Abstract
Pre-harvest sprouting (PHS) is a constrain problem in hybrid rice production. The present study was conducted to investigate the inhibitory effect of eugenol on seed germination and PHS of hybrid rice variety (Qian You 1). The results showed that seed germination speed and the activities of α-amylase were inhibited by eugenol pre-soaking and these effects enhanced with the increasing of eugenol concentrations; while seedling growth was not negatively affected. In field trials, eugenol application caused a significant decline in PHS as compared with control, whereas no sustained inhibition in post-harvested seed germination was observed. The HPLC analysis indicated that eugenol raised the internal ABA content by 1–4 times more than control, and seeds treated with eugenol had relatively lower OsABA8OH2 and higher transcript levels of OsNCED2 expression during early stages of seed imbibitions. In addition, seed germinated faster after GA3 application than eugenol alone, and seed endogenous ABA content decreased obviously. It suggested that eugenol strongly delayed seed germination and the PHS in the field, which might be mainly due to the increased ABA contents caused by eugenol. However, the phenomenon of delayed germination and high ABA content caused by eugenol could be effectively recovered by exogenous GA3.
Introduction
Rice is one of the most important staple foods worldwide. Hybrid rice varieties possess many remarkable advantages over conventional rice such as higher yield, stronger stress tolerance and better grain quality1. Nevertheless, there still exist some disadvantages in hybrid rice. For instance, in hybrid rice, seeds germinated easily in ears before timely field harvest under high humidity and appropriate temperature condition. Pre-harvest sprouting happened much easier during production of hybrid rice varieties than conventional ones2–4. Pre-harvest sprouting generally reduces the cereal yield and degrades seed quality for planting, resulting in severe economic losses in seed production5. Therefore, determining the ways and solutions to reduce or eliminate the pre-harvest sprouting is crucial for hybrid rice seed production.
Seed germination is a complex process involving physically metabolism and recovery, essential cellular events to enable the embryo to emerge, and preparation for subsequent seedling growth6. Whether seeds could germinate well is an important issue for agricultural production. Although seeds are expected to germinate rapidly for field establishment, pre-harvest sprouting is not what farmers and seed companies expected. In addition to genetic improvement, application of exogenous chemical compounds is considered as effective method to prevent pre-harvest sprouting. So far, some substances including plant hormones and plant growth regulators had been reported for pre-harvest sprouting inhibition, like abscisic acid (ABA), ethephon, maleic hydrazide, paclobutrazol and uniconazole, etc7, 8. Specifically, abscisic acid (ABA) exhibits strong inhibitory effect on seed germination, but was not widely applied in practice due to its high cost. For this reason, we tried to seek an economical and environmental friendly product for practically reducing pre-harvest sprouting of rice in replacement for traditional chemical compounds.
A type of natural plant extracts, eugenol, is the major constituent of clove (Syzygium aromaticum L.) essential oil and possesses various biological activities. Eugenol is extracted and widely applied in multiple products and has found application in various industries such as food flavoring, fragrance, pharmaceutical9–11. Recently, eugenol was reported to have special herbicidal activity for some specific grassy weeds and might be developed as a new herbicide product to be widely used practically12. Although the inhibitory effect of eugenol on seed germination has been reported in wheat13, up to now, the internal mechanism underlying the effects of eugenol for reducing the germination in hybrid rice is not completely clarified.
Therefore, a comprehensive analysis was performed to better understand the underlying mechanism of inhibitory effects of eugenol on germination of rice seed. Different concentrations of eugenol were applied to rice seeds to determine the inhibitory effect of eugenol on seed germination. Furthermore, related physiological index and subsequent seedling growth were analyzed. Finally, a time course assay of endogenous hormones amounts as well as corresponding metabolism gene expression profiles of ABA during seed imbibition processes was also studied at the molecular level. In addition, we also performed field trial to evaluate the influence of eugenol on reducing pre-harvest sprouting and seed quality of hybrid rice after harvest. Then an environmentally safe inhibitor and its appropriate concentration would be developed to effectively delay seed germination and to reduce the damage caused by pre-harvest sprouting of hybrid rice.
Results
Inhibitory effect of eugenol on germination and seedling growth of Qian You 1
Eugenol application decreased seed germination speed of hybrid rice, and the inhibitory effect enhanced with the increasing of eugenol concentrations (Table 1). The mean germination time (MGT) of hybrid rice seeds increased and a reverse trend in case of germination index (GI) was observed with the advancement of eugenol concentration. The application of eugenol with 2.0 g · L−1 concentration recorded the highest level of MGT and lowest value of GI. On the contrary, the lowest values of MGT and highest values of GI were observed in control. However, no significant differences in GP were observed among control and eugenol treatments, indicating that the inhibitory effect of eugenol just on seed germination speed other than final germination percentage.
Table 1.
Effects of different concentrations of eugenol on seed germination and seedling characteristics of hybrid rice Qian You 1.
| Treatment | MGT (d) | GP (%) | GI | Shoot Height (cm) | Root Length (cm) | Seedling Dry Weight (g) |
|---|---|---|---|---|---|---|
| Control (water) | 2.93 ± 0.05a | 96.0 ± 0.6a | 17.0 ± 0.6a | 6.96 ± 0.03ab | 6.20 ± 0.05a | 89.0 ± 2.6b |
| Eugenol (1.0 g · L−1) | 3.04 ± 0.04a | 97.0 ± 1.0a | 16.8 ± 0.2a | 7.28 ± 0.01a | 6.41 ± 0.03a | 94.7 ± 2.3ab |
| Eugenol (1.5 g · L−1) | 3.05 ± 0.04ab | 94.3 ± 2.2a | 15.7 ± 0.4b | 7.11 ± 0.02a | 6.18 ± 0.08a | 101.0 ± 3.6a |
| Eugenol (2.0 g · L−1) | 3.19 ± 0.04b | 95.7 ± 1.4a | 15.4 ± 0.2b | 6.32 ± 0.06b | 6.86 ± 0.06a | 90.7 ± 4.1ab |
Each value represented the mean (n = 3); small letters following the values within each column indicated significant differences (α = 0.05, LSD) among control and eugenol treatments.
In addition, eugenol applications had no negative inhibitory effect on seedling growth as compared with control on day 14 (Table 1). Both highest shoot height and root length were recorded in 1.0 g · L−1 of eugenol treatment. Seedling dry weight was promoted by eugenol compared with control, the highest dry weight of whole seedling (101.0 mg) was obtained from the 1.5 g · L−1 of eugenol, while the lowest value (89.0 mg) was observed in control.
Inhibitory effect of eugenol on α-amylase activity
As shown in Fig. 1, the α-amylase activity continuously increased from 24 to 96 h of seed imbibitions, eugenol applications had lower α-amylase activity as compared with control. In which, the high eugenol concentration (2.0 g · L−1) dramatically reduced the α-amylase activity at each sampling time during seed imbibition as compared with control and the other two eugenol treatments. After 96 h of imbibition, 1.0 and 1.5 g · L−1 of eugenol treatments had no significant differences in α-amylase activity as compared with control, however, 2.0 g · L−1 of eugenol still showed a significantly lower α-amylase activity than control.
Figure 1.
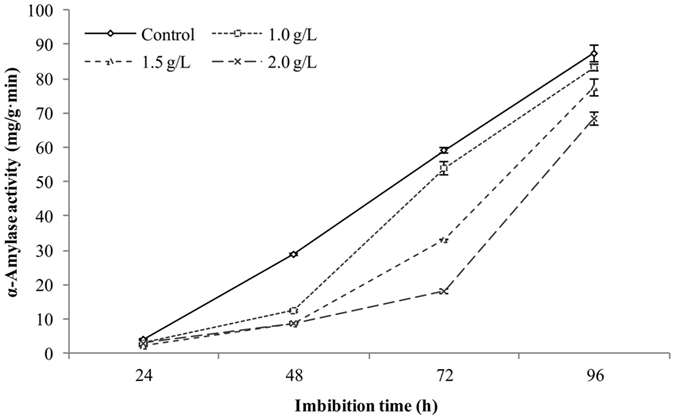
Effects of different concentrations of eugenol on α-amylase activities of Qian You 1. Control: seeds soaked in water; 1.0, 1.5 and 2.0 g · L−1: seeds soaked in the indicated concentrations of eugenol. The data were the means of three replications, and the standard deviation was indicated by bars.
Effect of eugenol on PHS in the field and quality of freshly harvested seeds
The effect of eugenol on the pre-harvest sprouting in the field was tested in 2013 year. As shown in Fig. 2, sprouting rate and sprouting index of seeds from the ears decreased obviously with the increasing of eugenol concentration as compared with control, and 1.5 and 2.0 g · L−1 of eugenol solutions reached significant levels. The sprouting rates of 1.0, 1.5 and 2.0 g · L−1 of eugenol treatments reduced by 9.3%, 36.5% and 43.4%, respectively; while the sprouting index decreased by 10.4%, 35.1% and 45.5%, respectively in comparison with control (Fig. 2). Whereas, no significant difference was observed among eugenol treatments and control in seed germination and seedling growth of freshly harvested seeds in laboratory (Table 2).
Figure 2.
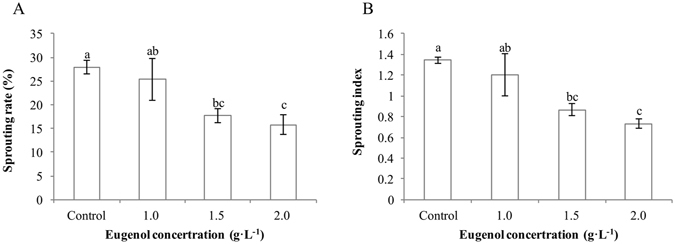
Effects of different concentrations of eugenol on the sprouting rate and sprouting index of Qian You 1 in the field. The data were the means of three replications, and the standard deviation was indicated by bars. Small letter (s) on top of bars indicated significant differences (α = 0.05, LSD) among control and different eugenol treatments at a certain imbibition time.
Table 2.
Effects of different concentrations of eugenol on the germination and seedling growth of the freshly harvested hybrid rice seeds of Qian You 1 from the field trial.
| Treatment | MGT (d) | GP (%) | GI | Shoot Height (cm) | Root Length (cm) | Seedling Dry Weight (g) |
|---|---|---|---|---|---|---|
| Control (water) | 3.02 ± 0.03a | 99.3 ± 0.7a | 17.0 ± 0.2a | 7.27 ± 0.87a | 5.36 ± 0.43a | 100.3 ± 5.5ab |
| Eugenol (1.0 g · L−1) | 3.16 ± 0.13a | 97.3 ± 1.8a | 16.7 ± 0.9a | 7.55 ± 0.11a | 5.27 ± 0.29a | 100.0 ± 3.1ab |
| Eugenol (1.5 g · L−1) | 3.13 ± 0.04a | 98.7 ± 0.7a | 16.3 ± 0.2a | 8.07 ± 0.16a | 5.64 ± 0.38a | 104.0 ± 1.0a |
| Eugenol (2.0 g · L−1) | 3.18 ± 0.02a | 97.3 ± 1.3a | 16.0 ± 0.1a | 7.16 ± 0.25a | 5.27 ± 0.52a | 89.3 ± 3.4b |
Each value represents the mean (n = 3); small letters following the values within each column indicated significant differences (α = 0.05, LSD) among control and eugenol treatments.
Effect of eugenol alone and eugenol-GA3 combined treatment on seed sprouting
The results showed that 2.0 g · L−1 of eugenol delayed seed germination by 12 hours in comparison with control; while the eugenol-GA3 combined treatment had similar germination process with control did. It suggested that 50 mg · L−1 of GA3 could relieve the inhibitory effect of 2.0 g · L−1 of eugenol and recover seed germination rate to its original speed (Fig. 3). The maximum percentage of seeds with level 2 happened at 72 h in eugenol treatment; while those of control and combined treatment recorded at 60 h. Similar delayed effect was observed in seeds with level 3, which percentage at 72 h was already 40% in control and combined treatment, while only 7% in eugenol treatment. Moreover, as shown in Fig. 4, the eugenol treatment significantly delayed seed sprouting; however, both of the sprouting rate and sprouting index increased obviously after combined treatment and reached to the control levels at 72 h of imbibition. Similar results were observed in the germination test, which showed that the increased MGT and decreased GI caused by 2.0 g · L−1 of eugenol were effectively recovered by 50 mg · L−1 of GA3 (Table 3).
Figure 3.
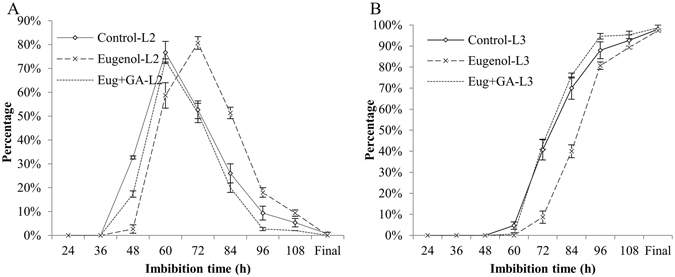
Effects of eugenol and eugenol-GA3 combined treatment on the percentage of Level 2 (A) and Level 3 (B) seeds of Qian You 1. Control: seeds soaked in water; Eugenol: seeds soaked in 2.0 g · L−1 of eugenol; Eug + GA: seeds soaked in 2.0 g · L−1 of eugenol + 50 mg · L−1 of GA3 mixed solution. L2: the radicle had extended to less than half the length of the whole seed; L3: the radicle length had extended to more than half the length of the whole seed. The data were the means of three replications, and the standard deviation was indicated by bars.
Figure 4.
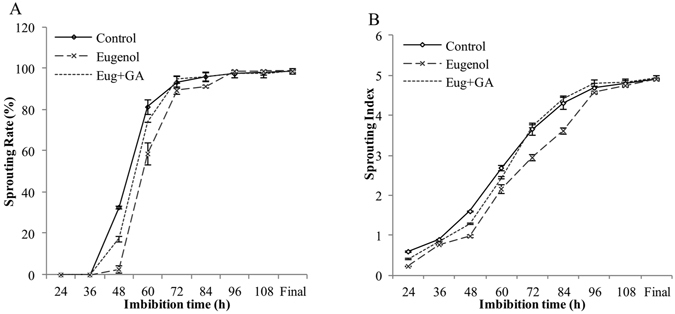
Effects of eugenol alone and eugenol + GA3 combined treatment on the sprouting rate (A) and sprouting index (B) of Qian You 1. Control: seeds soaked in water; Eugenol: seeds soaked in 2.0 g · L−1 of eugenol; Eug + GA: seeds soaked in 2.0 g · L−1 eugenol + 50 mg · L−1 of GA3 mixed solution. The data were the means of three replications, and the standard deviation was indicated by bars.
Table 3.
Effects of eugenol alone and eugenol-GA3 combined treatment on seed germination of hybrid rice Qian You 1.
| Treatment | MGT (d) | GP (%) | GI |
|---|---|---|---|
| Control (water) | 3.01 ± 0.08b | 98.7 ± 0.7a | 17.44 ± 0.49a |
| Eugenol (2.0 g · L−1) | 3.36 ± 0.02a | 97.3 ± 0.7a | 14.84 ± 0.11c |
| 2.0 g · L−1 Eug + 50 mg · L−1 GA3 | 3.13 ± 0.03b | 98.7 ± 1.3a | 16.07 ± 0.05b |
Each value represents the mean (n = 3); small letters following the values within each column indicated significant differences (α = 0.05, LSD) among control and eugenol treatments.
Effects of eugenol alone and eugenol + GA3 combined treatment on endogenous ABA content
In our study, internal ABA contents decreased from 3 h to 18 h of seed imbibition and always kept in lowest levels in control. The internal ABA contents were significantly improved by eugenol alone as compared with the control, which, however, declined significantly after seeds treated with eugenol + GA3 from 3 h to 18 h of seed imbibitions (Fig. 5). So it became reasonable that the delayed germination was associated with the enhancement of ABA synthesis at early seed imbibition.
Figure 5.
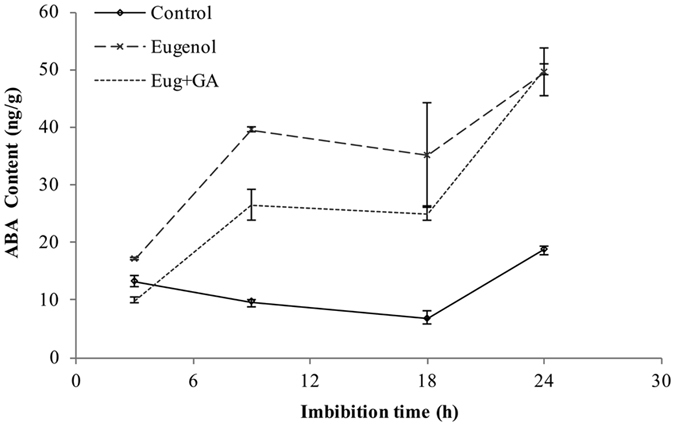
Changes of ABA contents in the presence of eugenol and eugenol + GA3 combined treatments during seed germination of Qian You 1. Control: seeds soaked in water; Eugenol: seeds soaked in 2.0 g · L−1 of eugenol; Eug + GA: seeds soaked in 2.0 g · L−1 eugenol + 50 mg · L−1 of GA3 mixed solution. The data were the means of three replications, and the standard deviation was indicated by bars.
Effects of eugenol alone and eugenol + GA3 combined treatment on expression of ABA genes
Expression levels of three members of OsABA8OH involved in ABA catabolism and five members of OsNCED involved in ABA synthesis were measured and each of them showed different expression patterns during seed imbibition (Fig. 6). The expression analysis of five OsNCEDs showed that the OsNCED1, OsNCED3 and OsNCED5 had similarly changed trends in different treatments during seed imbibition. Their expression levels at 3 h were control > eugenol + GA3 > eugenol, which decreased dramatically from 3 h to 9 h and then kept very low levels from 9 h to 24 h. Differ from OsNCED1, OsNCED3 and OsNCED5, the higher expressions of OsNCED2 in eugenol and eugenol + GA3 treatments than in control were found during seed imbibitions except for 3 h. For OsNCED4, eugenol improved its expression from 3 h to 9 h and reached the highest level at 9 h as compared with control and combined treatment. The eugenol + GA3 decreased OsNCED4 expression and had the lowest values except for 9 h from 9 h to 24 h of seed imbibition. For control, three OsABA8OHs expressions at 3 h were higher than those in eugenol and eugenol + GA3. OsABA8OHs transcript levels declined from 3 h to 9 h, in which the decrease of OsABA8OH2 expression reached a very significant level. For eugenol treatment, OsABA8OH1 and OsABA8OH3 expressions increased obviously from 3 h to 9 h as compared with control, while the expression of OsABA8OH2 always stayed in an extremely low level. For eugenol + GA3 combined treatment, OsABA8OH1 level was higher than control but lower than eugenol. In contrast, OsABA8OH2 level was significantly lower than control but a little higher than eugenol. The OsABA8OH3 expressions in seeds treated by eugenol + GA3 stayed in lowest levels among three treatments. In addition, the expression ratio of OsNCED2/OsABA8OH2 at 3 h in seeds treated with eugenol was obviously higher than those in eugenol + GA3 and control (Fig. S1 in Supplementary Data).
Figure 6.
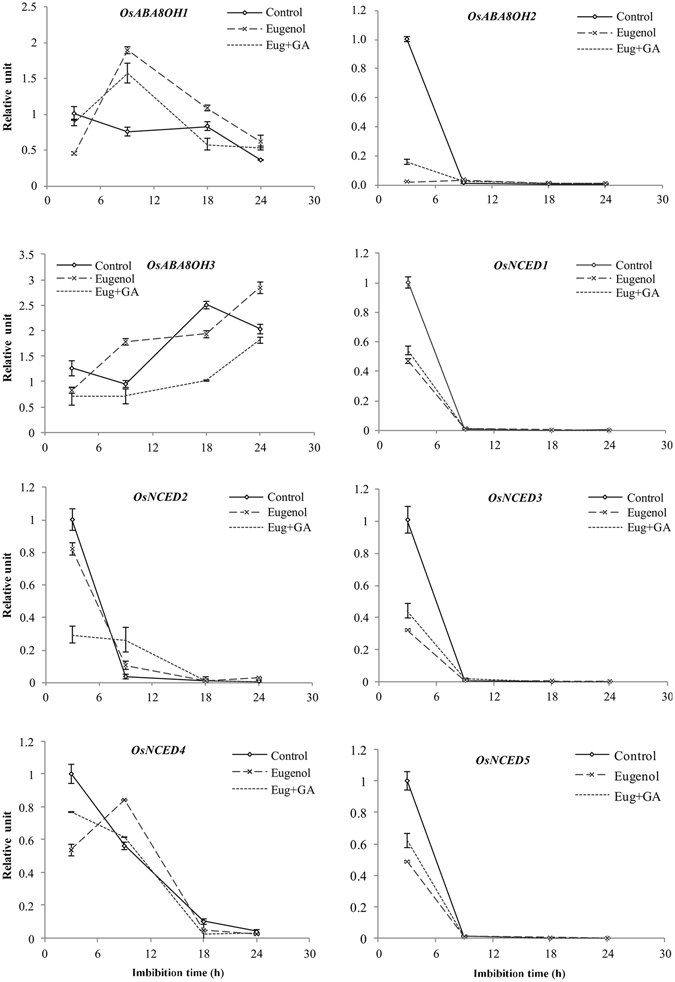
Changes of gene expression profiles of ABA metabolism during seed germination in Qian You 1. Gene expression profiles of ABA metabolism genes (OsNCED1, OsNCED2, OsNCED3, OsNCED4, OsNCED5, OsABA8OH1, OsABA8OH2, OsABA8OH3) were measured from 3 h to 24 h in rice cultivar Qian You 1. Control: seeds soaked in water; Eugenol: seeds soaked in 2.0 g · L−1 of eugenol; Eug + GA: seeds soaked in 2.0 g · L−1 of eugenol + 50 mg · L−1 of GA3 mixed solution. The data were the means of three replications, and the standard deviation was indicated by bars.
Discussion
Eugenol is the major constituent of clove [Syzygium aromaticum (L.)] essential oil, which is extracted and widely applied in multiple chemical industries. Recently, eugenol was reported to have special herbicidal activity for some specific grassy weeds and might be developed as a new herbicide product to be widely used practically12. Its inhibitory effect on seed germination was also recorded in wheat for the first time. The results were consistent with the previous research which reported that 0.4 g · L−1 of eugenol could inhibit the germination of wheat seeds13. Whereas in the present study, the inhibitory concentration was 1.5–2.0 g · L−1 (four or five times of 0.4 g · L−1), it might due to that wheat seeds germinated in eugenol solution throughout the whole experiment, while hybrid rice seeds were just pre-soaked in eugenol solution for one hour and then germinated in water. It indicated as well that the applied concentration of eugenol should be determined according to the type of crop and the treatment methods on seed or seedling. Among the three eugenol treatments, 2.0 g · L−1 of eugenol recorded maximum in MGT and minimum in GI. Eugenol treatment resulted in no significant difference in the germination percentage and seedling length. Furthermore, seedling weight was even promoted by eugenol compared with control, indicating that eugenol delayed seed germination but don’t affect seedling growth negatively.
During seed germination, activation of α-amylase plays a predominant role in hydrolyzing endosperm starch to soluble forms as the principal energy for radical elongation and seedling growth14, 15. Therefore, higher α-amylase activity generally contributes to faster germination progress. In present study, the maximum value of α-amylase activity was found in control and the minimum was in 2.0 g · L−1 of eugenol, indicating that the synthesis of α-amylase was postponed by eugenol. The decrease of α-amylase activity caused by eugenol treatment was highly coincident with the delayed germination speed.
We further applied eugenol in the field trial to examine whether eugenol could reduce the pre-harvest sprouting in the field condition. As results show, the rice ears sprayed with eugenol had significantly lower sprouting rates and indexes than those treated with water, and the inhibitory effects increased with the increasing concentration of eugenol. In addition, there was no significant difference between the eugenol treatments and control in the germination test of seeds freshly harvested from the hybrid line, which suggested that the inhibitory effect of eugenol on seed germination could be relieved over a period of time. This phenomenon might be related to the volatile characteristic of eugenol and the similar results were found in the test on the seeds directly treated by eugenol in laboratory.
In order to examine whether the inhibitory effect of eugenol could be restored by gibberellins, a co-treatment of 2.0 g · L−1 of eugenol and 50 mg · L−1 of GA3 was applied to rice seeds. The results showed that 2.0 g · L−1 of eugenol delayed seed germination by 12 hours in comparison with control; while the eugenol-GA3 combined treatment had similar germination process with control did, which suggested that the delayed sprouting induced by eugenol could be relieved by GA3 to a certain degree.
Plant hormones especially ABA and GA profoundly influence the transition from seed dormancy to germination. The internal level of ABA in plants depends on the balance of its biosynthesis and catabolism. The decrease in ABA content is considered beneficial to seed germination3. In our study, internal ABA contents decreased from 3 h to 18 h of seed imbibition and always kept in lowest levels in control. This variation tendency was mostly consistent with the results reported by Jacobsen et al.16 who found that ABA level decreased during early stage of seed imbibitions16. In contrast, both eugenol alone and combined treatment increased ABA levels from 3 h to 9 h, and eugenol alone treatment raised more than combined treatment. It was suggested that GA3 could partially inhibit the increase of ABA content caused by eugenol.
ABA 8’-hydroxylase (ABA8′-OH) and 9-cis-epoxycarotenoid dioxygenase (NCED) are thought to play predominant roles in ABA catabolism and synthesis respectively17, 18. Compared with control, ABA accumulation occurred at the early stage of seed germination in seeds treated with eugenol and combined treatment might be due to a high synthesis of ABA or low catabolism of ABA. Biosynthesis of ABA in seeds is mainly regulated by the rate-limiting enzyme NCED. Induction of NCED increased ABA content and promoted possibly seed dormancy19. Except for 3 h, OsNCED2 had higher expression levels in eugenol and combined treatment compared with control, which was consistent with the increased trends of ABA in seeds, suggesting that OsNCED2 might contribute more to seed germination postponement induced by eugenol than the other OsNCEDs in our study. After eugenol treatment, the expression profile of OsABA8OH2 at 3 h was significantly lower than control, which may contribute to lower catabolism of ABA and result in the higher ABA content. Based on the changed trends of OsABA8OHs and ABA content among the three treatments, OsABA8OH2 was thought to have more contribution to internal ABA catabolism than OsABA8OH1 and OsABA8OH3 genes did. In addition, the results of OsNCED2/OsABA8OH2 expression ratios in seeds imbibed for 3 h showed that eugenol has the higher ratio than the combined treatment and the Control (Fig. S1 in Supplementary Data). It highlighted that the delay of germination caused by eugenol was mainly induced by the suppression of ABA catabolism more than enhancement of ABA synthesis, which was similar with the study reported by Zhu et al.8 that the delay of rice seed germination induced by glucose mainly caused by suppressing OsABA8OH expression level8. Taken together, it concluded that eugenol could delay seed germination and reduce pre-harvest sprouting. However, there were no negative effects on seedling growth and quality of seeds harvested from eugenol treatments. Eugenol might promote content of ABA by mainly regulating relative genes involved in ABA catabolism. In addition, the inhibitory effects imposed by eugenol could be partially recovered by GA3.
Methods
Materials and seed treatment
Seeds of the hybrid rice variety Qian You 1 were obtained from the Zhejiang Nongke Seed Industry Co., Ltd., Hangzhou, P.R. China. Eugenol and Tween-20 were obtained from the Sinopharm Chemical Reagent Co., Ltd., Shanghai, P.R. China. ABA was obtained from Sigma-Aldrich Co., Ltd., St. Louis, Missouri USA. Eugenol was resolved in emulsifying agent Tween-20 to make eugenol solutions with different concentrations. A combined treatment consisted of eugenol and GA3 was made of equal volume of 2.0 g · L−1 of eugenol and 50 mg · L−1 of GA3. Before seed soaking, seeds for each treatment were sterilized with 0.1% NaClO for 15 min and then washed with tap water. Subsequently, seeds were treated with eugenol solutions or the combined treatment for 1 hour and dried over night at room temperature. Seeds were soaked in distilled water were used as control.
Seed germination and seedling growth assay
Seeds were soaked in distilled water (control), 1.0 g · L−1, 1.5 g · L−1 and 2.0 g · L−1 of eugenol solutions for 1 hour and dried over night at room temperature. Then seeds were germinated in germination boxes (12 cm × 12 cm × 6 cm) containing 3 layers of moistened filter paper, and placed in germination chambers under a diurnal cycle of 8 h of light at 30 °C and 16 h of darkness at 20 °C for 14 days20. Seeds were considered as germinated when the radicle reached half the seed length21, and germinated seeds were counted daily until the 14th day of germination. The germination percentage (GP) was calculated on day 14. The germination index (GI) and mean germination time (MGT) were calculated as GI = Σ(Gt/Dt) and MGT = Σ(Gt × Dt)/ΣGt, where Gt is the number of germinated seeds on Day t, and Dt is the time corresponding to Gt in days. Root length and shoot height were manually measured in ten randomly selected seedlings on day 14. The dry weight of seedlings was measured after drying at 80 °C for 24 h22.
Determination of α-amylase activity
Seed samples pre-treated with distilled water (control), 1.0, 1.5 and 2.0 g · L−1 of eugenol solutions were taken respectively at 24, 48, 72 and 96 h of imbibition, and were then quickly frozen in liquid nitrogen and stored at −80 °C. Each sample was ground into fine powder, homogenized with distilled water and then centrifuged at 5000 × g for 10 min. The supernatants were used for chromogenic reaction. The activity of α-Amylase was measured according to the 3, 5-dinitrosalicylic acid colorimetric (DNS) method as described by Li (2003)23 and calculated as enzyme activity = M × T/[R × W × t], where “M” is the maltose content (mg), “T” is the total volume of the extract, “R” is the volume of the extract used for the reaction, “W” is the weight of the seed sample and “t” is the reaction time.
Field trial
The field trial was carried out from Sep 4, 2013 to Sep 17, 2013 at the experimental farm of Zhejiang University, Hangzhou, P.R. China. The hybrid rice cultivar, Qian You 1 was used to evaluate the inhibitory effects of eugenol on PHS of hybrid rice. Four rows of the female parent and one row of the male parent were grown. Control and three eugenol treatments, with three replications were arranged randomly in a complete randomized block design (CRBD). A total of 450 kg/ha of eugenol was applied to rice ears at early yellow maturity stage, and control was sprayed with an equal amount of water. One day after eugenol treatment, rain simulators sprayed water for 10 min every 30 min from 6:00 am to 6:00 pm till harvest time to establish a moist environment with an 85% relative humidity (the rice canopy temperature and humidity data in field from September 5 to September 17, 2015 was listed in Table S1 in Supplementary Data). Fifty ears harvested from the female plants of Qian You 1 in each replicate were used for the PHS analysis. As described by Hu et al.24, seeds threshed from spikes were classified into four levels to calculate sprouting rate and sprouting index. Level 0: un-germinated seeds; Level 1: the micropyle had broken, but the embryo had not yet penetrated; Level 2: the radicle had extended to less than half the length of the whole seed; Level 3: the radicle length had extended to more than half the length of the whole seed. Sprouting rate = (number of level 2 + number of level 3)/total number of seeds, sprouting index = (1 × number of level 1 + 3 × number of level 2 + 5 × number of level 3)/total number of seeds. The un-germinated seeds harvested from control and eugenol treatment were used for germination tests to verify the persistently inhibitory effects of eugenol.
Seed sprouting Assay
In this portion, seeds were pretreated with distilled water (control), 2.0 g · L−1 of eugenol and the combined solution of eugenol and GA3 for 1 h. Seeds in each treatment were germinated as described for seed germination assay and counted every 12 h until all seeds germinated. At each time point, seeds were classified into four levels to calculate sprouting rate and sprouting index.
Extraction and measurement of ABA
After imbibed in control, 2.0 g · L−1 of eugenol and eugenol + GA3 combined solution for 3 h, 9 h, 18 h and 24 h, seed samples were collected and quickly frozen in liquid nitrogen and stored at −80 °C. Approximately, 500 mg of powdered rice seeds were used for ABA measurement with HPLC following the method described by Qin (2013)25 and Tombesi et al.26 with some modification25, 26, 20 μL of extraction for each sample was injected into a High Performance Liquid Chromatography system consisting of Binary HPLC Pump (Model 1525, Waters) and Dual λ absorbance detector (Model 2487, Waters). Sample was separated through a C18 reversed-phase chromatographic column (SunFire, 5 μm, 46 × 250 mm, Waters) with flow velocity of 0.8 mL/min. ABA content were calculated according to the standard curve.
Extraction of total RNA and qRT-PCR
Seeds were treated as described in extraction and measurement of ABA hormone. Total RNA was extracted from frozen seeds with an RNeasy Plant Mini Kit (Waryong, Beijing, China), and then 1 μg of RNA was reverse-transcribed into cDNA using a Hiscript QRT SuperMix for Qpcr (+gDNA wiper) (Vazyme, Nanjing, China). Transcript levels of each gene were measured by real-time PCR (qRT-PCR) using a LightCycler (Roche, Switzerland) and SYBR Green Master Mix (Vazyme, Nanjing, China) according to the manufacturers’ instructions. The cycling conditions included annealing at 95 °C for 120 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 1 min, and a final cycle of 95 °C for 10 s, 65 °C for 60 s and 97 °C for 1 s. qRT-PCR primers used in this study are listed in Table S2 in Supplementary Data. Transcript abundance was analyzed using the 2−ΔΔCT method as described by Livak and Schmittgen (2000)27 and the experiments were repeated three times. The relative mRNA expression level was normalized to the amplification of a rice ACTIN gene (internal standard gene)28.
Statistical analysis
Analysis of variance (ANOVA) of the obtained data was performed with SAS version 8.0 software (SAS Institute, Cary, NC). Prior to analysis, the percentage data were transformed according to y = arcsin [sqr (×/100)]. When a significant difference occurred in the treatments, the least significant difference was calculated (α = 0.05, LSD).
Electronic supplementary material
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation (No. LZ14C130002, LY15C130002), the Special Fund for Agro-scientific Research in the Public Interest (No. 201203052), National Natural Science Foundation of China (No. 31371708, 31201279), Project of Science and Technology Department of Zhejiang Province (No. 2013C02005, 2013C32023) and Jiangsu Collaborative Innovation Center for Modern Crop Production, P. R. China.
Author Contributions
H.J., H.Q. and H.W. initiated, designed the research and wrote the manuscript, H.Q. and L.C. performed the experiments, S.M.S. and H.W. analyzed the data, H.J. and G.Y. revised and edited the manuscript and also provided advice on the experiments.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04104-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yajing Guan, Email: vcguan@zju.edu.cn.
Jin Hu, Email: jhu@zju.edu.cn.
References
- 1.Dekkers BJW, Bentsink L. Regulation of Seed Dormancy by Abscisic Acid and DELAY of GERMINATION 1. Seed Sci Res. 2015;25:82–98. doi: 10.1017/S0960258514000415. [DOI] [Google Scholar]
- 2.Bykova NV, et al. Thiol Redox-Sensitive Seed Proteome in Dormant and Non-Dormant Hybrid Genotypes of Wheat. Phytochemistry. 2011;72:1162–1172. doi: 10.1016/j.phytochem.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Gubler F, Millar AA, Jacobsen JV. Dormancy Release, ABA and Pre-Harvest Sprouting. Curr Opin Plant Biol. 2005;8:183–187. doi: 10.1016/j.pbi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Chono M, et al. Field Studies On the Regulation of Abscisic Acid Content and Germinability During Grain Development of Barley: Molecular and Chemical Analysis of Pre-Harvest Sprouting. J Exp Bot. 2006;57:2421–2434. doi: 10.1093/jxb/erj215. [DOI] [PubMed] [Google Scholar]
- 5.Gualano NA, Benech-Arnold RL. Predicting Pre-Harvest Sprouting Susceptibility in Barley: Looking for “Sensitivity Windows” to Temperature Throughout Grain Filling in Various Commercial Cultivars. Field Crop Res. 2009;114:35–44. doi: 10.1016/j.fcr.2009.06.016. [DOI] [Google Scholar]
- 6.Nonogaki H, Bassel GW, Bewley JD. Germination-Still a Mystery. Plant Sci. 2010;179:574–581. doi: 10.1016/j.plantsci.2010.02.010. [DOI] [Google Scholar]
- 7.Wang Y, Zhang Y, Jin X, Wei Y, Zhu J. The Selection of Inhibitors Combination to Inhibit Pre-Harvest Sprouting in Rice and Study on Eletrophoretic Analysis. Seed. 2008;27:18–21. [Google Scholar]
- 8.Zhu G, Ye N, Zhang J. Glucose-Induced Delay of Seed Germination in Rice is Mediated by the Suppression of ABA Catabolism Rather than an Enhancement of ABA Biosynthesis. Plant Cell Physiol. 2009;50:644–651. doi: 10.1093/pcp/pcp022. [DOI] [PubMed] [Google Scholar]
- 9.Khalil AA, Deraz SF, Abd Elrahman S, El-Fawal G. Enhancement of Mechanical Properties, Microstructure, and Antimicrobial Activities of Zein Films Cross-Linked Using Succinic Anhydride, Eugenol, and Citric Acid. Prep Biochem Biotech. 2015;45:551–567. doi: 10.1080/10826068.2014.940967. [DOI] [PubMed] [Google Scholar]
- 10.Sudarma IM, Kusnandini A, Darmayanti MG. Chemical Transformation of Eugenol Isolated From Leaves of Syzygium Aromaticum to its New Isothiocyanate Derivatives. J Nat Prod. 2015;8:27–32. [Google Scholar]
- 11.Kamatou GP, Vermaak I, Viljoen AM. Eugenol-From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules. 2012;17:6953–6981. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahuja N, Batish DR, Singh HP, Kohli RK. Herbicidal Activity of Eugenol Towards some Grassy and Broad-Leaved Weeds. J Pest Sci. 2015;88:209–218. doi: 10.1007/s10340-014-0570-x. [DOI] [Google Scholar]
- 13.Darabi HR, et al. Clove Bud Oil: An Efficient, Economical and Widely Available Oil for the Inhibition of Wheat Seed Germination. Environ Chem Lett. 2011;9:519–524. doi: 10.1007/s10311-011-0312-8. [DOI] [Google Scholar]
- 14.Liao D, Zhang H, Li Z, Bennett J. Characterization of Evolution and Tissue-Expression of Rice (Oryza Sativa L.) α-Amylase Genes. Acta Agronomica Sinica. 2010;36:17–27. doi: 10.3724/SP.J.1006.2010.00017. [DOI] [Google Scholar]
- 15.Kato-Noguchi H. Effects of Four Benzoxazinoids On Gibberellin-Induced α-Amylase Activity in Barley Seeds. J Plant Physiol. 2008;165:1889–1894. doi: 10.1016/j.jplph.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN. Abscisic Acid, Phaseic Acid and Gibberellin Contents Associated with Dormancy and Germination in Barley. Physiol Plantarum. 2002;115:428–441. doi: 10.1034/j.1399-3054.2002.1150313.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, et al. Expression Patterns of ABA and GA Metabolism Genes and Hormone Levels during Rice Seed Development and Imbibition: A Comparison of Dormant and Non-Dormant Rice Cultivars. J Genet Genomics. 2014;41:327–338. doi: 10.1016/j.jgg.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular Aspects of Seed Dormancy*. Annu Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- 19.Graeber K, et al. Cross-Species Approaches to Seed Dormancy and Germination: Conservation and Biodiversity of ABA-regulated Mechanisms and the Brassicaceae DOG1 Genes. Plant Mol Biol. 2010;73:67–87. doi: 10.1007/s11103-009-9583-x. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Relationship Between Endogenous Salicylic Acid and Antioxidant Enzyme Activities in Maize Seedlings Under Chilling Stress. Exp Agr. 2013;49:295–308. doi: 10.1017/S0014479712001329. [DOI] [Google Scholar]
- 21.Hu, Q. et al. Inhibitory Effect of Chemical Combinations On Seed Germination and Pre-Harvest Sprouting in Hybrid Rice. Plant Growth Regul. 1–9 (2016).
- 22.Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A. Seed Priming with Brassinolide Improves Lucerne (Medicago Sativa L.) Seed Germination and Seedling Growth in Relation to Physiological Changes Under Salinity Stress. Australian Journal of Agricultural Research. 2007;58:811. doi: 10.1071/AR06253. [DOI] [Google Scholar]
- 23.Li, H. Principle and Technology of Plant Physiological and Biochemical Experimentso Higher Education Press, Beijing 2000.
- 24.Hu, W. M., He, C. X. & Ma, H. S. Genetic Effects of Pre-harvest Sprouting in Hybrid Rice (Oryza sativa L.). Chinese J Rice Sci. 3, 76–79 (In Chinese with English abstract) (2002).
- 25.Qin, G. Changes of Endogenous Hormone ABA Content, Antioxidant Enzyme Activities and Seed Quality During Seed Development in Sponge Gourd: Zhejiang University 2013.
- 26.Tombesi S, et al. Stomatal Closure is Induced by Hydraulic Signals and Maintained by ABA in Drought-Stressed Grapevine. Sci Rep. 2015;5:12449. doi: 10.1038/srep12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(T) (-Delta Delta C) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Zhong, M. et al. Effects of Exogenous Putrescine On Glycolysis and Krebs Cycle Metabolism in Cucumber Leaves Subjected to Salt Stress. Plant Growth Regul. 1–12 (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


