Abstract
Analysis of affective picture processing by means of EEG has invaded the literature. The methodology of event-related EEG coherence is one of the essential methods used to analyze functional connectivity. The aims of the present study are to find out the long range EEG connectivity changes in perception of different affective pictures and analyze gender differences in these long range connected networks. EEGs of 28 healthy subjects (14 female) were recorded at 32 locations. The participants passively viewed emotional pictures (IAPS, unpleasant, pleasant, neutral). The long-distance intra-hemispheric event-related coherence was analyzed for delta (1–3.5 Hz), theta (4–7.5 Hz), and alpha (8–13 Hz) frequency ranges for F3–T7, F4–T8, F3–TP7, F4–TP8, F3–P3, F4–P4, F3–O1, F4–O2, C3–O1, C4–O2 electrode pairs. Unpleasant pictures elicited significantly higher delta coherence values than neutral pictures (p < 0.05), over fronto-parietal, fronto-occipital, and centro-occipital electrode pairs. Furthermore, unpleasant pictures elicited higher theta coherence values than pleasant (p < 0.05) and neutral pictures (p < 0.05). The present study showed that female subjects had higher delta (p < 0.05) and theta (p < 0.05) coherence values than male subjects. This difference was observed more for emotional pictures than for neutral pictures. This study showed that the brain connectivity was higher during emotional pictures than neutral pictures. Females had higher connectivity between different parts of the brain than males during emotional processes. According to these results, we may comment that increased valence and arousal caused increased brain activity. It seems that not just single sources but functional networks were also activated during perception of emotional pictures.
Keywords: EEG, Event related coherence, EEG connectivity, Emotion, IAPS, Gender
Introduction
In the last decade, the analysis of emotional processes by means of Electroencephalography (EEG) invaded the literature. Numerous studies have been published in this research area. The emotional stimuli used in the experiments differed, including visual, auditory, or audio-visual. The visual stimuli were most commonly used in the literature; these stimuli were faces, facial expressions, and pleasant–unpleasant–neutral picture groups. The International Affective Picture System (IAPS) introduced by Lang et al. (1999) was one of the most used standardized picture groups in the research of Affective picture processing.
A majority of the studies performed analysis of Event Related Potentials (ERPs) upon application of emotional stimuli. In the ERP research of affective picture processing, the authors reported differences in short latency, middle latency, and long latency potentials among different picture groups (Please see reviews of Eimer and Holmes 2007; Olofsson et al. 2008; Palermo and Rhodes 2007). The ERP research on affective picture processing already presented strong conclusions by many researchers. However, in addition to the time and amplitude components of ERP signals, these signals also have frequency properties. A wide variety of methodologies could be used in the analysis of time and frequency components of ERP signals. These methodologies could be listed as evoked/induced/event-related power spectrum analysis by means of FFT (Fast Fourier Transform) or Wavelet analysis, filtered responses in different frequency bands, phase locking or inter-trail phase coherence between epochs in different frequency bands, cross frequency couplings between different frequency bands, and the coherence analysis between different electrode sites in different frequency bands. In the research of brain dynamics upon presentation of affective pictures, the authors published results on delta, theta, alpha, beta, and gamma band activities (Klados et al. 2009; Balconi et al. 2009a, b, Güntekin and Başar 2010b; Güntekin and Tülay 2014; Woodruff et al. 2011, 2016; Garcia-Garcia et al. 2010; Keil et al. 2001, 2007; Martini et al. 2012; Müller et al. 1999; Oya et al. 2002). In these studies, mostly the evoked/event related power spectrums, filtered oscillatory responses, and/or phase locking (inter-trial phase coherence) analysis was performed.
Among these methods, event-related coherence analysis is one of the most important methodologies that could show functional connectivity between different pair of electrodes. Our group previously analyzed event-related coherences in healthy subjects during a cognitive task (Güntekin and Başar 2010a); we have also analyzed event-related coherence in different patient groups such as Alzheimer’s disease (Başar et al. 2010; Güntekin et al. 2008) and bipolar disorder (Özerdem et al. 2010, 2011). In these papers, we have shown that event-related coherence values increased in delta and theta frequency bands during cognitive load in healthy young subjects (Güntekin and Başar 2010a). On the other hand, in Alzheimer’s patients, delta and theta coherence values decreased during cognitive load in comparison to healthy elderly subjects (Başar et al. 2010; Güntekin et al. 2008), but there were no difference between groups during a simple visual stimulation (Başar et al. 2010). As shown by the mentioned studies, event-related coherence analysis could give key knowledge on how the functional connectivity changes during application of different paradigms.
To our knowledge, only one study analyzed the event-related coherences upon presentation of IAPS pictures and reported the significant results for beta and theta frequency bands (Miskovic and Schmidt 2010). These authors reported that beta coherence increased during viewing of affective images. These authors also found that long-distance interhemispheric coherence increased during viewing of affective pictures but only among females. In their study, Miskovic and Schmidt (2010) analyzed the late latency coherence values during perception of affective pictures. The authors removed the first second from the analysis and performed the analysis for 1–6 s time window. Our previous studies and many studies in the literature showed that the first 0–800 ms are very important, especially in the perception of unpleasant and pleasant pictures. Therefore the present study analyzes the coherence values for early time window (0–800 ms). The present study is the first study analyzing early time window (0–800 ms) event-related coherences during viewing of affective pictures.
Previous studies analyzing event related oscillations in single electrodes showed that delta and theta responses were increased upon high arousal pictures (Klados et al. 2009; Balconi et al. 2009a, b), whereas unpleasant pictures elicited higher beta (Güntekin and Başar 2010b; Woodruff et al. 2011, 2016) and gamma responses (Garcia-Garcia et al. 2010; Keil et al. 2001, 2007; Martini et al. 2012; Müller et al. 1999; Oya et al. 2002) than neutral or positive stimuli. On the other hand, the long-range connectivity in different frequency bands during perception of affective pictures remained unclear especially for the early time windows.
In general as mentioned in the previous paragraph, the event-related oscillatory responses are more sensitive to emotional stimuli than to neutral stimuli. There were increases of delta, theta, beta, and gamma responses during emotional pictures in comparison to neutral pictures. How will the long-range connectivity between different electrode pairs change during the perception of pleasant, unpleasant and neutral pictures? According to whole brain theory (Basar 2010), in perception of different stimuli the brain works as a whole, and the activities do exist in different areas of the brain. We hypothesize that in the long-range connections, emotional pictures will also elicit higher responses, especially for unpleasant pictures. In the literature, gender differences in ERPs or event-related oscillations were also found during perception of emotional pictures (IAPs, face expressions, etc.) (Klados et al. 2009; Güntekin and Başar 2007b). In long-range connectivity, it is also expected that the female subjects would have higher coherence values than males. According to the mentioned hypothesis, the aims of the present study are to find out the long-range EEG connectivity changes in perception of different affective pictures and analyze gender differences in these long-range connected networks.
Methods
Subjects
Twenty-eight healthy subjects volunteered for the experiments. All of them were university students or university staff. Fourteen of the subjects were women (mean age = 21.64, SD = 3.91), and fourteen of the subjects were men (mean age = 24.71, SD = 3.56). Nine of the female participants were in the follicular phase of menstrual cycle (days 1–14 of menstrual cycle), while five of them were in luteal phase (days 14–28 of menstrual cycle). None of the female participants were taking contraceptive pills. All participants were right-handed and were educated for at least 10 years. All participants were given a questionnaire on their demographic information, medical situation, drinking habits, and family history. No subjects reported any current or past neurological or psychiatric illness, none were using any neurological or psychiatric drugs, and all participants had normal or corrected to normal vision. Before starting the study informed consent was obtained from each participant. The study was approved by the local ethics committee.
Stimuli and experimental procedure
Thirty pictures were chosen from the IAPS. Images consisted of 10 “pleasant” pictures including babies and animals, 10 “neutral” pictures including objects, and 10 “unpleasant” pictures including attack scenes, violence, and wild animals. Delta OHM HD 2302.0 light meter was used to measure the luminance of the pictures. Light meter was placed 1 m away from the screen, and a 1 m distance between the subject and screen was measured. Pictures with similar luminance were chosen with the mean of 23.4 ± 5.2 cd/m2. Pictures were shown on a 19-inch computer screen with a refresh rate of 60 Hz and a visual angel of 8° horizontally. Images were presented for 1000 ms, with an inter-stimulus interval that differed randomly between 3 and 7 s. Block design was used in the study. 10 × 4 = 40 pleasant pictures, 10 × 4=40 unpleasant pictures and 10 × 4 = 40 neutral pictures were shown to participants.
Right after the experiment, participants were given a self-assessment manikin (SAM)’s paper and pen version and asked to rate the affective valance (1–9) and arousal (1–9). As a measure of arousal, subjects were asked to rate their reaction to the photographs on a 9-point rating scale spanning from “excited” to “calm”, with 1 representing the highest arousal (“excited”) and 9 representing the lowest arousal (“calm”). As a measure of valence, the subjects were asked to describe their mood during viewing each of the photographs on a 9-point rating scale, with 1 representing the most positive mood, 5 representing neither a negative nor a positive mood, and 9 representing the most negative mood.
EEG recordings
The EEG was recorded with a Brain Amp 32-channel DC system machine with band limits of 0.01–250 Hz and digitized on-line with a sampling rate of 500 Hz. EEG was recorded according to the international 10–20 system with 30 Ag–AgCl electrodes mounted in an elastic Easy-cap. Two earlobe electrodes (A1 and A2) were used as references. The EOG from the medial upper- and lateral orbital rim of the right eye was also registered. All electrode impedances were less than 10 kΩ.
Event-related coherence analysis
Brain Vision Analyzer software was used for the event-related coherence analysis. Artifacts were eliminated manually off-line and semi-automatically. All eye-blink and muscle artifacts were removed from the EEG data. The data then was segmented into 0–800 ms epochs. Epoch numbers were equalized randomly between three conditions (pleasant, unpleasant, neutral). Fast Fourier Transform (Hanning window 10%) was applied to all epochs for each subject and for each electrode. The cross-spectrum/autospectrum function was selected in the Brain Vision Analyzer software.
The mathematical formulation of coherence (cross spectrum/autospectrum coherence or magnitude-squared coherence) function is as follow:
where as Sxx and Syy are power spectral densities of x and y (signals of different locations) and Sxy is cross power spectral density function. Power spectral density function is calculated with the following formula,
where as Rxx is autocorrelation function and calculated with
(τ = latency time).
Cross power spectral density function is calculated with the following formula;
where as Cxy(f) is cospectral density function and Qxy(f) is quadspectral density function.
The coherence values for each frequency band, for each electrode pair, and for each subject were calculated. Event related delta (1–3.5 Hz), theta (5–7 Hz), alpha1 (7.8–9.8 Hz), and alpha2 (10.7–12.7 Hz) coherence values were analyzed as the maximum coherence value in the specified frequency range. If there was more than one peak, the peak with the maximum coherence value was accepted as the coherence value. Then, Fisher’s Z-transformation was applied to the coherence values for each electrode pair and for each subject. The event related coherence were analyzed for long-range intra-hemispheric (F3–T7, F4–T8, F3–TP7, F4–TP8, F3–P3, F4–P4, F3–O1, F4–O2, C3–O1 and C4–O2) electrode pairs.
The effect of volume conduction on EEG coherence
Coherence analysis in the EEG is sometimes criticized due to the effect of volume conduction on coherence values. However, we have previously published several manuscripts showing that the effect of volume conduction may be eliminated while comparing different group of subjects or different stimulations (Başar et al. 2010; Güntekin and Basar 2010a; Güntekin et al. 2008; Özerdem et al. 2010, 2011). The effect of volume conduction is mostly seen in the comparison of different electrode pairs. The distance between electrode pairs matters; fronto-temporal electrode pairs usually had higher coherence values than fronto-occipital electrode pairs. On the other hand, comparing two different stimulations for same electrode pair (exp. F4–P4, etc.) gives important knowledge on how these stimulations affect the connectivity between two different brain regions. We have previously shown that during auditory cognitive stimulation, subjects had higher coherence values in comparison to simple auditory stimulation (Güntekin and Basar 2010a). Alzheimer’s subjects had reduced event-related coherences during cognitive load (Başar et al. 2010; Güntekin et al. 2008) in comparison to healthy controls, but if the stimulation is just sensory, there was no difference between healthy elderly subjects and Alzheimer’s patients (Başar et al. 2010). These results show that event-related coherence analysis is an important methodology in the understanding of connectivity between different cortical sources during sensory, cognitive, and emotional processes. Analysis of event related coherences has outstanding advantages: first of all EEG has a very good time resolution and event related coherences may show us how the connectivity changes in very early time windows during different functions of the brain. Furthermore, as we also could show in our earlier reports the connectivity differences between conditions and subject groups could be easily analyzed with event related coherence analysis. The increased coherence values show increased brain activity and connectivity between different cortical regions. In this manuscript, we did not analyze the inter-hemispheric electrode pairs; we also did not analyze the intra-hemispheric electrode pairs that have a distance less than 8 cm.
As we also mentioned above and in our previous reports (Başar et al. 2010; Güntekin et al. 2008; Güntekin and Basar 2010a), “Volume conduction is important when two electrode pairs (F3–T7 vs F3–O1) are compared. However, it could be neglected in different conditions: (1) When two different paradigms are compared for the same electrode pair (i.e. Comparing target coherence value to simple auditory coherence value for F3–T7 electrode pair. (2) When two different groups of subjects are compared for same electrode pair (i.e. comparing Alzheimer’s subjects’ coherence values to healthy subjects’ coherence values for F3–T7 electrode pair). In these two conditions, the volume conduction for F3–T7 is same; however the paradigm and group change influences the coherence values” (Başar et al. 2010; Güntekin et al. 2008).
Besides all these issues in the present study in order to minimize the effect of volume conduction we have analyzed coherence for long distance electrode pairs. According to Srinivasan et al. (2007) and Nunez (1997) the optimal distance between electrodes must be around 10–20 cm in human EEG-recordings to minimize the effect of volume conduction.
Statistical analysis
Statistical analyses were performed using Statistica software. A repeated measures ANOVA was used to investigate the event-related coherence differences during different types of pictures. Repeated measures of ANOVA included within-subject factors as 3 stimuli (pleasant, unpleasant and neutral), 5 locations (fronto-parietal, fronto-temporal, fronto-temporoparietal, centro-occipital and fronto-occipital), and 2 lateralizations (left and right hemispheres). Gender was included as a between-subjects factor.
The observation of the grand averages and the mean coherence values showed that the parietal and occipital areas were highly involved in perception of emotional pictures in comparison to neutral pictures, especially in the delta band. Accordingly, in order to show this difference, we had run a separate ANOVA including within-subject factors as 3 stimuli (pleasant, unpleasant and neutral) × 3 locations (fronto-parietal, centro-occipital and fronto-occipital) and 2 lateralizations (left and right hemispheres) and between-subjects factor as gender. The results of this ANOVA analysis were described in the results section whenever significant results were found for stimuli effect. Greenhouse-Geisser corrected p values were reported. Post-hoc comparisons were analyzed by Bonferroni test. All the results were reported with the significance level p < 0.05.
Results
Behavioral results
The subjects’ mean valence score for unpleasant pictures was 6.78 ± 1.13, and the valance values were significantly higher than for pleasant (2.93 ± 0.94; p < 0.05) and neutral images (5.16 ± 0.44; p < 0.05). Pleasant pictures had significantly lower valence scores than neutral pictures (p < 0.05). The mean of arousal scores for neutral images were 7.49 ± 1.42, and this value was significantly higher than for unpleasant pictures (4.79 ± 1.94; p < 0.05) and pleasant pictures (6.32 ± 1.82; p < 0.05). These results were in good accordance with previous findings related to IAPS research (Lang et al. 1999). Females’ mean valence score for unpleasant pictures (7.07 ± 0.87) was higher than the males’ mean valence score for unpleasant (6.47 ± 1.30) pictures. However, this difference was not statistically significant (p > 0.05). The mean valence scores for pleasant (male: 2.93 ± 0.55; female: 2.92 ± 1.23) and neutral pictures (male: 5.10 ± 0.39; female: 5.22 ± 0.50) were found to be very similar between genders. Females’ mean arousal score for pleasant pictures (6.60 ± 1.54) was higher than the males’ mean valence score for unpleasant (6.04 ± 2.09) pictures. However, this difference was not statistically significant (p > 0.05). The mean arousal scores for unpleasant (male: 4.96 ± 2.15; female: 4.61 ± 1.77) and neutral pictures (male: 7.42 ± 1.34; female: 7.56 ± 1.53) were found to be very similar between genders.
As we mentioned in the methods section, we also considered the luminance effect on the EEG recordings. Therefore, we were careful about having similar luminance levels for all types of stimuli. For the unpleasant pictures, the mean luminance value was 23.90 ± 4.48; for the pleasant pictures the mean luminance value was 22.50 ± 4.42; for the neutral pictures the mean luminance value was 24.00 ± 6.79. Luminance levels of different types of stimuli were not statistically different [F(2, 18) = 0.239; p > 0.05].
Results of the coherence analysis
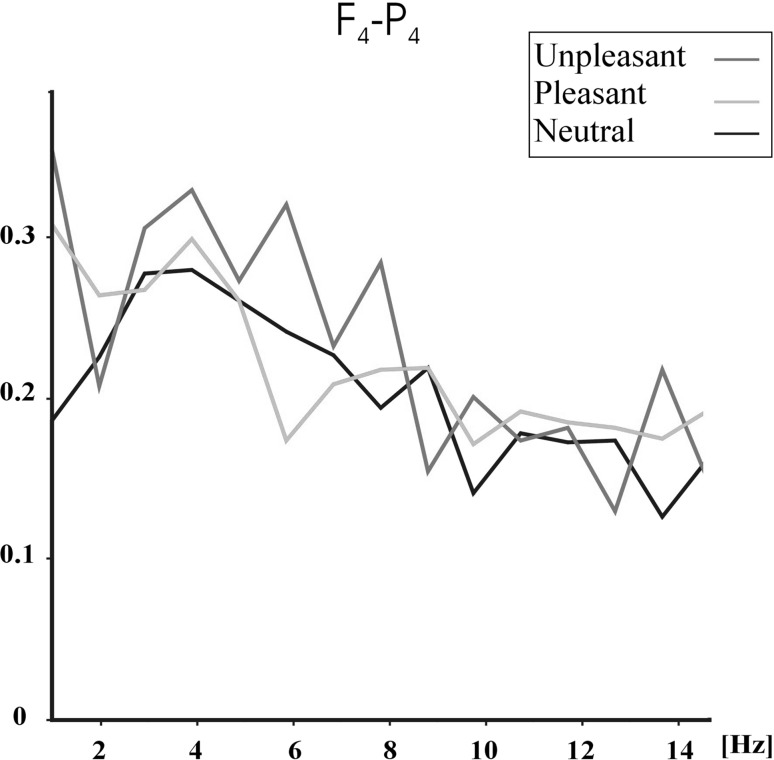
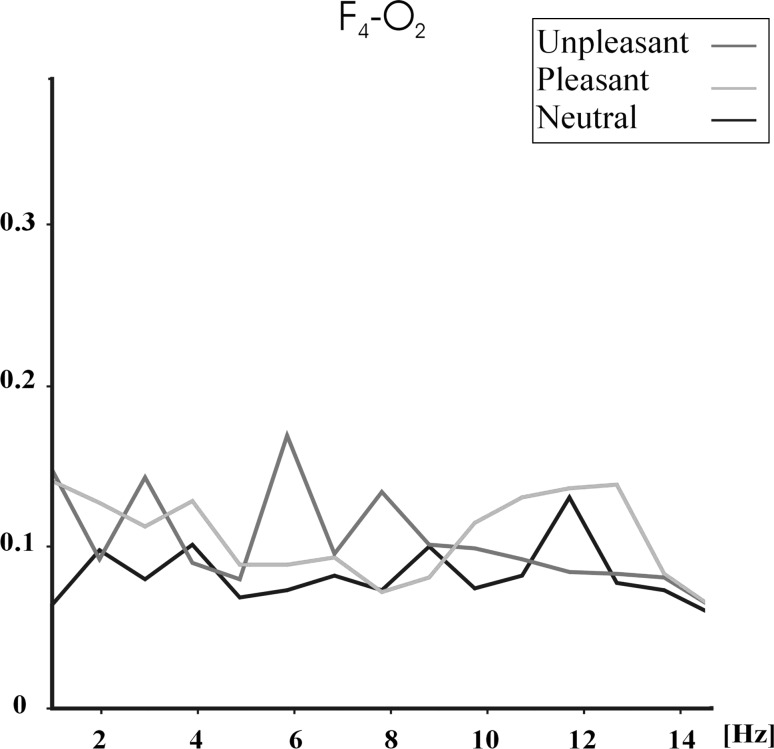
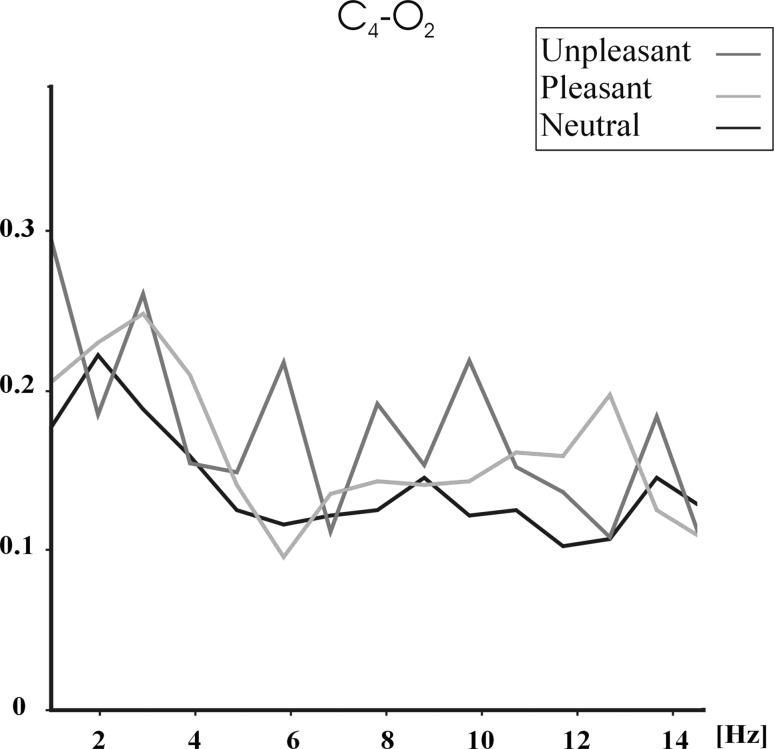
Figures 1, 2 and 3 show the grand average (N = 28) of event-related coherences upon application of unpleasant (red line), pleasant (blue line) and neutral (black line) pictures for F4–P4 (Fig. 1), F4–O2 (Fig. 2) and C4–O2 (Fig. 3) electrode pairs. Figures 1, 2 and 3 show that unpleasant pictures elicited higher theta (5–7 Hz) and alpha (7.8–12.7 Hz) coherence values than pleasant and neutral pictures over F4–P4, F4–O2 and C4–O2 electrode pairs. In the delta (1–3.5 Hz) frequency band, unpleasant pictures elicited higher coherence values than pleasant and neutral pictures over F4–P4 and F4–O2 electrode pairs. Unpleasant and pleasant pictures both elicited higher delta coherence than neutral pictures over C4–O2 electrode pair. Furthermore, the pictures show that unpleasant pictures mostly elicit higher alpha coherences in 7.8–9.8 Hz, while pleasant pictures elicit higher coherence values in 10.7–12.7 Hz.
Fig. 1.
Grand average of event-related coherences for F4–P4 electrode pair upon application of unpleasant, pleasant, and neutral IAPS pictures. Red line indicates unpleasant pictures, blue line indicates pleasant pictures, and black line indicates neutral pictures. (Color figure online)
Fig. 2.
Grand average of event-related coherences for F4–O2 electrode pair upon application of unpleasant, pleasant, and neutral IAPS pictures. Red line indicates unpleasant pictures, blue line indicates pleasant pictures, and black line indicates neutral pictures. (Color figure online)
Fig. 3.
Grand average of event-related coherences for C4–O2 electrode pair upon application of unpleasant, pleasant, and neutral IAPS pictures. Red line indicates unpleasant pictures, blue line indicates pleasant pictures, and black line indicates neutral pictures. (Color figure online)
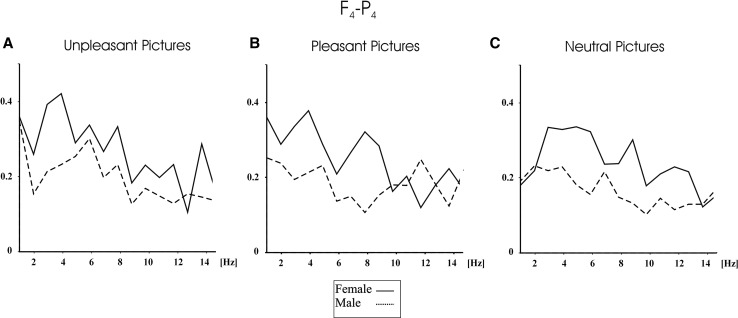
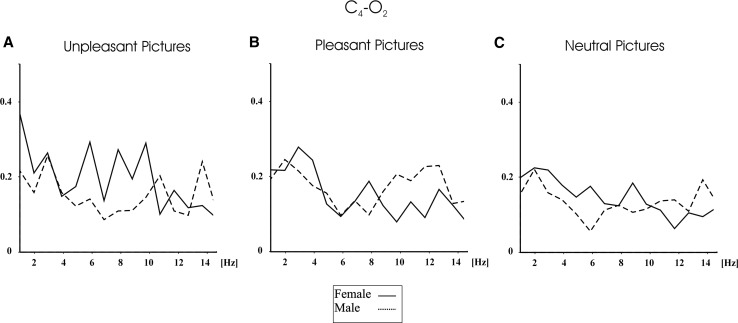
Furthermore, the grand averages of coherence measures showed that females had higher event-related coherence values than males, especially for the emotional pictures. Figure 4 shows the grand average of event-related coherences for female (N = 14) and for male subjects (N = 14) upon application of pleasant (upper part of the figure), neutral (middle of the figure), and unpleasant pictures (lower part of the figure) for F4–P4 electrode pairs, and Fig. 5 shows the grand average of event-related coherences for C4–O2 electrode pair. As can be seen in Figs. 4 and 5, female subjects had higher delta, theta, and alpha coherence values than males for pleasant, unpleasant, and neutral pictures. As can be seen in Fig. 5, the difference between males and females are more prominent for unpleasant pictures than neutral pictures. Figure 5 shows that the pleasant pictures elicited higher alpha coherence values in the 10.7–12.7 Hz for male subjects than female subjects.
Fig. 4.
Grand average of event-related coherences for F4–P4 electrode pair for male and female upon application of unpleasant (a), pleasant (b), and neutral (c) IAPS pictures. Dashed line indicates male participants, and bold line indicates female participants
Fig. 5.
Grand average of event-related coherences for C4–O2 electrode pair for male and female upon application of unpleasant (a), pleasant (b), and neutral (c) IAPS pictures. Dashed line indicates male participants, and bold line indicates female participants
The statistical analysis performed was in good accordance with the observation of grand averages described above. The significant results for the ANOVA analysis including 10 electrodes (F3–T7, F4–T8, F3–TP7, F4–TP8, F3–P3, F4–P4, F3–O1, F4–O2, C3–O1 and C4–O2) are as follows.
Statistical results of event related delta (1–3.5 Hz) coherence
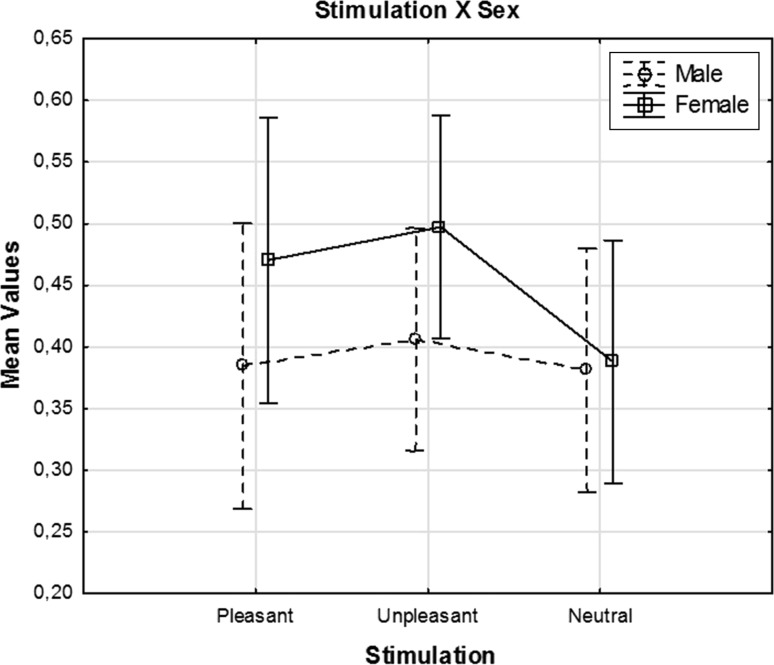
ANOVA revealed significant results for location [F(4, 104) = 86.00; p < 0.001]. Post-hoc comparisons showed that event-related coherence of fronto-temporal location was higher than all other locations (p < 0.05). In addition, coherence at fronto-temporo parietal was significantly higher than coherence at fronto-occipital (p < 0.05); coherence at fronto-parietal was significantly higher than coherence at fronto-occipital (p < 0.05); and coherence at centro-occipital location was significantly higher than coherence at fronto-occipital (p < 0.05). Test of between-subjects factors showed that females had significantly higher delta coherence values than males (p < 0.03). Figure 6 shows the mean delta coherence values of all electrodes for unpleasant, pleasant, and neutral stimulation. Solid line represents the mean delta coherence values for females, and dashed line represents the delta coherence values for males. As can be seen in the figure, the differences between females and males are mostly found in the emotional stimulations especially during the unpleasant stimulation. On the other hand, this difference is very small during neutral stimulation.
Fig. 6.
The mean delta coherence values upon presentation of unpleasant, pleasant, and neutral pictures. Solid line represents the mean delta coherence values for females, and dashed line represents the mean delta coherence values for males. Vertical bars denote ±standard errors
The observation of the grand averages and the mean coherence values showed that the parietal and occipital areas were highly involved in perception of emotional pictures in comparison to neutral pictures, especially in the delta band. Accordingly as we mentioned in the description of statistics, we had run a separate ANOVA (3 stimuli × 3 locations (fronto-parietal, centro-occipital and fronto-occipital) × 2 lateralizations; between-subjects factor: gender). The ANOVA analysis confirmed our observations by indicating that the effect of stimuli was significant. Delta coherence values over fronto-parietal, centro-occipital, and fronto-occipital were higher in response to unpleasant pictures in comparison to neutral pictures (p < 0.05). Location and gender effects were also significant showing similar results in the first ANOVA analysis.
Statistical results of event related theta (5–7 Hz) coherence
ANOVA revealed significant results for stimulation [F(2, 52) = 3.79; p < 0.04]. Post-hoc analysis showed that unpleasant pictures elicited significantly higher coherence values than pleasant and neutral pictures (p < 0.04). ANOVA revealed significant results for location [F(4, 104) = 47.35; p < 0.001]. Post-hoc analysis showed that fronto-temporal theta coherence values were higher than all other locations (p < 0.05 for all comparisons). In addition, theta coherence values at fronto-parietal electrode pairs were significantly higher than coherence values of fronto-temporoparietal, fronto-occipital, and centro-occipital locations (p < 0.05; p < 0.05; p < 0.05). Also, theta coherence values of fronto-temporoparietal location were significantly higher than theta coherence values at fronto-occipital electrode pairs (p < 0.05). Test of between-subjects showed that females had higher theta coherence values than men (p < 0.04).
Statistical results of event related alpha (7.8–12.7 Hz) coherence
ANOVA revealed significant results for location [F(4, 104) = 33.04; p < 0.001]. Post-hoc analysis showed that fronto-temporal alpha coherence values were higher than all other locations (p < 0.05 for all comparisons). In addition, alpha coherence values at fronto-parietal electrode pairs were significantly higher than coherence values of fronto-temporoparietal, fronto-occipital, and centro-occipital locations (p < 0.05; p < 0.05; p < 0.05). ANOVA revealed significant results for gender (p < 0.05). Post-hoc comparisons showed that females had higher alpha coherence values than males.
As can be seen in Fig. 5b, in the grand averages we observed that male subjects had higher alpha coherence values in 10.7–12.7 Hz frequency bands than females during pleasant pictures. Accordingly we also analyzed the alpha frequency in two sub-bands (7.8–9.8 and 10.7–12.7 Hz). ANOVA results showed similar results for within subject effects. However between subject effects showed differential results for lower and upper alpha frequency ranges. For lower alpha, ANOVA showed significant results for gender indicating that females had higher (7.8–9.8 Hz) alpha coherence values than males (p < 0.008). On the other hand, for the 10.7–12.7 Hz frequency band, there was a stimulation × sex effect (p < 0.05). Male subjects had higher 10.7–12.7 Hz alpha coherence values than females upon pleasant stimulation. The mean value of 10.7–12.7 Hz alpha coherence values for male subjects upon presentation of pleasant pictures was 0.360 (SE = 0.031) and it was 0.271 (SE = 0.055) for female subjects.
Discussion
The present study is one of the first studies to analyzed event-related coherence upon application of IAPS pictures. The results of the present study showed that, similar to local circuits, in the long-range connections of the brain, emotional stimuli elicited higher responses than neutral stimuli. Unpleasant stimuli elicited higher delta coherence values than neutral stimuli, especially over fronto-parietal, centro-occipital and fronto-occipital electrode pairs. Furthermore, unpleasant pictures elicited higher theta coherence values than pleasant and neutral pictures. The present study showed that female subjects had higher delta, theta, and alpha coherence values than male subjects. This difference was observed mostly for emotional pictures than for neutral pictures as presented in Fig. 6.
Increase of delta coherence during application of unpleasant pictures
Several studies in the literature showed that delta responses are involved in attention, perception, and decision-making (Başar et al. 2001; Bernat et al. 2007; Demiralp et al. 2001; Güntekin and Başar 2016; Knyazev 2012). Topological differences were reported according to the stimuli. In cognitive paradigms, frontal, central, and parietal delta responses were increased, and delta responses in occipital areas were relatively small in comparison to frontal and central areas. However, when the stimulus is a complex visual paradigm like perception of faces, facial expressions, and IAPS pictures, then an increase of occipital responses were reported (Balconi and Lucchiari 2006; Güntekin and Başar 2009, 2016). In the analysis of event-related coherences, increased delta coherence values upon application of cognitive load were reported for healthy young adults (Güntekin and Başar 2010a). While the event-related delta coherence increased upon cognitive load in healthy elderly subjects, this was not the case for Alzheimer’s disease patients (Başar et al. 2010; Güntekin et al. 2008).
In a recent review (Güntekin and Başar 2014), we have indicated that delta, theta, alpha, beta, and gamma responses were involved in the perception of affective pictures. According to the literature, high arousing (both pleasant and unpleasant) pictures elicited higher delta, theta, alpha, beta, and gamma responses than low arousing pictures. On the other hand, in the perception of unpleasant pictures, beta and gamma responses were more increased in very early time windows in comparison to neutral and/or pleasant pictures. Klados et al. (2009) and Balconi et al. (2009a, b) showed that high arousing pictures elicited greater event-related delta responses than low arousing pictures. Furthermore, these authors showed that females had higher delta responses than males. The results of the present manuscript are in good accordance with the work of Klados and co-workers. The present manuscript for the first time showed that, like in local circuits, in the long-range circuits of the brain, delta responses increased upon presentation of emotional pictures, especially upon presentation of unpleasant pictures. Delta coherence values of female subjects were higher than male subjects, and this difference was more prominent for emotional pictures than for neutral pictures. Miskovic and Schmidt (2010) analyzed event-related delta coherence in 1–6 s upon application of IAPS pictures. These authors found the significance level of the emotional effect for delta band as p > 0.09, which is very near to the significance level of p < 0.05. The number of subjects was 10 in their experiments. As they also discussed in their study, if they would have had more subjects included in their study, they might have found significant results for the delta frequency range.
Increase of theta coherence during application of unpleasant pictures
Theta responses were reported to be the dominant rhythms of the frontal cortex (Başar 1998; Westphal et al. 1990). On the other hand, increased occipital theta responses were reported on presentation of emotional paradigms (Aftanas et al. 2001, 2002; Başar et al. 2006; Güntekin and Başar 2009).
The present study showed that unpleasant pictures elicited significantly higher theta coherence values than pleasant and neutral pictures. On the contrary, Miskovic and Schmidt (2010) showed decreased theta coherence for pleasant and unpleasant compared to neutral picture viewing. This controversy could be due to difference of the time window in both studies. We have analyzed the first 800 ms after the picture viewing, while Miskovic and Schmidt (2010) analyzed the 1–6 s time window. The authors also mentioned this point in their study and commented that theta synchronization could be occurring in the initial stages of perception, which is the case in our study. In the literature, increase of event-related theta power upon application of emotional stimuli was reported for IAPS pictures and for facial expression stimuli (Balconi and Lucchiari 2009a, b; Knyazev et al. 2009; Aftanas et al. 2001; Sun et al. 2012; DeLaRosa et al. 2014). Aftanas et al. (2001) analyzed power spectrum upon application of IAPS pictures and showed greater left hemisphere theta power for unpleasant pictures and greater left hemisphere theta power for pleasant pictures. Sun et al. (2012) analyzed the power spectrum of theta response during viewing of Chinese Affective Picture System (CAPS). These authors indicated that theta synchronization was stronger for threatening cues than pleasant cues with significant effect in posterior regions, suggesting that the posterior theta synchronization reflects the evaluation of emotional significance of stimuli. DeLaRosa et al. (2014) showed that viewing an object that is perceived as either threatening or nonthreatening results in an early (300–475 ms) increase in theta-band EEG power over the occipital lobes, with the greatest power changes for the threatening images. Furthermore, these authors showed that there is a later (600 ms) theta power increase over the frontal region for both threatening and nonthreatening images, again with the greatest theta increase for the threatening images. The results of the mentioned studies showed that there were increases in theta responses in early time windows during emotional pictures. These studies analyzed event-related power spectrum for single electrodes. Our study further showed that connectivity in the brain increased in the theta frequency range during perception of unpleasant pictures, and this difference is mostly significant for female subjects.
Differentiation of alpha coherence between genders
In the spontaneous EEG analysis, increased left frontal alpha activity was associated with greater positive affect, and increased right frontal alpha activity was associated with negative affect (Davidson et al. 1979; Fox 1991; Hagemann et al. 2002; Harmon-Jones and Allen 1997; Jaworska et al. 2012, 2013; Sutton and Davidson 1997). Approach and withdrawal motivation were also reported to be related with alpha oscillations (Fox 1991; Harmon-Jones and Allen 1997; Sutton and Davidson 1997).
On the other hand, in the event-related potential studies, there was a lack of evidence for alpha asymmetry. Harmon-Jones (2007) indicated that emotional pictures did not evoke reliable shifts in asymmetrical cortical activation. Ponkänen and Hietanen (2012) also indicated that there was no alpha asymmetry during perception of happy and neutral facial expressions. In the present study, we did not find any asymmetry in the alpha frequency range. The main and interesting finding was that female subjects had higher alpha coherence values in 7.8–9.8 Hz than males. On the other hand, for the 10.7–12.7 Hz frequency band, there was a stimulation × sex effect (p < 0.05). Male subjects had higher 10.7–12.7 Hz alpha coherence values than females upon presentation of pleasant stimulation. To our knowledge, there was no previous study showing this frequency difference between males and females. Accordingly, this frequency difference between males and females should be further analyzed with application of different paradigms by trying to answer the question whether this difference is specific to emotional paradigms or does it also exist in different paradigms.
Previous studies showed that alpha responses were related with emotional processes. In the facial expression paradigms, increased alpha responses over temporal and occipital areas were reported (Güntekin and Başar 2007a). On the contrary, Balconi et al. (2009a, b) also found decrease of alpha power. Application of IAPS pictures also showed changes in alpha power. Aftanas et al. (2002) showed that moderate and high arousing IAPS pictures elicited higher alpha power than low arousing pictures. Huster et al. (2009) reported decreased alpha power during perception of negative IAPS pictures. Balconi et al. (2015) did not find any significant results between picture groups in the alpha power during perception of IAPS pictures. Up to now, there are no consistent results in the literature for either facial expression paradigms or for IAPS pictures as we also indicated in our recent review article in more detail (Güntekin and Başar 2014). This could be the result of different methodologies used in the experiments. More research is needed to clarify how the alpha responses change during application of IAPS pictures. The present report did not find differences between picture groups in long-range coherence values. However, we found significant gender effects; gender effects were mostly neglected in experiments in the literature.
In the present study, event-related beta and gamma coherence values were not analyzed. Previous research showed that both beta and gamma responses were involved in the emotional picture processing in very early time windows (0–300 ms); emotional pictures, especially negative emotional pictures (facial expressions or unpleasant pictures of IAPS), elicited higher beta and gamma responses. In a recent study, Başar et al. (2015) also concluded that there were multiple frequency and time windows in the gamma frequency range during application of more complex paradigms. Accordingly, beta and gamma oscillations should be investigated in multiple time and frequency windows. This conclusion makes it difficult to analyze the low and high frequencies together with same methodology. Delta responses mostly occur in the 0–600 or 0–800 ms time period, changing according to the paradigm. Theta responses also mostly occur in the 0–500 ms time window. On the other hand, alpha responses could occur in the early time window (0–400 ms), and there may also be second discharge of alpha oscillations in the late time windows. Therefore, in the present study in order to successfully analyze delta, theta and alpha coherences, the 0–800 ms time window was chosen. Since beta and gamma responses have more than one or two peaks in the 0–800 ms time window, analyzing beta and gamma coherences in 0–800 ms would not give the right conclusions. In the future, detailed analysis of beta and gamma coherences in multiple time and frequency windows is needed. This detailed analysis is strongly recommended and could show important results on how emotional picture processing is reflected in the high frequency connected networks.
Conclusions
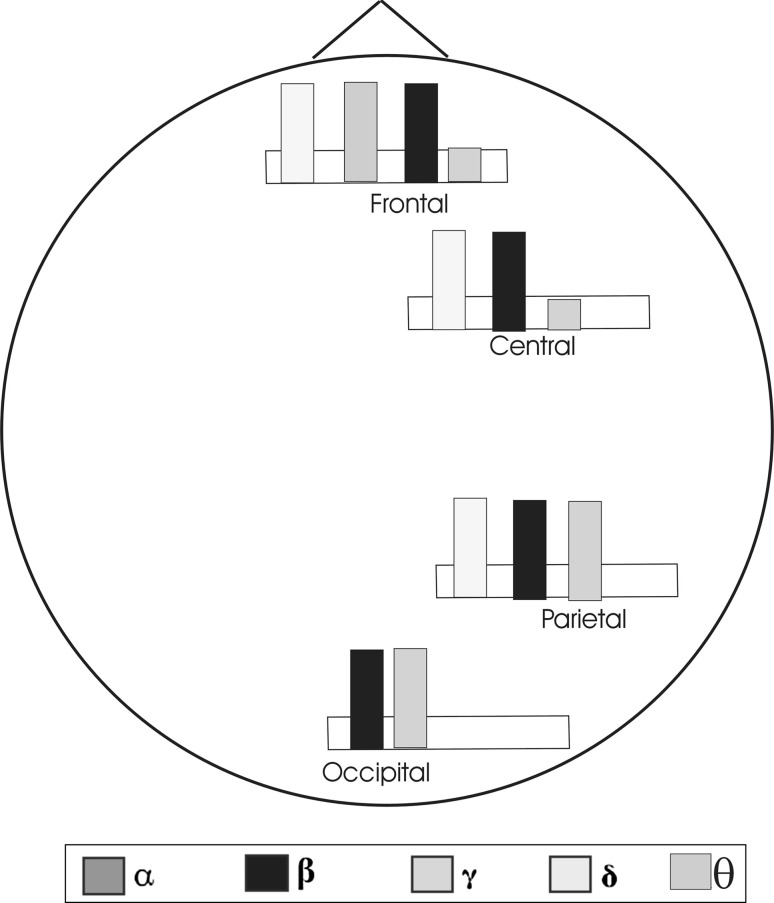
Figure 7 represents a diagram of event related oscillatory responses upon presentation of unpleasant pictures (Clair diagrams, see Başar et al. 2014; Başar and Düzgün 2016; for further information). The diagram was shaped according to the results of previous studies (Balconi et al. 2009b; Güntekin and Başar 2014; Güntekin and Tülay 2014; Klados et al. 2009; Woodruff et al. 2011). In general unpleasant pictures elicited higher delta, theta, beta and gamma responses than neutral and/or pleasant pictures. There are some contradictory results on alpha response during presentation of IAPS pictures that is why diagram does not include alpha responses elicited during perception of unpleasant pictures. As we also mention in the previous section Aftanas et al. (2002) showed that moderate and high arousing IAPS pictures elicited higher alpha power than low arousing pictures. Huster et al. (2009) reported decreased alpha power during perception of negative IAPS pictures. On the other hand Balconi et al. (2015) did not find any significant results between picture groups in the alpha power during perception of IAPS pictures. There are also few studies analyzing theta responses during perception of IAPS pictures. Future studies are needed to complete the whole diagram including all frequency bands in different time windows. The general increase of oscillatory responses during perception of unpleasant pictures could be explained from a phylogenetic view point. There could be a phylogenetic advantage associated with faster processing of and heightened physiological response to negative emotional stimuli.
Fig. 7.
Diagrams for event related oscillatory responses (delta, theta, alpha, beta and gamma) upon presentation of unpleasant pictures. The diagram was shaped according to the results of previous studies (Balconi et al. 2009b; Güntekin and Basar 2010a, b; Güntekin and Tülay 2014; Klados et al. 2009; Woodruff et al. 2011)
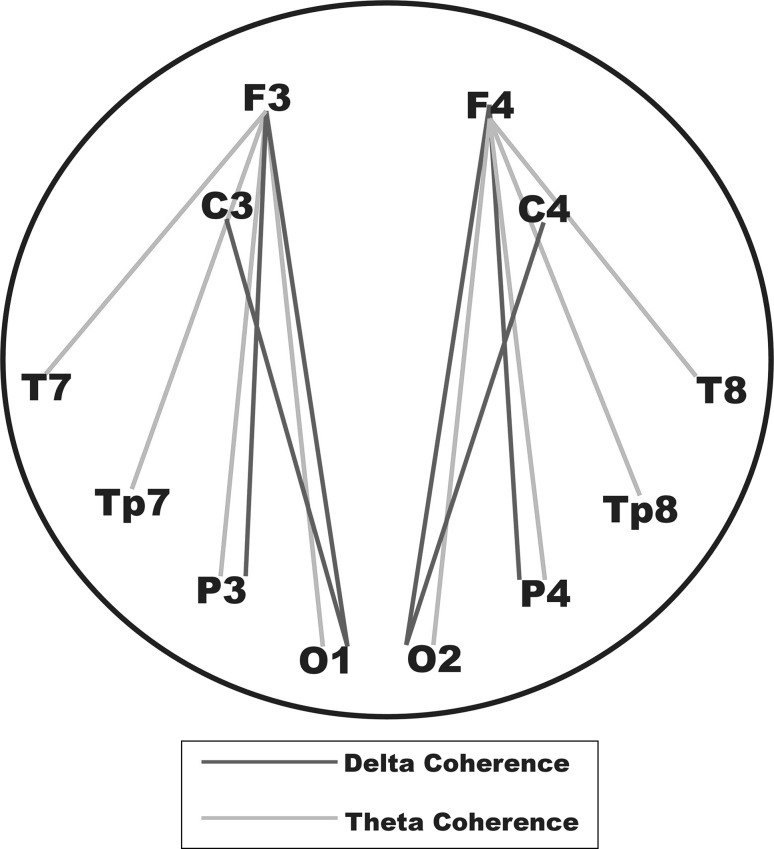
Figure 8 represents the most important findings of the present study schematically for unpleasant picture processing. In general, unpleasant pictures elicited higher coherence values than neutral and/or pleasant pictures. Unpleasant pictures elicited higher delta coherence mostly over fronto-parietal, centro-occipital, and fronto-occipital electrode pairs. On the other hand, there were no specific electrode pairs for the increase of theta coherences. ANOVA results showed significant results for stimulation (see “Results” section), which means that theta coherence values were higher in general for unpleasant pictures than for neutral and pleasant pictures.
Fig. 8.
Schematic representation of results of delta and theta coherence for the unpleasant picture processing
The present study shows that:
Brain’s connectivity changes according to the emotional pictures presented to the subjects.
Unpleasant pictures elicited higher delta and theta coherence values than neutral pictures.
Furthermore, unpleasant pictures had increased theta coherence than pleasant pictures.
Overall, female subjects had higher delta and theta coherence values than males.
There was frequency differentiation between genders in the alpha frequency.
In the present study, it was shown that the differentiation of emotional pictures is manifested in delta, theta, and alpha frequency ranges. According to this manifold of results, we conclude that emotional signal processing and differentiation of emotional pictures elicits a very complicated selectivity in the brain. In future more detail analysis could be performed by analyzing all frequency bands in different time windows. The present study mostly focused on early responses during presentation of emotional pictures. Early and late time windows could be analyzed to understand how the brain connectivity change in different time windows during perception of emotional pictures. The present study found significant gender differences. Accordingly analysis of gender difference is strongly recommended in the research of emotional paradigms.
According to these results, we may comment that increased valence and arousal caused increased brain activity. It seems that not just single sources but functional networks were also activated during perception of emotional pictures (Aftanas et al. 1998a, b; Bornas et al. 2015; Güntekin and Başar 2014; Yuvaraj and Murugappan 2016; Yuvaraj et al. 2015, 2016). Perception of emotional pictures is a notion of whole brain work.
Footnotes
This work was presented as poster abstract in the conferences: 70th Annual Scientific Meeting of the Society-of-Biological-Psychiatry, Toronto, Canada and 55th Annual meeting of Society for Psychophysiological Research, 29 September–04 October 2015, Seattle, USA.
References
- Aftanas LI, Lotova NV, Koshkarov VI, Makhnev VP, Mordvintsev YN, Popov SA. Non-linear dynamic complexity of the human EEG during evoked emotions. Int J Psychophysiol. 1998;28(1):63–76. doi: 10.1016/S0167-8760(97)00067-6. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Lotova NV, Koshkarov VI, Popov SA. Non-linear dynamical coupling between different brain areas during evoked emotions: an EEG investigation. Biol Psychol. 1998;48(2):121–138. doi: 10.1016/S0301-0511(98)00015-5. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Varlamov AA, Pavlov SV, Makhnev VP, Reva NV. Affective picture processing: event-related synchronization within individually defined human theta band is modulated by valence dimension. Neurosci Lett. 2001;303:115–118. doi: 10.1016/S0304-3940(01)01703-7. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Varlamov AA, Pavlov SV, Makhnev VP, Reva NV. Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int J Psychophysiol. 2002;44:67–82. doi: 10.1016/S0167-8760(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Balconi M, Lucchiari C. EEG correlates (event-related desynchronization) of emotional face elaboration: a temporal analysis. Neurosci Lett. 2006;392:118–123. doi: 10.1016/j.neulet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Balconi M, Brambilla E, Falbo L. BIS/BAS, cortical oscillations and coherence in response to emotional cues. Brain Res Bull. 2009;80:151–157. doi: 10.1016/j.brainresbull.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Balconi M, Brambilla E, Falbo L. Appetitive vs. defensive responses to emotional cues. Autonomic measures and brain oscillation modulation. Brain Res. 2009;1296:72–84. doi: 10.1016/j.brainres.2009.08.056. [DOI] [PubMed] [Google Scholar]
- Balconi M, Grippa E, Vanutelli ME. What hemodynamic (fNIRS), electrophysiological (EEG) and autonomic integrated measures can tell us about emotional processing. Brain Cogn. 2015;95:67–76. doi: 10.1016/j.bandc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Basar E. Why the concept of “quantum brain” was not discovered in 1940s. NeuroQuantology. 2010;8:322–336. [Google Scholar]
- Başar E. Brain Functions and Oscillations: I. Brain oscillations. Principles and approaches. Berlin: Springer; 1998. [Google Scholar]
- Başar E, Düzgün A. The CLAIR model: extension of Brodmann areas based on brain oscillations and connectivity. Int J Psychophysiol. 2016;103:185–198. doi: 10.1016/j.ijpsycho.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/S0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B, Oniz A. Principles of oscillatory brain dynamics and a treatise of recognition of faces and facial expressions. Prog Brain Res. 2006;159(06):43–62. doi: 10.1016/S0079-6123(06)59004-1. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B, Tülay E, Yener GG. Evoked and event related coherence of Alzheimer patients manifest differentiation of sensory-cognitive networks. Brain Res. 2010;1357:79–90. doi: 10.1016/j.brainres.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Başar E, Düzgün A, Güntekin B. A proposal to extend Brodmann’s areas concept to a new model. Neuroquantology. 2014;12:1–9. [Google Scholar]
- Başar E, Tülay E, Güntekin B. Multiple gamma oscillations in the brain: a new strategy to differentiate functional correlates and P300 dynamics. Int J Psychophysiol. 2015;95(3):406–420. doi: 10.1016/j.ijpsycho.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time–frequency ERP activity from a visual oddball task using PCA. Int J Psychophysiol. 2007;64:62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornas X, Fiol-Veny A, Balle M, Morillas-Romero A, Tortella-Feliu M. Long range temporal correlations in EEG oscillations of subclinically depressed individuals: their association with brooding and suppression. Cogn Neurodyn. 2015;9(1):53–62. doi: 10.1007/s11571-014-9313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Schwartz GE, Saron C, Bennett J, Goldman DJ. Frontal versus parietal EEG asymmetry during positive and negative affect. Psychophysiology. 1979;16:202–203. [Google Scholar]
- DeLaRosa BL, Spence JS, Shakal SK, Motes MA, Calley CS, Calley VI, Hart J, Jr, Kraut MA. Electrophysiological spatiotemporal dynamics during implicit visual threat processing. Brain Cogn. 2014;91:54–61. doi: 10.1016/j.bandc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Başar-Eroğlu C, Başar E. Wavelet analysis of oddball P300. Int J Psychophysiol. 2001;39(2–3):221–227. doi: 10.1016/S0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA. If it’s not left, it’s right. Electroencephalograph asymmetry and the development of emotion. Am Psychol. 1991;46:863–872. doi: 10.1037/0003-066X.46.8.863. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M, Yordanova J, Kolev V, Domínguez-Borràs J, Escera C. Tuning the brain for novelty detection under emotional threat: the role of increasing gamma phase-synchronization. NeuroImage. 2010;49:1038–1044. doi: 10.1016/j.neuroimage.2009.07.059. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Emotional face expressions are differentiated with brain oscillations. Int J Psychophysiol. 2007;64:91–100. doi: 10.1016/j.ijpsycho.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Gender differences influence brain’s beta oscillatory responses in recognition of facial expressions. Neurosci Lett. 2007;424:94–99. doi: 10.1016/j.neulet.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Facial affect manifested by multiple oscillations. Int J Psychophysiol. 2009;71(1):31–36. doi: 10.1016/j.ijpsycho.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. A new interpretation of P300 responses upon analysis of coherences. Cogn Neurodyn. 2010;4:107–118. doi: 10.1007/s11571-010-9106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Event-related beta oscillations are affected by emotional eliciting stimuli. Neurosci Lett. 2010;483(2010):173–178. doi: 10.1016/j.neulet.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia. 2014;58:33–51. doi: 10.1016/j.neuropsychologia.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Review of evoked and event-related delta responses in the human brain. Int J Psychophysiol. 2016;103:43–52. doi: 10.1016/j.ijpsycho.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Tülay E. Event related beta and gamma oscillatory responses during perception of affective pictures. Brain Res. 2014;1577:45–56. doi: 10.1016/j.brainres.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Saatçi E, Yener G. Decrease of evoked delta, theta and alpha coherences in Alzheimer patients during a visual oddball paradigm. Neurosci Lett. 2008;424(2):94–99. doi: 10.1016/j.neulet.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting EEG asymmetry reflect a trait? An application of latent state-trait theory. J Pers Soc Psychol. 2002;82:619–641. doi: 10.1037/0022-3514.82.4.619. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Trait anger predicts relative left frontal cortical activation to anger-inducing stimuli. Int J Psychophysiol. 2007;66:154–160. doi: 10.1016/j.ijpsycho.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol. 1997;106:159–163. doi: 10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Stevens S, Gerlach AL, Rist F. A spectral analytic approach to emotional responses evoked through picture presentation. Int J Psychophysiol. 2009;72(2):212–216. doi: 10.1016/j.ijpsycho.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Berrigan L, Fisher D, Ahmed AG, Gray J, Bradford J, Korovessis A, Fedoroff P, Knott V. A pilot study of electrocortical activity in dysfunctional anger: decreased frontocortical activation, impaired attention control, and diminished behavioral inhibition. Aggress Behav. 2012;38(6):469–480. doi: 10.1002/ab.21449. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Berrigan L, Ahmed AG, Gray J, Korovessis A, Fisher DJ, Bradford J, Federoff P, Knott VJ. The resting electrophysiological profile in adults with ADHD and comorbid dysfunctional anger: a pilot study. Clin EEG Neurosci. 2013;44:95–104. doi: 10.1177/1550059412465607. [DOI] [PubMed] [Google Scholar]
- Keil A, Müller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin Neurophysiol. 2001;112:2057–2068. doi: 10.1016/S1388-2457(01)00654-X. [DOI] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Moratti S, Ray WJ. Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. NeuroImage. 2007;36:472–479. doi: 10.1016/j.neuroimage.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klados MA, Frantzidis C, Vivas AB, Papadelis C, Lithari C, Pappas C, Bamidis PB (2009) A framework combining delta event-related oscillations (EROs) and synchronization effects. Compu Intell Neurosci 16. Article ID 549419 [DOI] [PMC free article] [PubMed]
- Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic an motivational processes. Neurosci Biobehav Rev. 2012;36(1):677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskoj-Plusnin JY, Bocharov AV. Event-related delta and theta synchronization during explicit and implicit emotion processing. Neuroscience. 2009;164:1588–1600. doi: 10.1016/j.neuroscience.2009.09.057. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): technical manual and affective ratings. Gainesville: University of Florida, Center for Research in Psychophysiology; 1999. [Google Scholar]
- Martini N, Menicucci D, Sebastiani L, Bedini R, Pingitore A, Vanello N, Milanesi M, Landini L, Gemignani A. The dynamics of EEG gamma responses to unpleasant visual stimuli: from local activity to functional connectivity. NeuroImage. 2012;60:922–932. doi: 10.1016/j.neuroimage.2012.01.060. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA. Cross-regional cortical synchronization during affective image viewing. Brain Res. 2010;1362:102–111. doi: 10.1016/j.brainres.2010.09.102. [DOI] [PubMed] [Google Scholar]
- Müller MM, Keil A, Gruber T, Elbert T. Processing of affective pictures modulates right-hemispheric gamma band EEG activity. Clin Neurophysiol. 1999;110:1913–1920. doi: 10.1016/S1388-2457(99)00151-0. [DOI] [PubMed] [Google Scholar]
- Nunez PL. EEG coherence measures in medical and cognitive science: a general overview of experimental methods, computer algorithms, and accuracy. In: Witte H, Zwiener U, Schack B, Doering A, editors. Quantitative and topological EEG and MEG analysis. Jena: Universitätsverlag Druckhaus Mayer; 1997. pp. 385–392. [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol Psychol. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, 3rd, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22:9502–9512. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Saatçi E, Tunca Z, Basar E. Disturbance in long distance gamma coherence in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):861–865. doi: 10.1016/j.pnpbp.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Atagün I, Turp B, Basar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord. 2011;132:325–332. doi: 10.1016/j.jad.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? Are view of how face perception and attention interact? Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Ponkänen LM, Hietanen JK. Eye contact with neutral and smiling faces: effects on autonomic responses and frontal EEG asymmetry. Front Hum Neurosci. 2012;6:122. doi: 10.3389/fnhum.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Sun B, Wang B, Gong H. The processing bias for threatening cues revealed by event-related potential and event-related oscillation analyses. Neuroscience. 2012;203:91–98. doi: 10.1016/j.neuroscience.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychol Sci. 1997;8:204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x. [DOI] [Google Scholar]
- Westphal KP, Grozinger B, Diekmann V, Scherb W, Reess J, Leibing U, Kornhuber HH. Slower theta activity over the midfrontal cortex in schizophrenic patients. Acta Psychiatry Scand. 1990;81:132–138. doi: 10.1111/j.1600-0447.1990.tb06465.x. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Daut R, Brower M, Bragg A. Electroencephalographic alpha-band and beta-band correlates of perspective-taking and personal distress. NeuroReport. 2011;22:744–748. doi: 10.1097/WNR.0b013e32834ab439. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Barbera D, Von Oepen R. Task-related dissociation of EEG β enhancement and suppression. Int J Psychophysiol. 2016;99:18–23. doi: 10.1016/j.ijpsycho.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Yuvaraj R, Murugappan M. Hemispheric asymmetry non-linear analysis of EEG during emotional responses from idiopathic Parkinson’s disease patients. Cogn Neurodyn. 2016;10(3):225–234. doi: 10.1007/s11571-016-9375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuvaraj R, Murugappan M, Ibrahim NM, Sundaraj K, Omar MI, Mohamad K, Palaniappan R, Satiyan M. Inter-hemispheric EEG coherence analysis in Parkinson’s disease: assessing brain activity during emotion processing. J Neural Transm. 2015;122(2):237–252. doi: 10.1007/s00702-014-1249-4. [DOI] [PubMed] [Google Scholar]
- Yuvaraj R, Murugappan M, Acharya UR, Adeli H, Ibrahim NM, Mesquita E. Brain functional connectivity patterns for emotional state classification in Parkinson’s disease patients without dementia. Behav Brain Res. 2016;298:248–260. doi: 10.1016/j.bbr.2015.10.036. [DOI] [PubMed] [Google Scholar]