Abstract
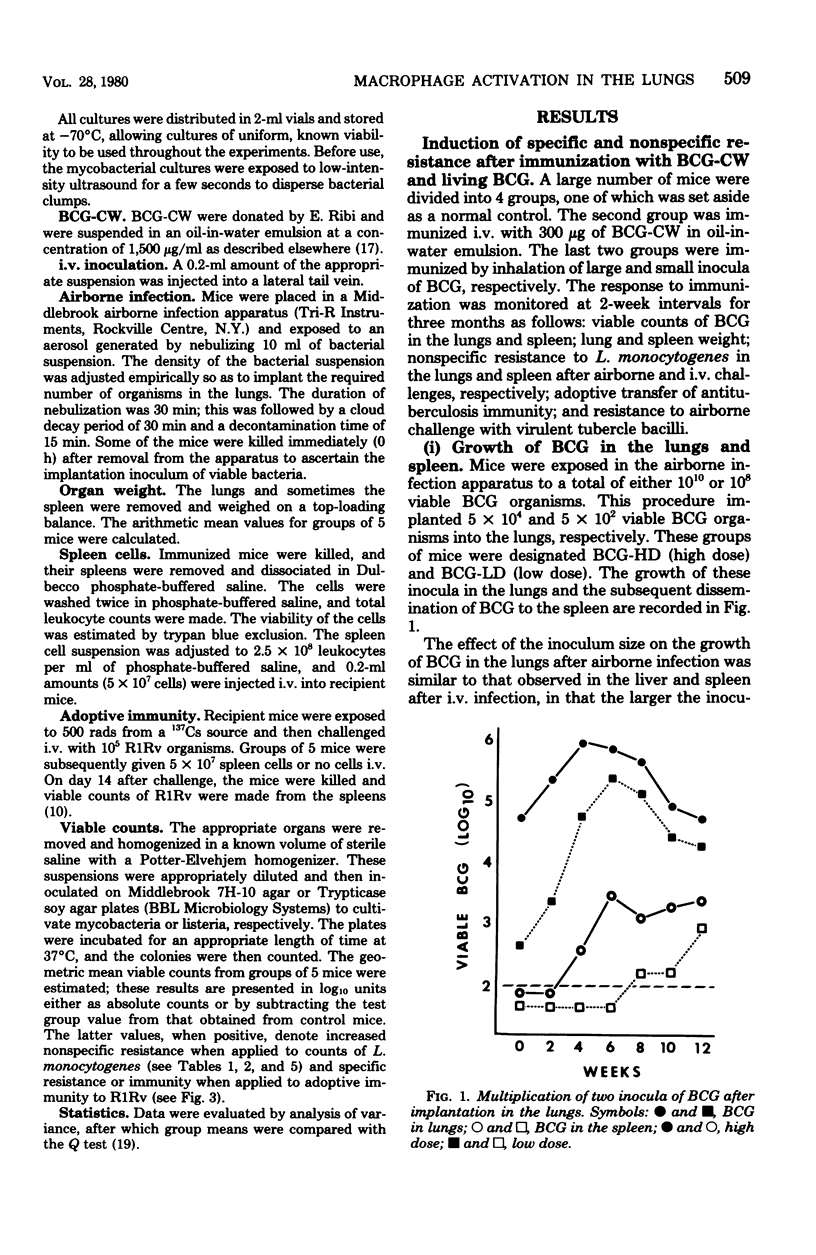
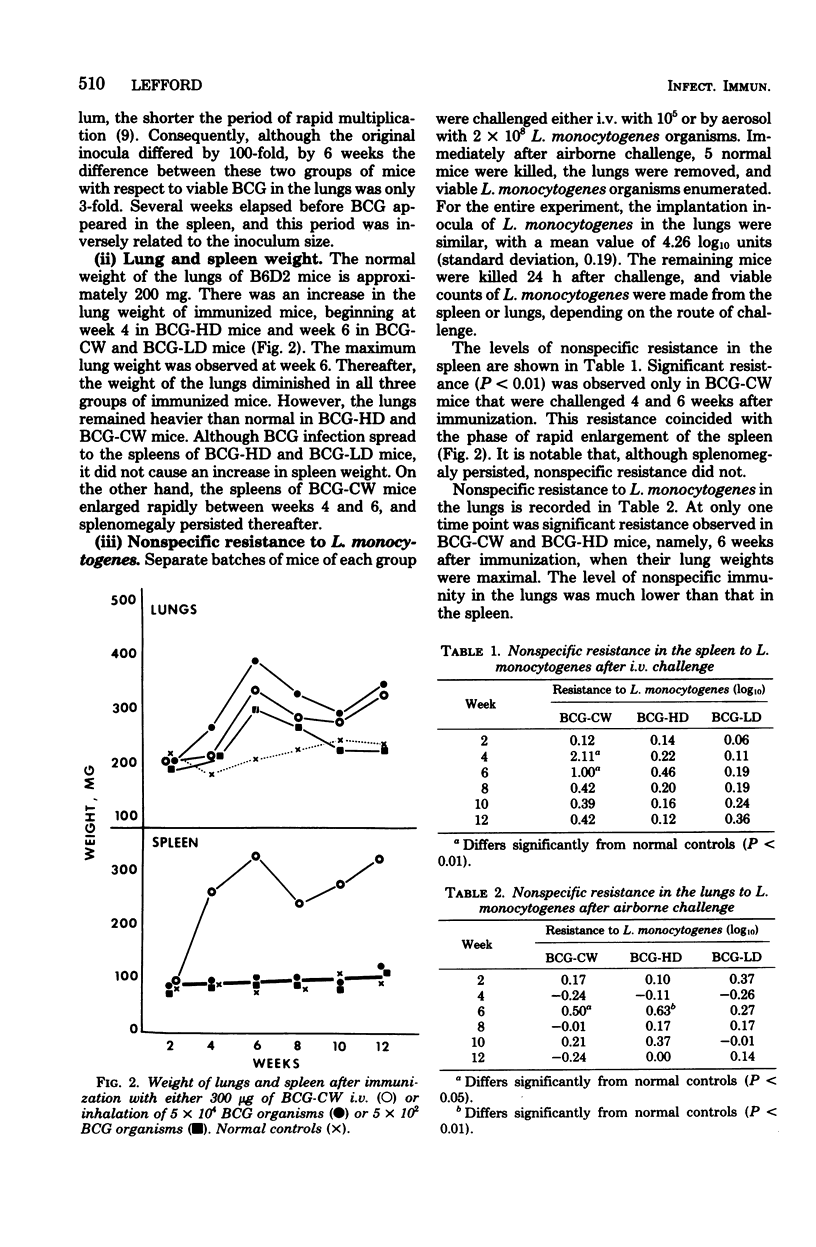
Mice were vaccinated with 300 micrograms of BCG cell walls (BCG-CW) in oil-in-water emulsion intravenously or with a high or low dose of living BCG by inhalation (BCG-HD or BCG-LD, respectively). The consequences of vaccination were evaluated in terms of the growth of BCG in the lungs and spleen, lung and spleen weight, resistance to intravenous and airborne challenge with Listeria monocytogenes, airborne challenge with virulent Mycobacterium tuberculosis H37Rv, and transfer of adoptive immunity. BCG-CW and BCG-HD mice developed increased lung weight, which was associated with transiet, low-level resistance to airborne L. monocytogenes and initial resistance to airborne H37Rv. Only BCG-CW mice developed splenomegaly, which was accompanied by high resistance to intravenous challenge with L. monocytogenes. The initial resistance of BCG-CW mice to H37Rv was not sustained, whereas that of BCG-HD mice persisted. There was no initial resistance to H37Rv in BCG-LD mice, but immunity was generated later. Overall, BCG-HD mice were most resistant to H37Rv, and BCG-CW and BCG-LD mice were less but equally resistant.
Full text
PDF
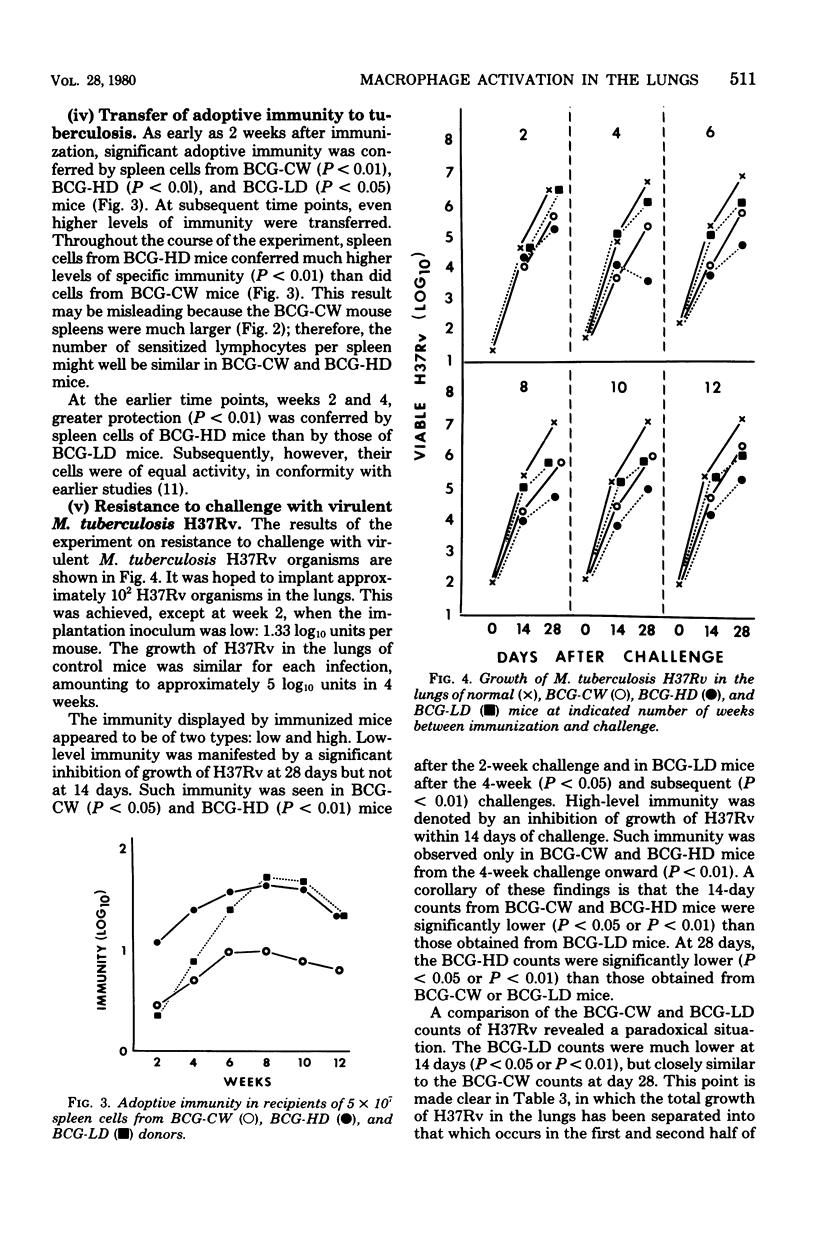

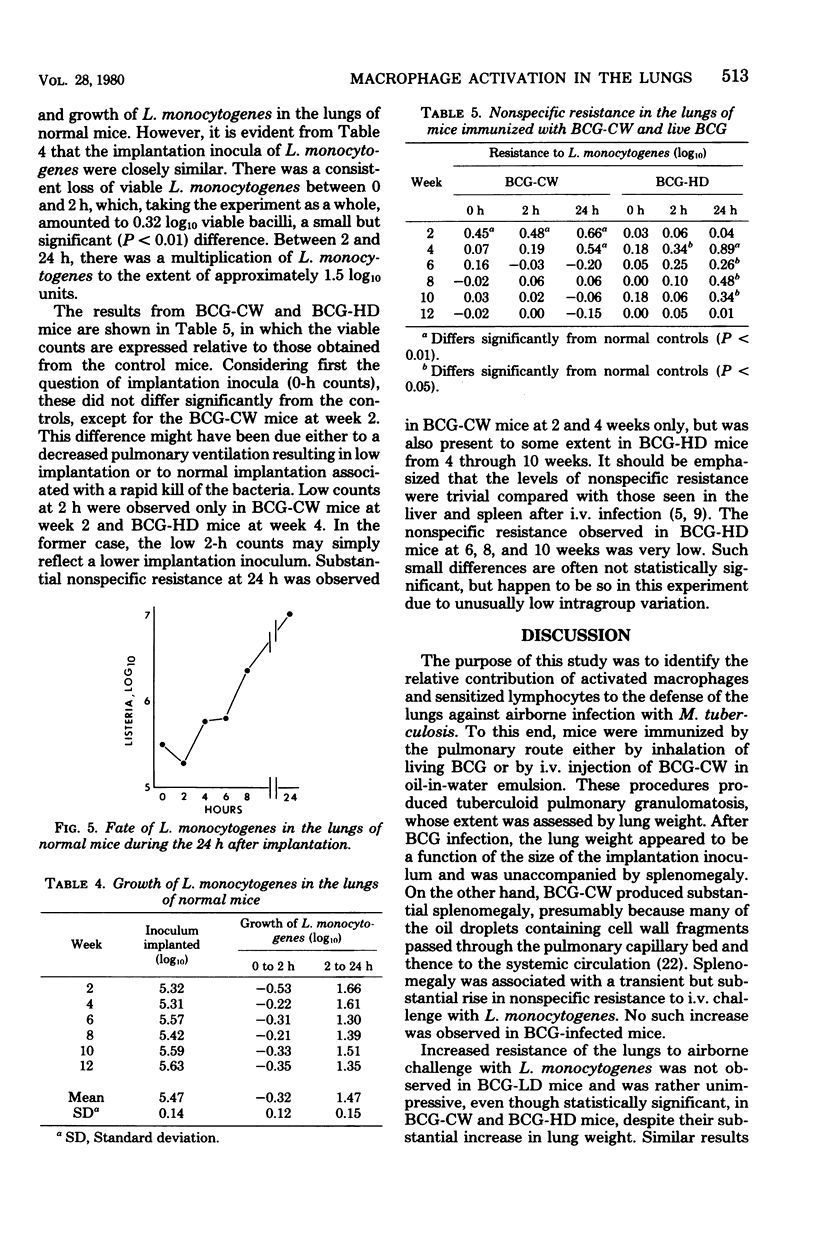


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am Rev Respir Dis. 1969 Feb;99(2):242–248. doi: 10.1164/arrd.1969.99.2.242. [DOI] [PubMed] [Google Scholar]
- Barclay W. R., Anacker R., Brehmer W., Ribi E. Effects of oil-treated mycobacterial cell walls on the organs of mice. J Bacteriol. 1967 Nov;94(5):1736–1745. doi: 10.1128/jb.94.5.1736-1745.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay W. R., Busey W. M., Dalgard D. W., Good R. C., Janicki B. W., Kasik J. E., Ribi E., Ulrich C. E., Wolinsky E. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis. 1973 Mar;107(3):351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Increased antibacterial resistance and immunodepression during graft-versus-host reactions in mice. Transplantation. 1969 Jun;7(6):484–497. doi: 10.1097/00007890-196906000-00005. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSON C. L., WICHT W. C. Studies of resistance to experimental tuberculosis in mice vaccinated with living attenuated tubercle bacilli and challenged with virulent organisms. Am Rev Respir Dis. 1962 Jun;85:833–846. doi: 10.1164/arrd.1962.85.6.833. [DOI] [PubMed] [Google Scholar]
- Lefford M. J. Induction and expression of immunity after BCG immunization. Infect Immun. 1977 Dec;18(3):646–653. doi: 10.1128/iai.18.3.646-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Immunological aspects of airborne infection: reactions to inhaled antigens. Bacteriol Rev. 1961 Sep;25:331–346. doi: 10.1128/br.25.3.331-346.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N. The role of normal alveolar macrophages in cell-mediated immunity. J Reticuloendothel Soc. 1977 Feb;21(2):131–139. [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E., Larson C., Wicht W., List R., Goode G. Effective nonliving vaccine against experimental tuberculosis in mice. J Bacteriol. 1966 Mar;91(3):975–983. doi: 10.1128/jb.91.3.975-983.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Stankus R. P., Cashner F. M., Salvaggio J. E. Bronchopulmonary macrophage activation in the pathogenesis of hypersensitivity pneumonitis. J Immunol. 1978 Mar;120(3):685–688. [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Yarkoni E., Rapp H. J. Granuloma formation in lungs of mice after intravenous administration of emulsified trehalose-6,6'-dimycolate (cord factor): reaction intensity depends on size distribution of the oil droplets. Infect Immun. 1977 Nov;18(2):552–554. doi: 10.1128/iai.18.2.552-554.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


