Abstract
ALS is a devastating disease resulting in degeneration of motor neurons (MNs) in the brain and spinal cord. The survival of MNs strongly depends on surrounding glial cells and neurotrophic support from muscles. We previously demonstrated that boundary cap neural crest stem cells (bNCSCs) can give rise to neurons and glial cells in vitro and in vivo and have multiple beneficial effects on co-cultured and co-implanted cells, including neural cells. In this paper, we investigate if bNCSCs may improve survival of MNs harboring a mutant form of human SOD1 (SOD1G93A) in vitro under normal conditions and oxidative stress and in vivo after implantation to the spinal cord. We found that survival of SOD1G93A MNs in vitro was increased in the presence of bNCSCs under normal conditions as well as under oxidative stress. In addition, when SOD1G93A MN precursors were implanted to the spinal cord of adult mice, their survival was increased when they were co-implanted with bNCSCs. These findings show that bNCSCs support survival of SOD1G93A MNs in normal conditions and under oxidative stress in vitro and improve their survival in vivo, suggesting that bNCSCs have a potential for the development of novel stem cell-based therapeutic approaches in ALS models.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-016-0505-8) contains supplementary material, which is available to authorized users.
Keywords: Amyotrophic lateral sclerosis, Neurodegeneration, Neuroglia, Oxidative stress, Transplantation
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive, neurodegenerative disorder affecting primarily upper and lower motor neurons (MNs), and usually leading to the death of the patient, most commonly due to respiratory failure. The glutamate signaling antagonist Riluzole has a measurable positive effect on disease progression and is relatively free of side effects. Riluzole acts by attenuating excitotoxic impact on endangered MNs, and is currently the only available approved ALS treatment, but offers not more than a few months extended life expectancy [1]. Riluzole exerts a wide range of neural effects which can influence neuronal activity and survival, although the mechanism of action still remains controversial [2]. About 10% of ALS cases are familial and several genetic mutations linked to this group of patients have been identified. Mutations in superoxide dismutase (SOD)1 are found in about 20% of familial cases and in a few percent of sporadic ALS cases [3]. SOD1 is an enzyme that helps in the catalysis of dismutation of superoxide to molecular oxygen and hydrogen peroxide [3]. The association between mutant SOD1 and ALS onset, and the subsequent generation of transgenic animals harboring human mutant and non-mutant forms of SOD1, have played a pivotal role and remains a cornerstone in research on the pathogenesis of ALS and in the search for novel therapeutic treatment of this disease [3]. To date, more than 150 SOD1 mutations have been identified and SOD1G93A is the most widely studied model for ALS pathogenesis [3].
Mutant SOD1 affects not only intrinsic properties of MNs, but also results in a neurotoxic astroglial phenotype, inducing degeneration of wild type MNs in vitro [4–7] and in vivo after transplantation into the spinal cord of rats [8]. Furthermore, astrocytes from patients with sporadic as well as familial ALS exert a toxic effect on primary MNs in vitro [9, 10]. Conversely, deletion of mutant SOD1 in astrocytes of transgenic mice significantly delays disease progression [11]. Wild type astrocytes release factors that promote survival of co-cultured mutant SOD1 MNs [12]. Implantation of stem/progenitor cell-derived astrocytes into the spinal cord or ventricular system of transgenic mice with mutant SOD1 promotes MN survival and delays disease progression [13, 14].
Boundary cap neural crest stem cells (bNCSCs) is a transient neural crest-derived group of cells that are located at the dorsal root entry zone (DREZ) [15]. These cells self-renew and show multipotency in culture and are able to differentiate into sensory neurons and Schwann cells in vitro and in vivo [15], as well as into astrocytes in vitro and after transplantation into the immature mouse brain [16]. We have previously shown remarkable, beneficial effects of bNCSCs on co-cultured [17, 18] and co-implanted pancreatic beta-cells [19], as well as excitotoxically challenged spinal cord neurons in vitro (unpublished observation). Interestingly, another type of NCSCs, the hair follicle stem cells, did not have corresponding positive effects on co-cultured cells [20].
These findings prompted us to test if bNCSCs have a beneficial effect on co-cultured and co-implanted SOD1G93AMNs, generated from SOD1G93A mouse embryonic stem cells (mESCs). These cell lines express green fluorescent protein (GFP) under the control of the promoter for the MN specific transcription factor HB9 (HB9::GFP cells), allowing their identification after directed differentiation to MN progenitors [21]. Furthermore, the survival of MN from the same SOD1G93A mESC line was previously analyzed in vitro under normal conditions [21]. Here, we investigate their survival in normal conditions and under oxidative stress and the effect of bNCSCs on SOD1G93A MN survival. Generation of MNs from mESCs results also in abundant generation of astrocytes. These cells express glutamate aspartate transporter (GLAST) and can be identified by anti-GLAST antibodies [22]. To exclude the negative effect from surrounding SOD1G93A astrocytes on SOD1G93A MNs, we used magnetic activated cell sorting (MACS) to eliminate GLAST-positive cells from SOD1G93A mESC cultures. To compare the effect of bNCSCs on SOD1G93A MN survival with mESC derived astrocytes, we used astrocytes differentiated from a non-SOD1 mutated glial fibrillary acidic protein (GFAP)::CD14 mESC line [23].
Our findings show that bNCSCs exert a significant survival promoting effect on co-cultured and co-implanted SOD1G93A MNs.
Material and methods
Mouse embryonic stem cell (mESC) cultures
Mouse embryonic stem cell (mESC) lines harboring human wild type SOD1 (SOD1WT) or mutant SOD1 (SOD1G93A) were a kind gift from Dr. Kevin Eggan (Harvard Stem Cell Institute). The risk of tumor formation for implanted cells from low passages is minimal. These cell lines carry green fluorescent protein (GFP) under the control of the promoter for the MN specific transcription factor HB9 (HB9::GFP cells) [21]. We used the SOD1WT and SOD1G93A mESC lines to derive GFP+ MNs.
For MN differentiation, a previously published protocol with small modifications was used [24]. Cells were cultured in ADFNB medium consisting of Advanced D-MEM/F12:Neurobasal (1:1), 1x GlutaMAX supplement (Invitrogen), 1x B27 supplement (Invitrogen), 1x N2 supplement (Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma) to form embryoid bodies (EBs), and supplemented with 0.1 μM of retinoic acid (RA, Sigma) and 0.5 μM of sonic hedgehog (Shh) agonist Ag1.3 (Curis) every other day. After 7 days of pre-differentiation, EBs were subjected to MACS (see below), and thereafter either cultured alone, co-cultured with bNCSCs, or with mESC-derived astrocytes.
For in vitro MN differentiation, EBs were enzymatically dissociated with TrypLE™ Express (Gibco) and seeded on pre-coated coverslips with 0.01% poly-l-ornithine (Sigma) followed by 10 μg/mL laminin (Sigma). Cells were seeded at a density of 5 × 104 cells/coverslip in 24 well plates with ADFNB cell medium supplemented with 10 ng/mL of CNTF (Miltenyi Biotec) and GDNF (Miltenyi Biotec). 50% of the medium was replaced with fresh medium every other day until the cultures were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 100 mM Na2HPO4, 18 mM KH2PO4) at the indicated time points.
bNCSC culture
bNCSCs were generated from transgenic mice harboring red fluorescent protein (RFP) under the universal actin promoter [25] according to previously published protocols [15, 26]. Neurospheres from passages 4 to 5 were trypsinized to obtain single cell suspensions for MACS for subsequent co-culture and co-implantation with SOD1G93A MNs.
Derivation of astrocytes from GFAP::CD14 mESC culture
Astrocytes were generated from GFAP::CD14 mESCs (kind gift from Dr. Ivo Lieberam, Kings College, London), using MACS as has been previously described [23] with minor modifications. At day 7 of differentiation, the EBs were re-plated into T75 flasks (Sarstedt) pre-coated with 10 μg/mL laminin and cultured until day 12 in ADFNB medium. The monolayer formed by EBs was treated with TrypLE™ Express on day 12 and a single cell suspension was prepared for MACS. Anti-CD14 antibody conjugated with magnetic microbeads was used according to the manufacturers protocol (Miltenyi Biotec).
Magnetic activated cell sorting (MACS)
Anti-GLAST (ACSA-1) antibodies were used for MACS according to the manufacturers protocol (Miltenyi Biotec). bNCSCs and GFAP::CD14 EBs were subjected to MACS to obtain astrocyte cultures. SOD1G93A EBs were subjected to MACS using anti-GLAST to deplete SOD1G93A MN cultures from SOD1G93A astrocytes.
Culture suspensions containing around 3 × 106 cells were labeled with anti-GLAST antibodies. Thereafter, anti-mouse IgG magnetic microbeads (Miltenyi Biotec) were applied to the cells for 15 minutes at 4 °C. Cells were re-suspended in MACS buffer (Miltenyi Biotec) and the cell suspension was loaded onto a MACS separation column (Miltenyi Biotec) and placed in the magnetic field of a MACS separator.
The column was rinsed three times with 0.5 mL of MACS buffer. In the case of the SOD1G93A cell line, the GLAST-positive fraction was removed to minimize the influence of SOD1G93A astrocytes on SOD1G93A MNs. For bNCSCs and GFAP::CD14 EBs, the GLAST positive fractions were used for the experiments.
For differentiation assays, 5 × 104 of SOD1G93A cells were seeded on 0.01% poly-l-ornithine and 10 μg/mL laminin coated coverslips. In case of co-cultures, around 2 x 104 bNCSCs or GFAP::CD14 mESC-derived astrocytes were seeded first followed by SOD1G93A MNs.
Oxidative stress assay
Oxidative stress was induced by hydrogen peroxide (H2O2) in SOD1WT MNs and SOD1G93A MNs plated alone or together with bNCSCs. In addition, bNCSCs were treated alone with H2O2 at similar concentrations to test their survival capacity under these conditions. On day 4 of differentiation assay, H2O2 (60 μM, 150 μM, 300 μM) was added for 3 hours to the cultures. Thereafter, the coverslips were fixed and analyzed for MN or bNCSC survival (GFP-positive or RFP-positive cells, respectively). For each condition at least three experiments were performed.
Animals
Transplantations were performed in Crl:NU(NCr)-Foxn1nu (nude; nu/nu) NMRI adult male mice (n = 12), (body weight 25–35 g; Möllegaard and Bomholgard Breeding and Research Centre, M&B A/S, Bomholt, Denmark, http://www.taconic.com). The serial sections were from the spinal cords of six animals − three of which received bNCSCs and SOD1G93A MN precursors (treatment group) and three only SOD1G93A MN precursors (control group) − were analyzed under confocal microscope for MN survival and glial response. All animal experiments were approved by the Local Ethical Committee for Animal Experimentation, Uppsala, as required by Swedish Legislation and in accordance with European Union Directives.
Surgery
The animals were anesthetized by spontaneous inhalation of Isoflurane. After onset of anesthesia the mouse was placed on its stomach on a heating pad (36 °C) and the skin cleaned with ethanol (70%). Following a procedure previously established in rats [27], an incision was made in the midline of the neck skin and local anesthetic (Xylocain©, 10 mg/ml) applied to the wound. After blunt dissection of the superficial and deep back muscles, the laminae of the cervical vertebrae were exposed. The vertebra prominens (C7) was held fixed with a pair of forceps, a partial laminectomy was made of vertebrae C3 to C5, and the exposed meninges were gently opened. 4 μL (2 injections of 2 μL each, around 50 000 cells per mouse) of equal proportion of bNCSCs and SOD1G93A MN precursors were injected into the left spinal cord using a Hamilton syringe with a metal needle (26 gauge) attached to a stereotactic frame and connected to an infusion pump (KD Scientific Legato 130). For the control group an equal amount of cells containing only SOD1G93A MN precursors were injected. The needle was kept in place for 2 minutes before removal. The wound was closed in layers using ethilon nylon suture 6.0 and 4.0, respectively. The animals were given 50 μl buprenorphine (Temgesic©) subcutaneously every 12 hours for 3 days after the operation.
Seven days after surgery the animals were re-anesthetized with an intraperitoneal injection of a mixture of ketamine, xylazine and acepromazine [28], and perfused via the left ventricle with warm saline solution (~38 °C) followed by a cold (~4 °C) fixative solution consisting of 4% formaldehyde (vol/vol), 14% saturated picric acid (wt/vol) in phosphate buffered saline (PBS; pH 7.35-7.45). The relevant part of the cervical spinal cord was removed, placed in fixative solution for 4 hours, and thereafter cryoprotected overnight in PBS containing 15% sucrose. The following day the tissue was placed in TissueTech™ and frozen in liquid nitrogen. Transverse sections (14 μm) were cut on a cryostat, collected on SuperFrost™ Plus slides (Menzel-Gläser, Braunschweig, Germany), and processed for immunohistochemistry and microscopic analysis as described below.
Immunohistochemistry
Cells were fixed in 4% phosphate-buffered paraformaldehyde at room temperature (RT) for 5 minutes, washed and left in blocking solution (1% bovine serum albumin, 0.3% Triton X-100 and 0.1% NaN3 in PBS) for 60 minutes, and then incubated with primary antibodies overnight at 4 °C, followed by the appropriate secondary antibodies for 1 hour at RT (Table 1). During the final wash, the cells were incubated with Hoechst 33342 (1:10000; Invitrogen) to label cell nuclei, and then mounted on a glass slide for analysis. bNCSC neurospheres were fixed for 30 minutes, immersed in 15% sucrose overnight, cryosectioned the next day and sections processed for immunohistochemistry.
Table 1.
Antibodies used in the study
| Antigen | Host | Catalog number | Source | Dilution |
|---|---|---|---|---|
| Primary | ||||
| Human SOD1 misfolded | Mouse | MM-0070-2 | Medimabs | 1:100 |
| GFAP | Rabbit | 2016-04 | Dako | 1:500 |
| Iba1 | Rabbit | 019-19741 | Wako | 1:500 |
| Beta-3-tubulin | Mouse | 32-2600 | Invitrogen | 1:500 |
| ACSA-1 (GLAST) | Mouse | 130-095-822 | Miltenyi Biotec | 1:100 |
| SOX2 | Goat | Sc-17320 | Santa Cruz | 1:200 |
| GFP-FITC | Goat | ab6662 | Abcam | 1:250 |
| Secondary | ||||
| Alexa 647 | Donkey α mouse | A31571 | Invitrogen | 1:500 |
| Alexa 647 | Donkey α rabbit | A31573 | Invitrogen | 1:500 |
| FITC | Donkey α goat | 705-095-147 | Jackson ImmunoResearch | 1:200 |
| AMCA 350 | Donkey α rabbit | 711-155-152 | Jackson ImmunoResearch | 1:100 |
| Cy3 | Donkey α mouse | 715-165-151 | Jackson ImmunoResearch | 1:500 |
Transverse sections from the C3-5 spinal cord were pre-incubated with blocking solution for 1 hr at RT and then incubated overnight at 4 °C with primary antibodies for the astroglial marker GFAP or microglia/macrophage marker Iba1 followed by the appropriate secondary antibodies and anti-GFP-FITC for 1 hour at RT (Table 1).
Microscopy and cell counting
Cells on coverslips and spinal cord cryosections were captured using a 20× objective (NA 0.75) of a Nikon Eclipse E800 epifluorescence microscope equipped with a Nikon DXM1200F CCD camera. For cultured cells, GFP and RFP positive cells were counted manually for each condition. GFP positive cells were counted in every 5th cryosection along the length of the transplant area (ranging from ~0.8 to 1.5 mm along the cranial-caudal axis between animals). Sections were analysed using a Zeiss LSM700 or a Zeiss LSM780 confocal laser scanning microscope. Images were captured using a LD LCI Plan-Apochromat 20x (NA 0.8) or a LD C-Apochromat 40x W Corr (NA 1.1) objective.
Statistical Analysis
Datasets for oxidative stress and survival assays were analyzed using two-way ANOVA followed by the Bonferroni multiple comparisons tests. MN survival was analyzed using a two-tailed Students t-test. All statistical analyses were performed in GraphPad Prism 5.04. The confidence interval was stated at the 95% confidence level, placing statistical significance at p < 0.05.
Results
Improved survival of SOD1G93A MNs in vitro
Both SOD1WT and SOD1G93A mESC lines formed EBs with abundant expression of HB9::GFP and gave rise to GFP+ MN precursors in culture (Supplementary Fig. 1).
bNCSCs neurospheres abundantly expressed RFP, the neural crest stem cell marker SOX2 and GLAST (after MACS purification), and continued to express RFP in differentiation assay in co-culture and co-implants (Supplementary Figs. 2, 3 and 4).
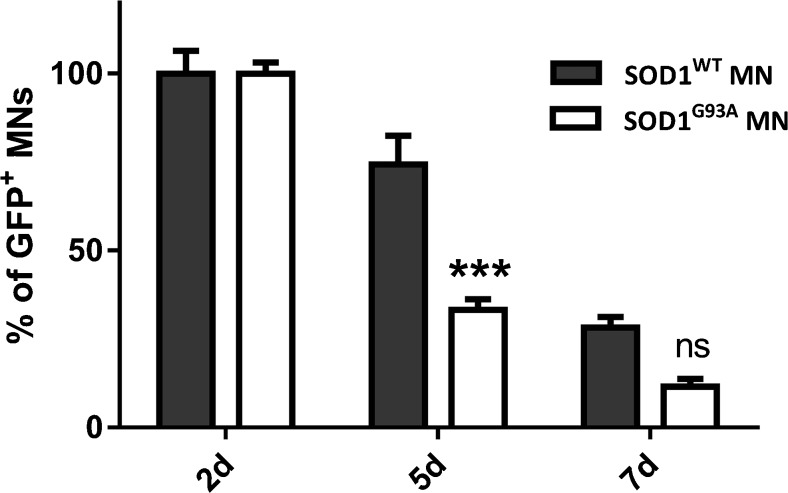
The poor survival of SOD1G93A MNs in vitro has been compared to MNs derived from the HB9::GFP cell line in a previous study [21]. Here we compare the survival of SOD1G93A MNs with SOD1WT MNs to confirm that the reduced survival is due to the SOD1G93A mutation. Cells from both cultures were subjected to differentiation assay and GFP+ MNs were counted after 2, 5 and 7 days of differentiation. The number of MNs declined more rapidly in SOD1G93A cultures at day 5 of differentiation (p < 0.001) (Fig. 1).
Fig. 1.
Survival of SOD1WT and SOD1G93A MNs in vitro. The SOD1G93A cell line shows a reduced MN survival compared to the SOD1WT cell line between days 2 and 7. Asterisks indicate the level of statistical significance by two-way ANOVA followed by Bonferroni multiple comparison test (*** p < 0.001). Data shown is in mean ± SEM of three independent experiments
During MN differentiation from mESCs, a population of astrocytes is also present. Previous studies have shown a negative effect of SOD1G93A astrocytes on MNs [21]. We therefore examined if a reduction of the astrocyte population in SOD1G93A cultures will improve MN survival and if co-culture with bNCSCs, or with GFAP::CD14 astrocytes will improve the survival of SOD1G93A MNs.
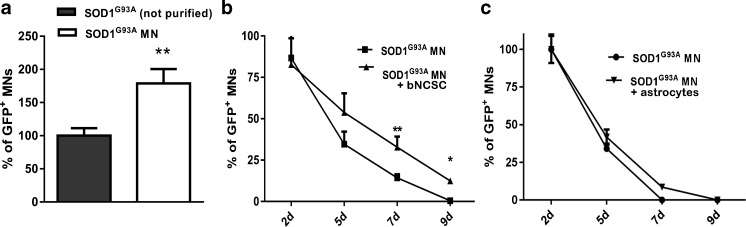
After reduction of the astrocyte population in SOD1G93A cultures there was an approximate 75% increase in the number of SOD1G93A MNs 12 hours after purification compared to non-purified cell cultures (Fig. 2a; p < 0.01). The survival of SOD1G93A MNs was significantly increased in co-culture with bNCSC by day 7 (Fig. 2b; p < 0.01 and p < 0.05), whereas co-culture of SOD1G93A MNs with GFAP::CD14 astrocytes did not affect MN survival (Fig. 2c).
Fig. 2.
Increased survival of SOD1G93A MNs in vitro by removal of SOD1G93A astrocytes and addition of bNCSC. Removal of astrocytes from SOD1G93A cell cultures results in an increased number of SOD1G93A MNs (a). Survival of SOD1G93A MNs increased when co-cultured with bNCSCs (b). SOD1G93A MN survival showed no improvement when co-cultured with GFAP::CD14 astrocytes (c). Asterisks indicate the level of statistical significance by two-tailed Student’s test (a) or two-way ANOVA (b) (** p < 0.01,*p < 0.05). Data shown is in mean ± SEM of three independent experiments
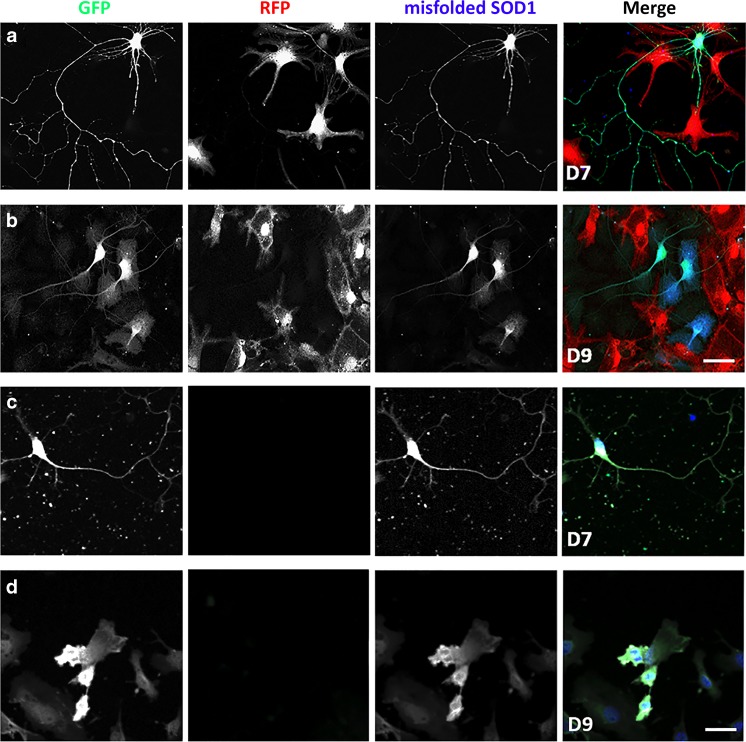
We detected close contact between SOD1G93A MNs and bNCSCs, but did not observe immunoreactivity for misfolded SOD1 in bNCSCs, suggesting that aggregated SOD1 was not transferred from SOD1G93A MNs to adjacent bNCSCs (Fig. 3).
Fig. 3.
SOD1G93A MNs in the presence or absence of bNCSCs in vitro. Co-cultured SOD1G93A cells (GFP) and bNCSCs (RFP) display close contact on day 7 (a) and 9 (b), misfolded SOD1 was only detected in SOD1G93A MNs. SOD1G93A MNs cultured alone show reduced survival and the presence of misfolded SOD1 on day 7 (c) and day 9 (d). Scale bar: 50 μm
bNCSCs improve survival of SOD1G93A MNs in vitro under oxidative stress
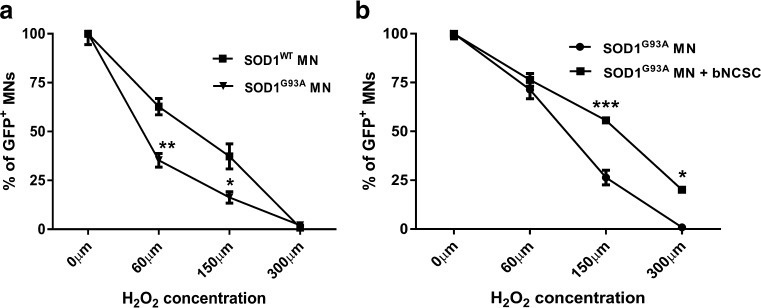
We compared the survival of SOD1G93A MNs and SOD1WT MNs under oxidative stress. Survival of SOD1G93A MNs was markedly decreased (p < 0.01 and p < 0.05) compared to SOD1WT MNs, indicating that SOD1G93A MNs are more susceptible than wildtype MNs to oxidative stress (Fig. 4a). Co-culture of SOD1G93A MNs with bNCSCs under oxidative stress showed a significant increase in SOD1G93A MN survival compared to SOD1G93A MNs cultured alone (p < 0.001 and p < 0.05, Fig. 4b).
Fig. 4.
Effect of hydrogen peroxide (H2O2) on SOD1G93A MN survival in vitro. Survival of SOD1G93A MNs was reduced compared to SOD1WT MNs under oxidative stress (a). Survival of SOD1G93A MNs increased when co-cultured with bNCSCs (SOD1G93A MNs + bNCSCs) compared to when cultured alone (SOD1G93A MN) (b). Asterisks indicate level of statistical significance by two-way ANOVA followed by Bonferroni multiple comparison test (*** p < 0.001, ** p < 0.01, *p < 0.05). Data shown is in mean ± SEM of three independent experiments
We next tested if bNCSCs are susceptible to oxidative stress under similar oxidative stress conditions and found no effect of H2O2 exposure on the survival of bNCSCs in vitro (Supplementary Fig. 3).
SOD1G93A MNs show increased survival in the presence of bNCSCs in vivo
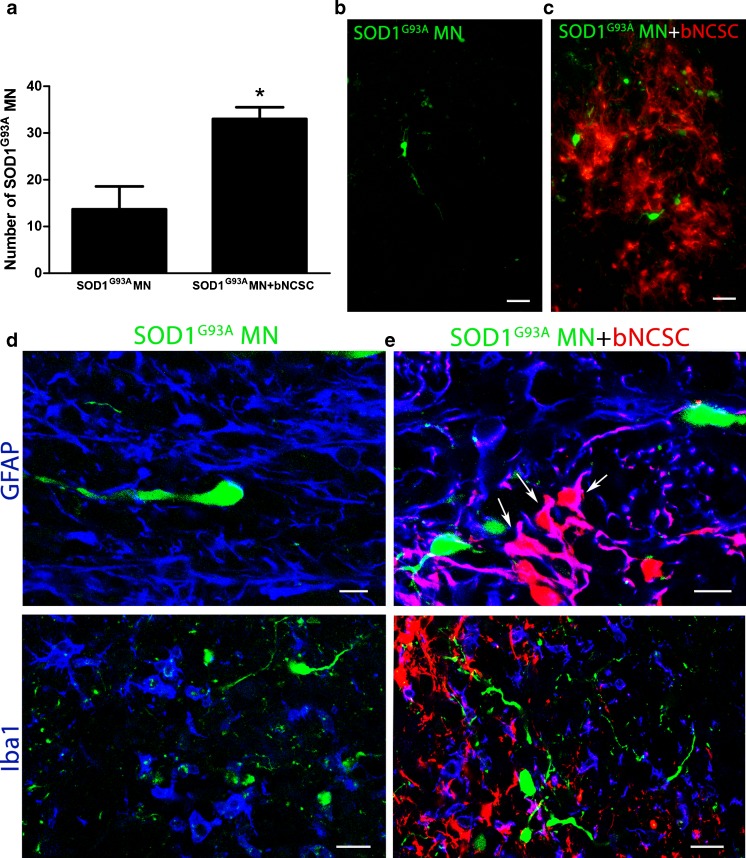
SOD1G93A MNs alone or together with bNCSCs were implanted into the left spinal cord of nude mice, and the survival of implanted MNs was assessed one week after implantation. In all animals which received SOD1G93A MN precursors together with bNCSCs, significantly more GFP+ SOD1G93A MNs were detected compared to animals that received SOD1G93A MN precursors alone (Student’s t-test, p < 0.05, Fig. 5a). The surviving MNs were located in the proximity of bNCSCs or localized as single cells in the spinal cord parenchyma (Fig. 5b,c). Immunolabeling with the astrocytic marker GFAP revealed that bNCSCs differentiated primarily to GFAP positive cells (Fig. 5e).
Fig. 5.
Increased survival of SOD1G93A MN after co-implantation with bNCSC to the spinal cord of mice. One week after implantation, SOD1G93A MN co-implanted with bNCSC showed significantly increased survival compared to SOD1G93A MN implanted alone. Asterisks indicate the level of statistical significance by two-tailed Student’s t-test (* p < 0.05). Data shown is in mean ± SEM of three animals per condition (a). SOD1G93A MNs when implanted alone are located as single cells in the spinal cord parenchyma (b). When implanted together with bNCSCs, SOD1G93A MNs can be found both spread out as single cells as well as in close proximity to bNCSCs (c). The injection site showed increased immunoreactivity for GFAP and Iba1 following implantation of either SOD1G93A MN alone (d) or co-implantation with bNCSCs (e). Arrows indicate bNCSCs differentiated to GFAP positive cells (e). Scale bars: b,c 25 μm; d,e 10 μm for GFAP and 20 μm for Iba1
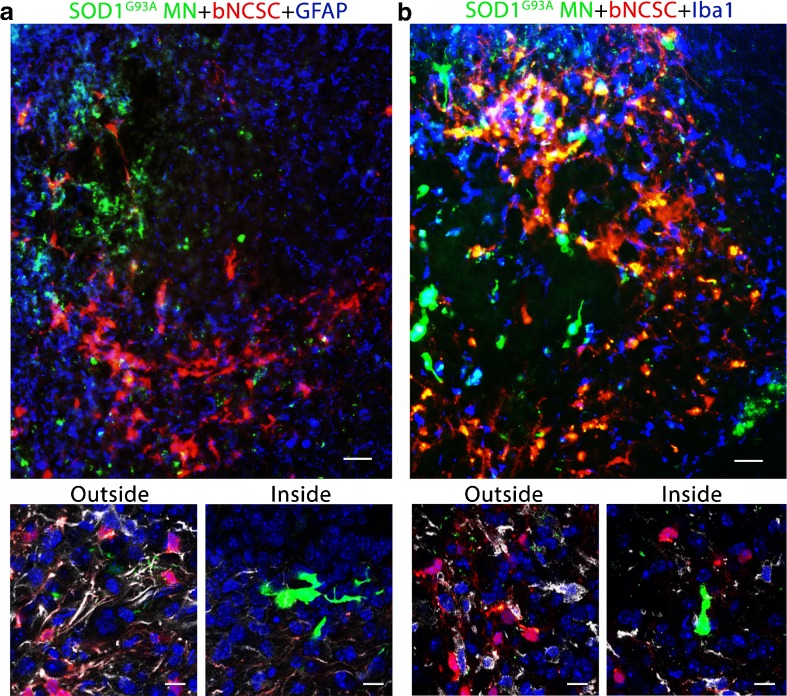
The improved survival of MNs in the presence of bNCSCs after implantation prompted us to investigate if bNCSCs affect the glial response in the recipient spinal cord in the vicinity of implanted MN precursors. As expected, there was an increased immunolabeling for GFAP and Iba1 from the minor injury at the injection site, but these changes were similar in the two experimental groups (Fig. 5d,e). However. the glial response for GFAP and Iba1 within the area where bNCSCs were located seemed reduced compared to adjacent areas lacking bNCSCs (Fig. 6a,b).
Fig. 6.
Transplanted bNCSCs might diminish the local glial response following implantation. Spinal cord areas partially surrounded by bNCSC transplants showed decreased immunoreactivity for GFAP (a, inside ) and Iba1 (b, inside ) and a high number of co-implanted SOD1G93A MN (a, b). Overviews show GFAP and Iba1 in blue, high magnification confocal images show GFAP and Iba1 in white and cell nuclei in blue for areas inside and outside the area of bNCSC transplantation. Scale bars: a,b 25 μm for overviews and 10 μm for high magnification images
Discussion
There is an urgent need for novel therapeutic strategies with enhanced effect for ALS patients. Here we show that bNCSCs exert a significant survival promoting effect on SOD1G93A MN in vitro as well as after co-implantation to the spinal cord. Previously, we detected several beneficial effects of bNCSCs on co-cultured and co-transplanted cells. Thus, bNCSCs strongly increase proliferation of insulin producing beta-cells in co-transplants of mouse pancreatic islets in streptozotocin diabetic mice [19, 29], and in co-culture with beta-cells [17]. bNCSCs protect insulin-producing cells from cytokine induced apoptosis in vitro [18], an effect which appears to require direct bNCSC- cell contact through catenin-cadherin junctions [30]. Co-implantation of bNCSCs with mouse and human pancreatic islets improves their survival after transplantation and increases beta-cell proliferation, vascularization of transplants and their re-innervation [29]. We also found their survival supporting effect in spinal cord slice cultures exposed to excitotoxic stress (unpublished observation).
The conditions for implanting healthy supportive cells into the spinal cord of ALS patients imply that these cells will have not only to survive in the harmful ALS environment, but also exert beneficial effects on diseased MNs. For this reason we have tested if bNCSCs are susceptible to oxidative stress and found that they are resistant to exposure to hydrogen peroxide in concentrations which significantly impair survival of SOD1G93A MNs. We next examined whether bNCSCs are “contaminated” with misfolded SOD1 from co-cultured SOD1G93A MNs. ALS appears to begin focally at a random location and progresses contiguously through two distinct types of neuroanatomic propagation: contiguous propagation through the extracellular matrix independent of synaptic connections, and network propagation, which is dependent on synaptic connections [31]. In many neurodegenerative disorders misfolded proteins appear to contribute to disease progression [32]. Thus, the presence of misfolded SOD1 may contribute to disease propagation in some forms of ALS [33]. We detected immunoreactivity for misfolded SOD1 in SOD1G93A MNs generated from SOD1G93A mESCs. Although astrocytes co-cultured with SOD1G93A MNs have been reported to be "contaminated" with aggregated SOD1 [34], we did not detect aggregated SOD1 in bNCSCs, suggesting that they are resistant to relocation of misfolded SOD1. Thus, bNCSCs display properties in vitro that may be useful for disease modifying cell therapy in ALS.
We explored these properties in co-cultures of bNCSCs with SOD1G93A MNs subjected to oxidative stress, and demonstrated significantly increased SOD1G93A MN survival compared to untreated SOD1G93A MN cultures. In agreement with previous studies [11], we found that depletion of the astrocyte population from SOD1G93A cultures, increases MN survival. The addition of bNCSCs, but not healthy astrocytes, to these cultures further improved the survival of SOD1G93A MNs under both normal and oxidative stress conditions. This finding provides additional evidence for the potency of bNCSCs in supporting diseased SOD1G93A MNs. The improved SOD1G93A MN survival coincided with the presence of direct contacts between bNCSCs and SOD1G93A MNs, suggesting that the positive effect of bNCSCs might be mediated through intercellular connections, as was shown previously in bNCSC-beta-cell cultures [29]. We have also shown that bNCSCs produce a broad range of trophic factors, as well as Wnt-1 and matrix metalloproteinases [35]. This trophic capacity, in co-operation with direct cell-cell interaction, may explain the survival promoting effects of bNCSCs on SOD1G93A MNs under stressful conditions in vitro.
Pre-differentiated neural cells are particularly vulnerable during the initial period after implantation into the central nervous system. At this stage environmental conditions that support survival and differentiation of implanted cells are likely to be critical for their subsequent integration into neural circuits and long-term survival. We therefore focused on the viability of SOD1G93A MNs alone, or in combination with bNCSCs, during the first week of their implantation. One week after implantation of SOD1G93A MN precursors to the spinal cord, only few surviving SOD1G93A MNs were found. Survival of SOD1G93A MNs was, however, significantly increased when co-implanted with bNCSCs, despite the fact that twice as many SOD1G93A MN precursors were implanted alone compared to the number of SOD1G93A MN precursors co-implanted with bNCSCs. These findings show that bNCSCs create environmental conditions for SOD1G93A MNs, which support their survival in the spinal cord.
Inflammatory components have been implicated in the neurodegeneration associated with ALS [36]. As expected, the injection procedure resulted in a local microglial and astroglial response at the site of cell implantation, but this response did not differ between the two experimental conditions. However, when areas harboring implanted cells were analyzed, immunoreactivity for host microglia/macrophages and astrocytes appeared reduced where SOD1G93A and bNCSCs were co-localized, suggesting that bNCSCs are able to attenuate the local inflammatory response associated with implanted SOD1G93A MNs. The survival support by bNCSCs may initially rely on direct cell-cell interactions at the site of cell implantation where bNCSCs and SOD1G93A MN precursors intermingle during the injection procedure. However, the presence of GFP+ MNs at a distance from bNCSCs and in the vicinity of the central canal one week after implantation indicates that some implanted SOD1G93A MN precursors rapidly become independent of close contact with bNCSCs. Survival of migrated SOD1G93A MNs may be supported by bNCSC-derived diffusible molecules, such as vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and ciliary neurotrophic factor (CNTF) [35], all of which have trophic effects on MNs [37]. bNCSC-derived matrix metalloproteinase-2 and -9 (MMP-2 and -9) [36], which have been implicated in cell migration [38, 39], may contribute to the migratory capacity of implanted SOD1G93A MNs to the spinal cord parenchyma and in some cases towards the central canal (Supplemental Fig. 4). The ependyma which surrounds this canal is a source of growth factors [40] and was previously designated as a stem cell region in the adult spinal cord [41]. Previous studies have shown that stem cells readily generate neurons when transplanted into neurogenic areas of the adult brain [42, 43]. The survival of SOD1G93A MN precursors in the central canal area suggests that this is a region in the spinal cord with neurogenic properties, which are beneficial for the survival of implanted MN precursors.
The ability of bNCSCs to promote vascularization of co-implanted tissue [29] may also have contributed to improved survival of co-implanted SOD1G93A MNs. Previous studies have shown that ALS is associated with disruption of the blood-CNS-barrier (B-CNS-B) [44–46], a process that can contribute to the inflammatory response in areas affected by ALS. An interesting aspect in this context is whether the angiogenetic properties of bNCSCs in combination with their preferential post-implantation differentiation to healthy SOD1G93A resistant astrocytes may contribute to the formation of new blood vessels with an intact B-CNS-B in the diseased spinal cord. Implantation of bNCSCs to animal models of ALS may clarify the potential of these cells to recover B-CNS-B.
In conclusion, we show that bNCSCs promote survival of SOD1G93A MNs in vitro and in vivo after co-implantation to the spinal cord. The beneficial effects by bNCSCs might be due to their resistance to oxidative stress and their neurotrophic and angiogenic support. These properties in combination with their resistance to SOD1G93A aggregates make them interesting candidates for further investigations on novel therapeutic approaches to achieve long-term neuroprotection in animal models of ALS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
SOD1WT and SOD1G93A EBs abundantly expressed HB9::GFP in MN precursors on day 7 (a, b) and generated GFP+ MNs in vitro (c, d), expressing Beta-3-tubulin (e, f, g, h). Scale bar: 50 μm (d), 20 μm (h) (GIF 137 kb)
Overview of RFP-expressing bNCSC neurospheres in pre-differentiation (a) and the bNCSC monolayer during differentiation stage (b). Immunostaining of bNCSC neurospheres shows expression of the glial specific marker GLAST (c) and the neural crest stem cell specific markers SOX2 (d). Scale bars: a,b 20 μm; c,d 50 μm. (GIF 164 kb)
Effect of hydrogen peroxide (H2O2) on the survival of bNCSCs in vitro. Cultures were exposed to different concentrations of H2O2 for 3 hours. The survival of bNCSCs is not affected by different concentrations of H2O2 (GIF 5 kb)
bNCSCs and SOD1G93A MNs one week after implantation to the ventral horn. SOD1G93A MNs (green) are located in the grey matter close to the central canal and together with bNCSCs (red) in the white matter of the ventral funiculus. Scale bar: 50 μm (GIF 127 kb)
(PDF 1224 kb)
Acknowledgments
We are grateful to Dr. Ivo Lieberam for providing us with the GFAP::CD14 cell line and for reading the manuscript, and to Dr. Kevin Eggan for the HB9::GFP cell lines. The study was supported by the Swedish Research Council (Project No 20716), under the frame of EuroNanoMed-II, Stiftelsen Olle Engkvist Byggmästare and Signhild Engkvists Stiftelse.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Tanya Aggarwal and Jan Hoeber contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-016-0505-8) contains supplementary material, which is available to authorized users.
References
- 1.Carlesi C, Pasquali L, Piazza S, Lo Gerfo A, Caldarazzo Ienco E, Alessi R, et al. Strategies for clinical approach to neurodegeneration in Amyotrophic lateral sclerosis. Arch Ital Biol. 2011 Mar;149(1):151–67. [DOI] [PubMed]
- 2.Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther. 2011 Feb;17(1):4–31. [DOI] [PMC free article] [PubMed]
- 3.Kaur SJ, McKeown SR, Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene. 2016 Feb 15;577(2):109–18. [DOI] [PubMed]
- 4.Rojas F, Cortes N, Abarzua S, Dyrda A, van Zundert B. Astrocytes expressing mutant SOD1 and TDP43 trigger motoneuron death that is mediated via sodium channels and nitroxidative stress. Front Cell Neurosci. 2014;8:24. doi: 10.3389/fncel.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007 May;10(5):615–22. [DOI] [PMC free article] [PubMed]
- 6.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008 Dec 4;3(6):637–48. [DOI] [PubMed]
- 7.Fritz E, Izaurieta P, Weiss A, Mir FR, Rojas P, Gonzalez D, et al. Mutant SOD1-expressing astrocytes release toxic factors that trigger motoneuron death by inducing hyperexcitability. J Neurophysiol. 2013 Jun;109(11):2803–14. [DOI] [PMC free article] [PubMed]
- 8.Papadeas ST, Kraig SE, O’Banion C, Lepore AC, Maragakis NJ. Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. Proc Natl Acad Sci USA. 2011 Oct 25;108(43):17803–8. [DOI] [PMC free article] [PubMed]
- 9.Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011 Aug 10;29(9):824–8. [DOI] [PMC free article] [PubMed]
- 10.Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci USA. 2014 Jan 14;111(2):829–32. [DOI] [PMC free article] [PubMed]
- 11.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008 Mar;11(3):251–3. [DOI] [PMC free article] [PubMed]
- 12.Thangavelu SR, Tripathi PP, Arya U, Mishra HK, Subramaniam JR. ALS associated mutant SOD1 impairs the motor neurons and astrocytes and wild type astrocyte secreted-factors reverse the impaired motor neurons. Ann Neurosci. 2011 Apr;18(2):48–55. [DOI] [PMC free article] [PubMed]
- 13.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008 Nov;11(11):1294–301. [DOI] [PMC free article] [PubMed]
- 14.Boucherie C, Schäfer S, Lavand’homme P, Maloteaux J-M, Hermans E. Chimerization of astroglial population in the lumbar spinal cord after mesenchymal stem cell transplantation prolongs survival in a rat model of amyotrophic lateral sclerosis. J Neurosci Res. 2009 Jul;87(9):2034–46. [DOI] [PubMed]
- 15.Hjerling-Leffler J, Marmigère F, Heglind M, Cederberg A, Koltzenburg M, Enerbäck S, et al. The boundary cap: a source of neural crest stem cells that generate multiple sensory neuron subtypes. Development. 2005 Jun;132(11):2623–32. [DOI] [PubMed]
- 16.Zujovic V, Thibaud J, Bachelin C, Vidal M, Deboux C, Coulpier F, et al. Boundary cap cells are peripheral nervous system stem cells that can be redirected into central nervous system lineages. Proc Natl Acad Sci USA. 2011 Jun 28;108(26):10714–9. [DOI] [PMC free article] [PubMed]
- 17.Grouwels G, Vasylovska S, Olerud J, Leuckx G, Ngamjariyawat A, Yuchi Y, et al. Differentiating neural crest stem cells induce proliferation of cultured rodent islet beta cells. Diabetologia. 2012 Jul;55(7):2016–25. [DOI] [PubMed]
- 18.Ngamjariyawat A, Turpaev K, Welsh N, Kozlova EN. Coculture of insulin-producing RIN5AH cells with neural crest stem cells protects partially against cytokine-induced cell death. Pancreas. 2012 Apr;41(3):490–2. [DOI] [PubMed]
- 19.Olerud J, Kanaykina N, Vasylovska S, Vasilovska S, King D, Sandberg M, et al. Neural crest stem cells increase beta cell proliferation and improve islet function in co-transplanted murine pancreatic islets. Diabetologia. 2009 Dec;52(12):2594–601. [DOI] [PubMed]
- 20.Kosykh A, Ngamjariyawat A, Vasylovska S, Konig N, Trolle C, Lau J, et al. Neural crest stem cells from hair follicles and boundary cap have different effects on pancreatic islets in vitro. Int J Neurosci. 2015;125(7):547–54. doi: 10.3109/00207454.2014.950373. [DOI] [PubMed] [Google Scholar]
- 21.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007 May;10(5):608–14. [DOI] [PMC free article] [PubMed]
- 22.Jungblut M, Tiveron MC, Barral S, Abrahamsen B, Knöbel S, Pennartz S, et al. Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia. 2012 May;60(6):894–907. [DOI] [PubMed]
- 23.Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J, et al. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science. 2014 Apr;344(6179):94–7. [DOI] [PMC free article] [PubMed]
- 24.Wichterle H, Lieberam I, Porter J a, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–97. [DOI] [PubMed]
- 25.Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, Biechele S, et al. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004 Dec;40(4):241–6. [DOI] [PubMed]
- 26.Aldskogius H, Berens C, Kanaykina N, Liakhovitskaia A, Medvinsky A, Sandelin M, et al. Regulation of boundary cap neural crest stem cell differentiation after transplantation. Stem Cells. 2009 Jul;27(7):1592–603. [DOI] [PMC free article] [PubMed]
- 27.Lepore AC. Intraspinal cell transplantation for targeting cervical ventral horn in amyotrophic lateral sclerosis and traumatic spinal cord injury. J Vis Exp. 2011 Sep 18;(55). [DOI] [PMC free article] [PubMed]
- 28.Arras M, Autenried P, Rettich A, Spaeni D, Rülicke T. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med. 2001;51:443–56. [PubMed] [Google Scholar]
- 29.Grapensparr L, Vasylovska S, Li Z, Olerud J, Jansson L, Kozlova E, et al. Co-transplantation of human pancreatic islets with post-migratory neural crest stem cells increases β-cell proliferation and vascular and neural regrowth. J Clin Endocrinol Metab. 2015 Apr;100(4):E583–90. [DOI] [PubMed]
- 30.Ngamjariyawat A, Turpaev K, Vasylovska S, Kozlova EN, Welsh N. Co-culture of neural crest stem cells (NCSC) and insulin producing beta-TC6 cells results in cadherin junctions and protection against cytokine-induced beta-cell death. PLoS One. 2013 Jan;8(4):e61828. [DOI] [PMC free article] [PubMed]
- 31.Ravits J. Focality, stochasticity and neuroanatomic propagation in ALS pathogenesis. Exp Neurol. 2014 Dec;262 Pt B:121–6. [DOI] [PubMed]
- 32.Recasens A, Dehay B. Alpha-synuclein spreading in Parkinson’s disease. Front Neuroanat. 2014;8:159. doi: 10.3389/fnana.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotunno MS, Bosco DA. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front Cell Neurosci. 2013 Dec 16;7:253. [DOI] [PMC free article] [PubMed]
- 34.Basso M, Pozzi S, Tortarolo M, Fiordaliso F, Bisighini C, Pasetto L, et al. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem. 2013 May 31;288(22):15699–711. [DOI] [PMC free article] [PubMed]
- 35.Lau J, Vasylovska S, Kozlova EN, Carlsson P-O. Surface coating of pancreatic islets with neural crest stem cells improves engraftment and function after intraportal transplantation. Cell Transplant. 2015;24(11):2263–72. doi: 10.3727/096368915X686184. [DOI] [PubMed] [Google Scholar]
- 36.Hooten KG, Beers DR, Zhao W, Appel SH. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015 Apr;12(2):364–75. [DOI] [PMC free article] [PubMed]
- 37.Tovar-Y-Romo LB, Ramírez-Jarquín UN, Lazo-Gómez R, Tapia R. Trophic factors as modulators of motor neuron physiology and survival: implications for ALS therapy. Front Cell Neurosci. 2014;8:61. doi: 10.3389/fncel.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanSaun MN, Matrisian LM. Matrix metalloproteinases and cellular motility in development and disease. Birth Defects Res C Embryo Today. 2006 Mar;78(1):69–79. [DOI] [PubMed]
- 39.Tonti GA, Mannello F, Cacci E, Biagioni S. Neural stem cells at the crossroads: MMPs may tell the way. Int J Dev Biol. 2009;53(1):1–17. doi: 10.1387/ijdb.082573gt. [DOI] [PubMed] [Google Scholar]
- 40.Moore SA. The spinal ependymal layer in health and disease. Vet Pathol. 2016 Jul;53(4):746–53. [DOI] [PubMed]
- 41.Meletis K, Barnabé-Heider F, Carlén M, Evergren E, Tomilin N, Shupliakov O, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008 Jul 22;6(7):e182. [DOI] [PMC free article] [PubMed]
- 42.Ormerod BK, Palmer TD, Caldwell MA. Neurodegeneration and cell replacement. Philos Trans R Soc Lond B Biol Sci. 2008 Jan 12;363(1489):153–70. [DOI] [PMC free article] [PubMed]
- 43.Butti E, Cusimano M, Bacigaluppi M, Martino G. Neurogenic and non-neurogenic functions of endogenous neural stem cells. Front Neurosci. 2014;8:92. doi: 10.3389/fnins.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MCO, Haller E, Frisina-Deyo A, Mirtyl S, et al. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 2012 Aug 21;1469:114–28. [DOI] [PubMed]
- 45.Garbuzova-Davis S, Sanberg PR. Blood-CNS barrier impairment in ALS patients versus an animal model. Front Cell Neurosci. 2014;8:21. doi: 10.3389/fncel.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, et al. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci USA. 2014 Mar 18;111(11):E1035–42. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SOD1WT and SOD1G93A EBs abundantly expressed HB9::GFP in MN precursors on day 7 (a, b) and generated GFP+ MNs in vitro (c, d), expressing Beta-3-tubulin (e, f, g, h). Scale bar: 50 μm (d), 20 μm (h) (GIF 137 kb)
Overview of RFP-expressing bNCSC neurospheres in pre-differentiation (a) and the bNCSC monolayer during differentiation stage (b). Immunostaining of bNCSC neurospheres shows expression of the glial specific marker GLAST (c) and the neural crest stem cell specific markers SOX2 (d). Scale bars: a,b 20 μm; c,d 50 μm. (GIF 164 kb)
Effect of hydrogen peroxide (H2O2) on the survival of bNCSCs in vitro. Cultures were exposed to different concentrations of H2O2 for 3 hours. The survival of bNCSCs is not affected by different concentrations of H2O2 (GIF 5 kb)
bNCSCs and SOD1G93A MNs one week after implantation to the ventral horn. SOD1G93A MNs (green) are located in the grey matter close to the central canal and together with bNCSCs (red) in the white matter of the ventral funiculus. Scale bar: 50 μm (GIF 127 kb)
(PDF 1224 kb)