioreactor tilt angle is enough to harvest maximum solar radiation for algal growth and development. For large algal cultivation, it’s perfect because this is not susceptible to contamination Drawbacks It has a gas exchange unit, but the main drawback in such a system is that gas transfer rate is low because of its large horizontal tube and small diameter |
Zittelli et al. (2013); Tredici and Zittelli (1998) |
| Helical tubular bioreactor |
Working It possesses flexible tubular pipe which is having coiled framework with heat exchange and gas exchange tower. It is coiled/conical shaped structure provides a significant sunlight for algal biomass productivity Drawbacks Removal of deposited algal biomass culture in the inner wall is a tedious task |
Rogers et al. (2014) |
| Alfa shaped bioreactor |
Working Surface and volume ratio is large, and it possesses airlift agitation system. Temperature can be maintained and for gas exchange injection can be done at the vertical unit. High unidirectional flow rate Drawbacks Foam formation due to high cell density |
Dasgupta et al. (2010); Aditya and Kunjapur Eldrige (2010) |
| Flat plat bioreactor |
Working Surface to volume ratio is medium, and gas exchange generally is done through bubbling. Open gas transfer avoids oxygen build up. Temperature can be maintained through heat exchange coil Drawbacks Shear due to entrainment of cells till bubbles burst |
Pohl et al. (1988) |
Table 7.
Descriptive features for different dark fermentation bioreactors with their specific remarks
| Types of bioreactor | Specifications | References |
|---|---|---|
| Continuous stirred tank reactor (CSTR) |
Working In CSTR, microorganisms are completely mixed and suspended in the liquid substrate; wastewater may be used as feeding material. This bioreactor is generally used for continuous hydrogen production Drawbacks higher electricity uses to maintain proper stirring |
Dasgupta et al. (2010) |
| Membrane bioreactor (MBR) |
Working Best in terms of higher biomass production. Best utilization of wastewater as feedstock. Avoid chances of contamination Drawbacks Membrane fouling and the high cost of membrane bioreactor are not economically feasible for bioenergy production |
Honda et al. (2012); Pohl et al. (1988) |
| Anaerobic sequencing batch reactor (ASBR) |
Working Flexible in operation, potential capital cost savings by eliminating clarifiers and other equipment, equalization, primary clarification, biological treatment, and secondary clarification can be achieved in a single reactor vessel Drawbacks A higher level of sophistication is required especially for larger systems, of timing units and controls, Higher level of maintenance associated with more sophisticated controls, automated switches, and automated valves |
Honda et al. (2012) |
The most crucial factors, while designing a bioreactor include light penetration efficiency, agitation and flow rate, area-to-volume ratio with different shape and size of bioreactors (Gao et al. 2014). Based on the principle of high area-to-volume ratio, flat-plate, tubular and fermenter type of bioreactors are designed which is capable to provide proper mixing, flow, and high light penetration (Dasgupta et al. 2010). Table 8 portraits the imperative factors associated with open and closed bioreactors.
Table 8.
Key parameters associated with open and closed type cultivating systems
| Parameters | Open system | Closed system |
|---|---|---|
| Area-to-volume ratio | Large (4–10 times higher than closed counterpart) | Small |
| Algal species | Restricted | Flexible |
| Main criteria for species selection | Growth competition | Shear-resistance |
| Population density | Low | High |
| Harvesting efficiency | Low | High |
| Cultivation period | Limited | Extended |
| Contamination | Possible | Unlikely |
| Water loss through evaporation | Possible | Prevented |
| Light utilization efficiency | Poor/fair | Fair/excellent |
| Gas transfer | Poor | Fair |
| Temperature control | None | Excellent |
| Most costly parameters | Mixing | Oxygen control, temperature control |
| Capital investment | Small | High |
At the commercial level, an efficient photobioreactor has not been engineered to achieve higher algal biomass. Both open raceway pond and close photobioreactor are not a matured technology to provide algal-based VAPs in huge amount. Many uncertainties such as optimum cultivation scale, heating and cooling, mixing and gaseous exchange remains to be optimized while producing high-value compounds as reported by Singh and Sharma (2012). The operating capital cost associated with commercial raceway pond and PBR is very important factor to understand the market potential of algal-based bio-power and bioproducts. Various estimates have been proposed for the production costs of microalgal biomass with different PBR and open raceway ponds in US$, as depicted in Table 9.
Table 9.
Production cost of microalgae in raceway pond and PBRs
| System | Operating costs (US$/kg) | Capital costs (US$/ha) | Total costs (US$/kg) |
|---|---|---|---|
| Commercial raceway ponds | – | 100,000 | 2–15 |
| 50,000 m2 raceway pond | 7–10 | 300,000 | 8–11 |
| Raceway ponds for phyco-remediation | – | 2–4 | |
| PBR (Chlorella) | – | – | 40–60 (selling price) |
| PBR (Haematococcus) | – | – | >30 2 |
| PBR (cost analysis) | 19.4 | 12.6 (capital costs 11% per year) | 32 |
The area of microalgae-based technology for production of different valuable products is being developed rapidly to sustain the economy and ecosystem of the world. The governments in the US, EU, Brazil, China, India, and Canada are funding to facilitate and enhance the algal-based VAPs as it has multiple applications in various sectors (Usher et al. 2014; Borowitzka 2013; Adarme-Vega et al. 2012). Table 10 indicates the range of different products that comes from microalgal biomass and includes the different methods of cultivation worldwide.
Table 10.
Cultivation of microalgae to produce value-added products (VAP) worldwide
| Algal sp. | Cultivation methods | Industry/products | Location |
|---|---|---|---|
| Dunaliella | Closed PBRs | Nutraceuticals (β-carotene) | Israel |
| Dunaliella | Open (raceway) | Nutraceuticals (β-carotene) | Australia |
| Haematococcus | Open (raceway) | Nutraceuticals (astaxanthin) | Israel |
| Haematococcus/Spirulina | Open (raceway) | Nutraceuticals (astaxanthin/dietary supplement) | Hawaii |
| Haematococcus | Closed PBRs | Nutraceuticals (Astaxanthin) application | Sweden |
| Spirulina | Open (raceway) | Nutraceuticals (dietary supplement) | USA |
| Spirulina, Chlorella | Open (centre pivot ponds) | Nutraceuticals (food supplement) | Taiwan |
| Cyanobacteria | Closed PBRs | Biofuel (ethanol, diesel, jet fuel) | USA |
| Chlorella | Closed PBRs | Nutraceuticals (dietary supplement) | Germany |
Large-scale cultivation of microalgae regarding the production of nutraceutical and other protein suppliants are predominant in the world market as these products are economically reasonable due to the high-value products such as pigments and nutrients. Adarme-Vega et al. 2012) reported that over 80% green algae producing industries are located in Taiwan, with Mongolia in China along with Israel are top three producers of Dunaliella in the World. Therefore, microalgal production from algae and entrepreneurship is highly required to magnify the business of algal benefits.
Enviro-economical assessments for VAPs from algal biomass
Although many studies are available on multidimensional aspects of algal biomass to contribute in the economy at international level, but still the role of environmental sustainability with system viability at the economic ground is to refrain from this concept. This section is focused on enviro-economical assessments associated with mass production of algal biomass for generation of VAPs at commercial scale and feasibility of reactors used for the production of algal biomass.
Challenges on environmental scale
Though it is well known that microalgae have importance regarding bioenergy generation yet it has some negative aspect about environmental impacts of large-scale microalgae cultivation (Smith et al. 2010; Wijffels and Barbosa 2010). Some important ones are delineated here:
Use of nutrient and fertilizer
For mass cultivation of algal biomass, additional nutrients supply such as nitrogen, phosphorus, and potassium (some species, e.g., diatoms, also require silicon) are highly required (Handler et al. 2012; Campbell et al. 2011; Wijffels and Barbosa 2010). Therefore, use of fertilizers cannot be ignored because the dry microalgal biomass possesses fraction of approximately 7% nitrogen and 1% phosphorus (Wijffels and Barbosa 2010). Substituting fossil fuels with algal biomass would require high extent of fertilizer consumption, which is economically not feasible, and its entrance into the food chain may impart adverse impact on living organisms. Consequently, such condition may change the stability of the ecosystem. Large-scale cultivation of microalgal biomass may impart both downbeat and upbeat impact. Downbeat impacts could arise if leftover nutrients in culture medium pass into the adjacent aquatic ecosystem because of which, the adjacent river receives tremendous amount of nutrient that causes microalgal bloom (Clarens et al. 2010; Lardon et al. 2009). It is reported by Agricultural Research Service Scientists and found that 60–90% of nitrogen and 70–100% of phosphorus runoff can be obtained from manure effluents using microalgal turf scrubber, which in turn leads to the eutrophication (Jorquera et al. 2010). Therefore, excessive use of nutrients should be avoided, and alternatives like different wastewater should be promoted for microalgal cultivation, as suggested by some research groups at the global level (Pathak et al. 2014; Chisti 2012; Kothari et al. 2010, 2012).
Algal toxicity
Algal species have potential to produce toxic substances ranging from simple ammonia to more complex physiologically active substances such as polypeptides and polysaccharides. These compounds have potential to harm the other native species present in adjacent system. Their effect may vary from acute to chronic for other native species as reported by Razon and Tan (2011). Toxins production usually varies from species to species and local environmental circumstances. As a result, appropriate algae selection for biomass cultivation is very crucial to avoid the algal toxicity in algal-based industrial sector.
Genetically modified algae
A large number of algal species produces valuable compounds in significant amounts, though their products are yet not competitive as compared to petroleum based fuels. In this regard, application of genetic and metabolic engineering approach is essential to improve microalgal species that can produce high biomass and lipid contents (Gangl et al. 2015). Although many genes controlling these events related to enhanced biofuel production is largely unknown. But recently, the availability of large number of bioinformatics tools and genome sequence of many algal species helped in addressing this issue. Genetic engineers forecast that microalgae will be redesigned to produce biofuels using insights from synthetic biology—an advanced method of creating genetically engineered algae (Dana et al. 2012). The main goal of algal engineering is to accelerate the evolution of strain which can convert solar energy into lipids or triglycerides that can further be refined into biofuels. Genetic engineers are attempting to do this by splicing new genes into strains of algae and manipulating their current genes. Advances in science and technologies and availability of new and improved genetic tools, enable scientists to analyze and manipulate the metabolic pathway of microalgal cells with extraordinary correctness. Metabolic engineering approach along with genetic engineering helps in identifying target pathway and its enzymes towards harnessing maximum benefits from the microalgal species. To achieve maximum production of biofuels from the algal strains, an effective biochemical pathway should be constructed with a proper selection of host and other essential requirements targeting and it’s modeling toward desired product formation.
Fossil fuel utilization
Cultivation process of microalgae biomass production required electricity consumption and drying process of microalgal biomass requires natural gas. In PBRs high microalgal biomass productivity has been reported, rather than open pond system and the former requires temperature control system using electricity (Khoo et al. 2011; Plappally and Lienhard 2011; Mata et al. 2010). Hence, temperature control, i.e., heating and cooling of the system boosts the demand for fossil fuel many folds. Significant microalgal biomass production at the cost of fossil fuel must be avoided with the use of renewable energy technologies (RETs).
Green house gas emission by algae
Microalgae also release CO2 and CH4 through respiration and anaerobic decomposition of the waste material. Therefore, research work should also focus on molecular level to suppress the production of green house gasses to minimize the global warming potential (GWP). There are some gasses emitted from microalgae and its impacts on the environment have been suggested in Table 11. There is negative energy balance obtained in microalgal-based VAPs production process. The essential nutrients required in microalgal cultivation impart a downbeat impact on the sustainability and economics of the process if artificial fertilizers are used. The most important concern is to make microalgal-based biofuel economically viable associating with wastewater treatment or the production of valuable by-products.
Table 11.
Emitted gases from algae and its environmental impact
| CO2 | CH4 | |
|---|---|---|
| Potential source | Via respiration | Anaerobic decomposition |
| Formation mechanism | C6H12O6 + O2 = CO2 + H2O + energy | CH2O + 1/2 + 1/2 CO2 |
| Impact fluctuation from microalgae | Ranges from negative to positive when offset by photosynthesis | Positive |
| Environmental impacts (direct) | Green house gas | Green house gas |
| Further reaction | Inhibits isoprene production | Decomposition to CO2 precursor for organ-halogens |
Challenges on economical scale
To know about the economic feasibility of microalgae biomass cultivation methods for mass production, cost-modeling approach is required. The production costs of algal cultivation must be decreased drastically, to one-tenth of the current level, if cost-modeling approaches stipulate with large-scale cultivation of algae. It can be achieved by applying improved reactor designs and also by use of more efficient/genetically modified microalgal strains. Also, a substantial saving on nutrients becomes possible by making use of waste, and residual flows and recycling of these nutrients can reduce the cost regarding nutrient supplement. Furthermore, a considerable reduction of energy consumption can be achieved by the use of energy-efficient pumps. A better harvest and downstream processing methods can significantly reduce the costs, without compromising quality of final product. The cost related analysis reveals that the operation cost regarding raceway pond system is associated with labor, utilities, and raw materials, but the production cost allied to the PBRs is dominated by the capital cost of PBRs. Open pond system is economically cheap, but there are so many inconveniences associated with it, such as less productive, extensive land area requirement, optimization of physico-chemical parameter, chances of contamination, etc. Photobioreactor is relatively expensive, but it is a favorable option for algal-based VAP generation because of easy optimization and less contamination in comparison to open system. Use of different photobioreactors for microalgal biomass cultivation is an effective and promising approach as it provides a wide range of solar receiver area, high area/volume ratio to receive maximum solar radiation for proper growth and development.
Noteworthy cost decline (>50%) may be obtained if CO2, nutrients, and water can be acquired at near to onsite spots. Subsequently, it could dramatically increase the demand for microalgal-based VAPs (Davis et al. 2011; Norsker et al. 2011). Regarding composition, a microalgal biomass is more potent, compared with another source of biomass, but it is expensive with respect to the production and operational cost. Therefore, a significant and economically feasible long-term research effort is needed to achieve a suitable and viable process for mass cultivation of microalgae. To resolve the challenges associated with algal-based product industry, more open data sharing and synchronization of analytical and methodical approaches for microalgal cultivation to product generation is highly desired. In addition to this techno-economic assessments, and life cycle analysis of derived product is also essential to determine the sustainability of the process.
Critical evaluation of VAPs on enviro-economical scale
To accomplish validated pragmatic goal regarding microalgal-based VAPs, microalgal cultivation and products yields for its development as a next generation renewable products poised a central role (Table 5). Nowadays, to scale up the microalgal market potential various microalgal platforms such as photoautotrophic, heterotrophic, mixotrophic, lignin-producing microalgae, and oil producing microalgae have been promoted to enhance the quality and quantity of microalgal-based VAPs. Though, a large number of microalgal-based VAPs have been generated, but only a few of them have the capability to replace their alternative, i.e., chemical based products. Regarding animal food, microalgal biomass density is higher as it is available in fresh and marine water both. In many countries, it is being sold as edible material. Microalgae-based value-added chemicals being used in cosmetics are herbal in nature and do not pose much risk to skin. To enhance the quality and quantity of microalgal-based VAPs, various key factors have been critically evaluated as an important part for processing, like:
Environmental assessment of VAPs from microalgal biomass evaluated by various authors and supported by various life cycle assessment reports (Quinn and Devis 2015; Vasudevan et al. 2012; Stratton et al. 2012; http://www.aquafuelis.eu/deliverableshtml). The environmental risk assessment report of genetically modified microalgae that is also in favor of its cultivation in open pond system after a lab scale testing period.
At economical scale, microalgal feedstock has a market potential in the trillion-dollar range. Nevertheless, market analysis shows that exploration of VAPs is not significantly achieved due to the lack of adequate extraction and purification approaches. Furthermore, the production cost is also difficult to estimate due to the wide variation in technologies, different strain-specific harvesting requirement and most important is a lack of published information/data from commercial sectors. The omega-3 market is estimated to be valued at USD 9.94 Billion in 2015. It is projected to grow at a CAGR (compound annual growth rate) of 13.8% from 2015 to 2020. The Omega-3 PUFA market is segmented on the basis of its types into docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and alpha linolenic acid (ALA) (Market and markets 2016). The expected market potential of microalgal-derived high-value compound, i.e., docosahexaenoic acid and eicosapentaenoic acid has been estimated $300 million and $1.5 billion, respectively. The worldwide production in terms of quantity of various microalgae like Spirulina, Chlorella, Dunaliella, Nostoc, and Aphanizomenon, etc, has been speculated 3000, 2000, 1200, 600, and 500 ton per year, respectively (Pulz and Gross 2004), and these microalgal biomass extracts is being used by various industries for its high-value chemical compounds.
Hence, advancement in processing systems is urgently needed to bring a significant breakthrough for greener and sustainable VAPs from microalgal biomass.
Future perspectives of VAPs
The sustainability of microalgal-derived value-added products depends on the development of sustainable and eco friendly technologies. Numerous microalgal-based value-added products would emerge that will replace the chemical based non-renewable products having severe environmental impacts. In future, microalgal-derived plastics, polymers, high-value chemical compounds, cosmetics, paints, lubricants, cosmetics, coatings, and paper would be able to sustain the green economy of the society, efficiently. The success of the microalgal-derived product requires industrial development and the optimum combination of technical innovation to achieve tremendous microalgal biomass. Economically feasible processes coupled with practical implementation and integrated scale up for commercial scale production is required to enhance the market potential of microalgal-based value-added products. The design of different kind of PBRs require innovative technologies to improve some key parameters like light capturing and distribution (e.g., spectral shifting and internal illumination), mass transfer (e.g., membrane PBRs), and reduction of construction costs (e.g., plastic bag PBRs) associated with PBRs to achieve maximum productivities. In the case of biofuel production, energy balance is a challenging parameter, as it requires huge energy for different processing units, therefore, intensive research has to be carried out to minimize energy demand for its cultivation and biofuel production. To minimize the cost of different processing units, an integration of wastewater with industrial source of carbon, i.e., flue gasses is a significant source while keeping the prices low. Therefore, innovative technology related to carbon capture and storage from various smoke stacks must be implemented for better growth and development of algal biomass. Extraction and purification of microalgal-derived high-value compounds have to be magnified many folds to dig out maximum output. Microalgae are the best suitable option since it does not compete with the production of food and feed and avoid fresh water and huge land area. Due to its versatility and tremendous potential, such a tiny microorganism sustains microalgal-based biofuel, value-added products, and bio-economy, to provide endless opportunities in the microalgal market worldwide. The prolonged development associated with microalgal-based value-added products will lead to high demands, development in new technology, and new economic opportunities. The contemporary research level, as well as industrial activity, is very encouraging for the concerned sector worldwide. The integration of technologies for the production of microalgal-based biofuel and value-added products would be more sustainable as it reduces the capital and operating cost of integration versus stand-alone value-added production along with location and local microalgal-based product market dynamics. There are numerous opportunities and challenges regarding large-scale application of microalgal-based value-added products worldwide. An effective utilization of value-added products is highly crucial for commercialization as well as future developments. It can be speculated that the prospects regarding microalgal biotechnology will lead to a diverse range of technical solution for cultivation in microalgae. It has been predicted that bio-prospecting can be helpful to identify desired microalgal traits with (i.e., high growth rate, high lipid content, high-value compounds, high growth densities) high-value co-products, even as growing on low costs. Therefore, research regarding the development of microalgal-based value-added products has to be done with the fast rate to conquer the chemical requirements, where microalgal-based high-value chemical compounds are alternative sources. Hence, biofuel, together with other VAPs can make the process economically feasible and expected to generate job opportunities along with a positive effect on overall sustainability. To maximize the economic viability of algal-based VAPs, ecological, genetic (biotechnological and genetic engineering), and biochemical developments of microalgal species associated with amalgamation of co-located inoculation, microalgal cultivation, primary and secondary harvesting, processing of system, post harvesting physiological variations of generated algal biomass and sustainability of whole operation should be explored and integrated with commercial applications.
Conclusion
The main aim of this article is to critically focus on the combined enviro-economical impacts with generation of value-added products from microalgal-based biomass. Major findings have concluded that production of VAPs, integrated with biofuel can be commercialized, but major challenges should not be ignored at the part of environment because they have the potential to recover in the long-term with sustainability and to contribute at bio-economy on behalf of chemical based products and fossil fuels. Similarly, reactors for mass production is also one of the major contributing factor to assess its feasibility on enviro-economical scale, because OPS type reactors can be made at low-cost for mass production and challenges associated with this need a proper R&D. Therefore, microalgal cultivation should be done at zero environmental cost as microalgal-based biofuel, and high-value compounds are being reached at a tipping point. The market value and its potential are expected to be double in the upcoming years, which directly show the future prospect of economic growth. A potential platform for algal-based value-added products is speculating to grow substantially over the next few years. Genetic manipulation and metabolic engineering of microalgal strains can be a way to meet immediate and long-term demands for food and liquid fuel production on a sustainable basis. In this regard, the main goal is to develop microalgae–microbial fuel cells that can effectively channelize the solar energy into electrical energy via algal metabolic pathways.
Acknowledgements
We would like to thank Mrs. Meghna Singh for their critical reading, evaluation, and suggestions for this article.
Compliance with ethical standards
Conflict of interest
Richa Kothari, Arya Pandey, Shamshad Ahmad, Ashwani Kumar, Vinayak V. Pathak, V.V. Tyagi declare that they have no conflict of interest in reference for funding and acknowledgement.
References
- Adarme-Vega TC, Lim DKY, Timmins M, Vernen F, Li Y, Schenk PM. Microalgal bio-factories: a promising approach towards sustainable omega-3 fatty acid production. Microb Microbe Cell Factories. 2012;11:1–10. doi: 10.1186/1475-2859-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aditya M, Kunjapur Eldrige B. Photobioreactor design for commercial biofuel production from microalgae. Ind Eng Chem. 2010;49(8):3516–3526. doi: 10.1021/ie901459u. [DOI] [Google Scholar]
- Akhtar A, Pareek V, Tade M. CFD simulations for continuous flow of bubbles through gases liquid columns: application of VOF method. Chem Prod Proc Mod. 2007;2(2):211–222. [Google Scholar]
- Aoyama K, Uemura I, Miyake J, Asada Y. Fermentative metabolism to produce hydrogen gas and organic compounds in a cyanobacterium, Spirulina platensis. J Fermentation Bioengg. 1997;83:17. doi: 10.1016/S0922-338X(97)87320-5. [DOI] [Google Scholar]
- Baltrėnas P, Misevičius A. Biogas production experimental research using algae. J Environ Health Sci Eng. 2015;13:18. doi: 10.1186/s40201-015-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013;2:164–173. doi: 10.1016/j.algal.2013.01.004. [DOI] [Google Scholar]
- Becker EW. Microalgae: biotechnology and microbiology. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Bhatt NC, Panwar A, Bisht TS, Tamta S (2014) Coupling of algal biofuel production with wastewater. Sci World J:1–10. doi: 10.1155/2014/210504 [DOI] [PMC free article] [PubMed]
- Borowitzka MA. High-value products from microalgae—their development and commercialization. J Appl Phycol. 2013;25:743–756. doi: 10.1007/s10811-013-9983-9. [DOI] [Google Scholar]
- Brennan L, Owende P. Biofuels from microalgae: a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev. 2010;14(2):557–577. doi: 10.1016/j.rser.2009.10.009. [DOI] [Google Scholar]
- Brown MR, Mc Causland MA, Kowalski K. The nutritional value of four Australian microalgal strains fed to Pacific oyster Crassostrea gigasspat. Aquaculture. 1998;165:281–293. doi: 10.1016/S0044-8486(98)00256-7. [DOI] [Google Scholar]
- Bunnag B (2009) Microalgae research in Thailand and southeast Asia. International workshop aquatic biomass sustainable bioenergy from algae. Berlin
- Campbell PK, Beer T, Batten D. Life cycle assessment of biodiesel production from microalgae in ponds. Biores Technol. 2011;102(1):50–56. doi: 10.1016/j.biortech.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25(3):294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Chisti Y. Raceways-based production of algal crude oil. In: Posten C, Walter C, editors. Microalgal biotechnology: potential and production. Berlin: de Gruyter; 2012. pp. 113–146. [Google Scholar]
- Clarens AF, Resurreccion EP, White MA, Colosi LM. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol. 2010;44(5):1813–1819. doi: 10.1021/es902838n. [DOI] [PubMed] [Google Scholar]
- Colla LM, Reinehr CO, Reichert C, Costa JAV. Production of biomass and nutraceuticals compounds by Spirulina platensis under different temperature and nitrogen regimes. Biores Technol. 2007;98(7):1489–1493. doi: 10.1016/j.biortech.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Dana GV, Kuiken T, Rejeski D, Snow AA. Synthetic biology: four steps to avoid a synthetic-biology disaster. Nature. 2012;483:29. doi: 10.1038/483029a. [DOI] [PubMed] [Google Scholar]
- Dasgupta CN, Gilbert JJ, Lindblad P, Heidorn T, Borgvang SA, Skjanes K, Das D. recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int J Hydrog Energy. 2010;35:10218–10238. doi: 10.1016/j.ijhydene.2010.06.029. [DOI] [Google Scholar]
- Davis R, Aden A, Pienkos PT. Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy. 2011;88(10):3524–3531. doi: 10.1016/j.apenergy.2011.04.018. [DOI] [Google Scholar]
- Demirbas A. Production of biodiesel from algae oils. Energy Sources Part A Recovery Util Environ Eff. 2008;31(2):163–168. doi: 10.1080/15567030701521775. [DOI] [Google Scholar]
- Gallezot P. Conversion of biomass to selected chemical products. Chem Soc Rev. 2012;41(4):1538–1558. doi: 10.1039/C1CS15147A. [DOI] [PubMed] [Google Scholar]
- Gangl D, Zedler JAZ, Rajakumar PD, Martinez EMR, Riseley A, Włodarczyk A. Biotechnological exploitation of microalgae. J Exp Bot. 2015;66:6975–6990. doi: 10.1093/jxb/erv426. [DOI] [PubMed] [Google Scholar]
- Gantar M, Simović D, Djilas S, Gonzalez WW, Miksovska J. Isolation, characterization and antioxidative activity of C-phycocyanin from Limnothrix sp. strain 37-2-1. J Biotechnol. 2012;159:21–26. doi: 10.1016/j.jbiotec.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Yang ZH, Li C, Wang YJ, Jin WH, Deng YB. Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Biores Technol. 2014;167:441–446. doi: 10.1016/j.biortech.2014.06.042. [DOI] [PubMed] [Google Scholar]
- Gebreslassie BH, Waymire R, You F. Sustainable design and synthesis of algae-based biorefinery for simultaneous hydrocarbon biofuel production and carbon sequestration. AIChE J. 2013;59(5):1599–1621. doi: 10.1002/aic.14075. [DOI] [Google Scholar]
- Gfeller RP, Gibbs M. Fermentative metabolism of Chlamydomonas reinhardtii: I. Analysis of fermentative products from starch in dark and light. Plant Physiol. 1984;75:212–218. doi: 10.1104/pp.75.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves AL, Pires JC, Simões M. Green fuel production: processes applied to microalgae. Environ Chem Lett. 2013;11:315–324. doi: 10.1007/s10311-013-0425-3. [DOI] [Google Scholar]
- Guan YF, Deng MC, Yu XJ, Zhang W. Two stage photobiological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem Eng J. 2004;19:69–73. doi: 10.1016/j.bej.2003.10.006. [DOI] [Google Scholar]
- Handler RM, Canter CE, Kalnes TN. Evaluation of environmental impacts from microalgae cultivation in open-air raceway ponds: analysis of the prior literature and investigation of wide variance in predicted impacts. Algal Res. 2012;1(1):83–92. doi: 10.1016/j.algal.2012.02.003. [DOI] [Google Scholar]
- Hirano A, Ueda R, Hirayama S, Ogushi Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy. 1997;1997(22):137–142. doi: 10.1016/S0360-5442(96)00123-5. [DOI] [Google Scholar]
- Honda R, Boonnorat J, Chiemchaisri C, Chiemchaisri W, Yamamoto K. Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Biores Technol. 2012;125:59–64. doi: 10.1016/j.biortech.2012.08.138. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hong A, Zhang D, Li L. Comparison of cell rupturing by ozonation and ultrasonication for algal lipid extraction from Chlorella vulgaris. Environ Technol. 2014;35:931–937. doi: 10.1080/09593330.2013.856954. [DOI] [PubMed] [Google Scholar]
- Ike N, Toda N, Tsuji K, Hirata K, Miyamoto K. Hydrogen photo production from CO2-fixing microalgal biomass: application of halotolerant photosynthetic bacteria. J Ferment Bioeng. 1997;84:606–609. doi: 10.1016/S0922-338X(97)81921-6. [DOI] [Google Scholar]
- İnan B (2014) Utilization of algae for bioethanol production. M.Sc. Thesis. Yıldız Technical University
- Jensen A. Present and future needs for algae and algal products. Hydrobiologia. 1993;260–261:15–23. doi: 10.1007/BF00048998. [DOI] [Google Scholar]
- Jorquera O, Kiperstok A, Sales E, Embiruçu M, Ghirardi ML. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Biores Technol. 2010;101(4):1406–1413. doi: 10.1016/j.biortech.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Khoo HH, Sharratt PN, Das P, Balasubramanian RK, Naraharisetti PK, Shaik S. Life cycle energy and CO2 analysis of microalgae-to-biodiesel: preliminary results and comparisons. Bioresour Technol. 2011;102(10):5800–5807. doi: 10.1016/j.biortech.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Kosourov S, Tsygankov A, Seibert M, Ghirardi ML. Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: effects of culture parameters. Biotechnol Bioeng. 2002;78:731–740. doi: 10.1002/bit.10254. [DOI] [PubMed] [Google Scholar]
- Kothari R, Tyagi VV, Pathak A. Waste-to-energy: a way from renewable energy sources to sustainable development. Renew Sustain Energy Rev. 2010;14:3164–3170. doi: 10.1016/j.rser.2010.05.005. [DOI] [Google Scholar]
- Kothari R, Pathak VV, Kumar V, Singh DP. Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: an integrated approach for treatment and biofuel production. Biores Technol. 2012;116(2012):466–470. doi: 10.1016/j.biortech.2012.03.121. [DOI] [PubMed] [Google Scholar]
- Kumar A, Agila E, Salam Z, Ponraj M, Din MFM, Ani FN. A study on large scale cultivation of Microcystis aeruginosa under open raceway pond at semi-continuous mode for biodiesel production. Biores Technol. 2014;172:186–193. doi: 10.1016/j.biortech.2014.08.100. [DOI] [PubMed] [Google Scholar]
- Kumar K, Mishra SK, Srivastava A, Park MS, Yang JW. Recent trends in the mass cultivation of algae in raceway ponds. Renew Sustain Energy Rev. 2015;51:875–885. doi: 10.1016/j.rser.2015.06.033. [DOI] [Google Scholar]
- Lardon L, Hélias A, Sialve B, Steyer JP, Bernard O. Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol. 2009;43(17):6475–6481. doi: 10.1021/es900705j. [DOI] [PubMed] [Google Scholar]
- Laurinavichene TV, Fedorov AS, Ghirardi ML, Seibert M, Tsygankov AA. Demonstration of sustained hydrogen photoproduction by immobilized, sulfur-deprived Chlamydomonas reinhardtii cells. Int J Hydrogen Energy. 2006;31:659. doi: 10.1016/j.ijhydene.2005.05.002. [DOI] [Google Scholar]
- Makri A, Bellou S, Birkou M, Papatrehas K, Dolapsakis NP, Bokas D. Lipid synthesized by micro-algae grown in laboratory- and industrial-scale bioreactors. Eng Life Sci. 2011;11:52–58. doi: 10.1002/elsc.201000086. [DOI] [Google Scholar]
- Market and markets (2016) Omega-3 PUFA Market by type (DHA, EPA, ALA), application (dietary supplements, functional foods & beverages, pharmaceuticals, infant formula), source (marine, plant), sub-source), & region—global forecasts to 2020. http://www.marketsandmarkets.com/Market-Reports/omega-3-omega-6-227.html
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev. 2010;14:217–232. doi: 10.1016/j.rser.2009.07.020. [DOI] [Google Scholar]
- Mendes A, Da Silva T, Reis A. DHA concentration and purification from the marine heterotrophic microalga Crypthecodinium cohnii CCMP 316 by winterization and urea complexation. Food Technol Biotechnol. 2007;45(1):38–44. [Google Scholar]
- Mortuza SM, Kommareddy A, Gent S, Anderson G (2011) Computational and experimental investigation of bubble circulation pattern within a column photobioreactor. ASME energy sustainability conference, Aug 07–10. Grand Hyatt Washington, Washington DC
- Mussgnug JH, Klassen V, Schlüter A, Kruse O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J Biotechnol. 2010;150:51–56. doi: 10.1016/j.jbiotec.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Norsker NH, Barbosa MJ, Vermuë MH, Wijffels RH. Microalgal production—a close look at the economics. Biotechnol Adv. 2011;29(1):24–27. doi: 10.1016/j.biotechadv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Órpez R, Martínez ME, Hodaifa G, El Yousfi F, Jbari N, Sánchez S. Growth of the microalga Botryococcus braunii in secondarily treated sewage. Desalination. 2009;246:625–630. doi: 10.1016/j.desal.2008.07.016. [DOI] [Google Scholar]
- Oswald WJ, Borowitzka M, Borowitzka L. Micro algal biotechnology. Cambridge: Cambridge University Press; 1988. Large scale culture systems: engineering aspects; pp. 357–392. [Google Scholar]
- Parmar A, Singh NK, Kaushal A, Madamwar D. Characterization of an intact phycoerythrin and its cleaved 14 kDa functional subunit from marine cyanobacterium Phormidium sp. A27DM. Process Biochem. 2011;46:1793–1799. doi: 10.1016/j.procbio.2011.06.006. [DOI] [Google Scholar]
- Pathak VV, Singh DP, Kothari R, Chopra AK. Phycoremediation of textile wastewater by unicellular microalga Chlorella pyrenoidosa. Cell Mol Biol. 2014;60(5):35–40. [PubMed] [Google Scholar]
- Pittman JK, Dean AP, Osundeko O. The potential of sustainable algal biofuel production using wastewater resources. Biores Technol. 2011;102:17–25. doi: 10.1016/j.biortech.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Plappally K, Lienhard VJH. Energy requirements for water production, treatment, end use, reclamation, and disposal. Renew Sustain Energ Rev. 2011;16(7):4818–4848. doi: 10.1016/j.rser.2012.05.022. [DOI] [Google Scholar]
- Pohl P, Kohlhase M, Martin M. Photobioreactors for the axenic mass cultivation of microalgae. In: Stadler T, Mollion J, Verdus MC, Karamanos Y, Morvan H, Christiaen D, editors. Algal biotechnology. New York: Elsevier; 1988. pp. 209–218. [Google Scholar]
- Priyadarshani I, Rath B. Commercial and industrial applications of micro algae—a review. J Algal Biomass Utln. 2012;3(4):89–100. [Google Scholar]
- Pulz O, Gross W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- Quinn JC, Davis R. The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling. Bioresour Technol. 2004;184:444–452. doi: 10.1016/j.biortech.2014.10.075. [DOI] [PubMed] [Google Scholar]
- Razon LF, Tan RR. Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl Energy. 2011;88(10):3507–3514. doi: 10.1016/j.apenergy.2010.12.052. [DOI] [Google Scholar]
- Reyes FA, Mendiola JA, Ibañez E, Del Valle JM. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J Supercrit Fluids. 2014;92:75–83. doi: 10.1016/j.supflu.2014.05.013. [DOI] [Google Scholar]
- Richardson JW, Johnson MD, Outlaw JL. Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res. 2012;1:93–100. doi: 10.1016/j.algal.2012.04.001. [DOI] [Google Scholar]
- Rittmann S, Herwig C. A comprehensive and quantitative review of dark fermentative biohydrogen production. Microbial Cell Factories. 2012;11(1):2–18. doi: 10.1186/1475-2859-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JN, Rosenberg JN, Guzman BJ, Oh VH, Mimbela LE, Ghassemi A, Betenbaugh MJ, Oyler GA, Donohue MDA. Critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res. 2014;4:76–88. doi: 10.1016/j.algal.2013.11.007. [DOI] [Google Scholar]
- Ross AB, Jones JM, Kubacki ML, Bridgeman T. Classification of macroalgae as fuel and its thermo chemical behavior. Biores Technol. 2008;99:6494–6504. doi: 10.1016/j.biortech.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Ryckebosch E, Muylaert K, Foubert I. Optimization of an analytical procedure for extraction of lipids from microalgae. J Am Oil Chem Soc. 2011;89:189–198. doi: 10.1007/s11746-011-1903-z. [DOI] [Google Scholar]
- Singh RN, Sharma S. Development of suitable photobioreactor for algae production—a review. Renew Sustain Energy Rev. 2012;16:2347–2353. doi: 10.1016/j.rser.2012.01.026. [DOI] [Google Scholar]
- Smith VH, Sturm BS, Denoyelles FJ, Billings S. The ecology of algal biodiesel production. Trends Ecol Evol. 2010;25(5):301–309. doi: 10.1016/j.tree.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Stratton RW, Wong HM, Hileman JI. Quantifying variability in life cycle greenhouse gas inventories of alternative middle distillate transportation fuels. Environ Sci Technol. 2012;45:4637–4644. doi: 10.1021/es102597f. [DOI] [PubMed] [Google Scholar]
- Sydney EB, Sturm W, de Carvalho JC, Thomaz-Soccol V, Larroche C, Pandey A, Soccol CR. Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol. 2010;101:5892–5896. doi: 10.1016/j.biortech.2010.02.088. [DOI] [PubMed] [Google Scholar]
- Sydney EB, da Silva TE, Tokarski A, Novak AC, de Carvalho JC, Woiciecohwski AL. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl Energy. 2011;88:3291–3294. doi: 10.1016/j.apenergy.2010.11.024. [DOI] [Google Scholar]
- Tong KL, You FQ, Rong G. Robust design and operations of hydrocarbon biofuel supply chain integrating with existing petroleum refineries considering unit cost objective. Comput Chem Eng. 2014;68:128–139. doi: 10.1016/j.compchemeng.2014.05.003. [DOI] [Google Scholar]
- Tredici MR, Zittelli GC. Efficiency of sunlight utilization: tubular versus flat photobioreactors. Biotechnol Bioeng. 1998;57(2):187–197. doi: 10.1002/(SICI)1097-0290(19980120)57:2<187::AID-BIT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Troshina O, Serebryakova LT, Sheremetieva M, Lindberg P. Int J Hydrogen Energy. 2002;27:1283. doi: 10.1016/S0360-3199(02)00103-9. [DOI] [Google Scholar]
- Trujilio FJ, Safinski T, Adesina AA. CFD analysis of the radiation distribution in a new immobilized catalyst bubble column externally illuminated photoreactor. J Solar Energy Eng. 2007;129:27–35. doi: 10.1115/1.2391013. [DOI] [Google Scholar]
- Usher PK, Ross AB, Camargo-Valero MA, Tomlin AS, Gale WF. An overview of the potential environmental impacts of large scale microalgae cultivation. Biofuels. 2014;5(3):331–349. doi: 10.1080/17597269.2014.913925. [DOI] [Google Scholar]
- Vasudevan V, Stratton RW, Pearlson MN, Jersey GR, Beyene AG, Weissman JC, Rubino M, Hileman JI. Environmental performance of algal biofuel technology options. Environ Sci Technol. 2012;46:2451–2459. doi: 10.1021/es2026399. [DOI] [PubMed] [Google Scholar]
- Wijffels RH. Potential of sponges and microalgae for marine biotechnology. Trends Biotechnol. 2008;26(1):26–31. doi: 10.1016/j.tibtech.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels. Science. 2010;329:796–799. doi: 10.1126/science.1189003. [DOI] [PubMed] [Google Scholar]
- Xu H, Miao X, Wu Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol. 2006;126:499–507. doi: 10.1016/j.jbiotec.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Zhu L. Microalgal culture strategies for biofuel production: a review. Biofuels Bioprod Biorefining. 2015 [Google Scholar]
- Yoo JJ, Choi SP, Kim JYH, Chang WK, Sim JS. Development of thin-film photo-bioreactor and its application to outdoor culture of microalgae. Bioprocess Biosyst Eng. 2013;36:729–736. doi: 10.1007/s00449-013-0898-2. [DOI] [PubMed] [Google Scholar]
- Zhang QH, Wu X, Xue SZ, Wang ZH, Yan CH, Cong W. Hydrodynamic characteristics and microalgae cultivation in a novel flat-plate photobioreactor. Biotechnol Prog. 2013;29:127–134. doi: 10.1002/btpr.1641. [DOI] [PubMed] [Google Scholar]
- Zhang X, Rong J, Chen H, He C, Wang Q. Current status and outlook in the application of microalgae in biodiesel production and environmental protection. Front Energy Res. 2014;2:1–15. [Google Scholar]
- Zhu L, Ketola T. Microalgae production as biofuel feedstock: risks and challenge. Int J Sustain Dev World Ecol. 2012;19:268–274. doi: 10.1080/13504509.2011.636083. [DOI] [Google Scholar]
- Zittelli GC, Biondi N, Rodolfi L, Tredici MR. Photobioreactors for mass production of microalgae. In: Richmond A, Hu Q, editors. Handbook of microalgal culture. 2. New York: Wiley; 2013. [Google Scholar]