Abstract
The infralimbic cortex (IL) is known to facilitate the formation of extinction memory through reciprocal interactions with the amygdala, which produces fear responses such as freezing. Thus, whether presynaptic input from the amygdala and post-synaptic output of IL neurons are functionally dissociated in extinction memory formation remains unclear. Here, we demonstrated that photostimulation of IL inputs from BLA did not change freezing responses to conditioned stimuli (CS) during training, but did facilitate extinction memory, measured as a reduction in freezing responses to the CS 1 day later. On the other hand, photostimulation of somata of IL neurons induced an immediate reduction in freezing to CS, but this did not affect extinction memory tested the next day. These results provide in vivo evidence for IL-dependent facilitation of extinction memory without post-synaptic modulation of freezing circuits.
Introduction
The infralimbic cortex (IL) has been implicated in the extinction of conditioned fear responses1–3. A mechanism of extinction memory has been proposed based on pioneering studies in rodents employing electrical stimulation4, 5 and pharmacological inactivation6, 7 of IL neurons, which facilitates or interferes with extinction memory formation. Given that electrical stimulation of the IL reduces freezing response to conditioned stimuli (CS)5 and the fact that the amygdala is the neural substrate for freezing responses to CS8, 9, it has been thought that the post-synaptic impact of IL neurons on the amygdala is critical for extinction memory. Indeed, connectome studies have shown that efferents of IL neurons innervate inhibitory neurons in the amygdala10, explaining how electrical stimulation can reduce the freezing response to CS.
IL neurons are known to receive input from a subset of excitatory neurons in the basolateral amygdala (BLA)11, 12. Recent studies suggests that these excitatory BLA neurons are involved in fear extinction13, 14. Considering that electrical stimulation activates axon terminals at the stimulation site15, 16, afferent presynaptic input from the BLA to the IL could contribute to the extinction memory formation facilitated by electrical stimulation of the IL17, 18. A recent study showed that optogenetically induced long-term depression (LTD) of the BLA-IL synapse facilitates extinction memory to CS rather than inhibition, suggesting an inhibitory role of this synapse19; thus, the role of presynaptic activity in the IL remains controversial. Here, we optogenetically stimulated input and output circuits separately under the same experimental conditions, then examined effects on freezing responses and retrieval of extinction memory in mice.
Results
To compare input and output circuits in the IL, we separately stimulated each circuit optogenetically, which enables stimulation of specific types of cells or projections20. This approach is premised on the idea that previous experiments using electrical stimulation might have stimulated both circuits simultaneously. To stimulate IL somata, we introduced an adeno-associated virus harboring a vector that expresses the light-gated cationic channel, channelrhodopsin (ChR2) under the control of the CamKII promoter (AAV-CamKII-ChR2) into the IL (Fig. 1a, ILChR2). This promoter specifies the expression of ChR2 in pyramidal neurons, except for interneurons21–24, and has been used to successfully induce action potentials in response to laser pulses delivered to the medial prefrontal cortex (mPFC)25. For illumination of the ChR2-expressing neurons, the optic fiber was located in IL as illustrated in Fig. 1a and Supplementary Fig. S1a. As a control, we used a viral vector expressing enhanced yellow fluorescent protein (EYFP) under the control of the CamKII promoter (ILEYFP). On day 1, mice were trained for acquisition of fear memory by coupling a CS (tone) with an unconditioned stimulus (electrical foot shock). On day 2, mice were repetitively exposed to CS coupled with laser stimulation (473 nm, 10 Hz, 20 ms), delivered to the IL by the optic fiber, in a different chamber. The next day, the level of extinction memory was tested in an extinction chamber by measuring the reduction in freezing responses to CS (Fig. 1b). As shown in Fig. 1c, ILChR2 mice showed a greater reduction in freezing responses to CS when coupled with photostimulation compared with control ILEYFP mice (ANOVA group effect: F 1,20 = 42.036, *P < 0.001; post hoc: P < 0.05 for all, except for trial 20 [P = 0.137]), as described in previous reports using an electrical stimulation paradigm5. Surprisingly, however, there was no significant difference in freezing responses between ILChR2 and ILEYFP mice tested the next day without laser stimulation (Fig. 1c; ANOVA group effect: F 1,20 = 0.584, P = 0.453). IL photostimulation could conceivably produce this effect by increasing overall locomotion, which can affect freezing, but a comparison of locomotor activity revealed no significant difference between ILChR2 and ILEYFP mice (Fig. 1d; ANOVA group effect: F 1,16 = 0.0000621, P = 0.994). These data suggest that, although neuronal output from the IL reduces freezing responses, it does not affect retrieval of extinction memory.
Figure 1.
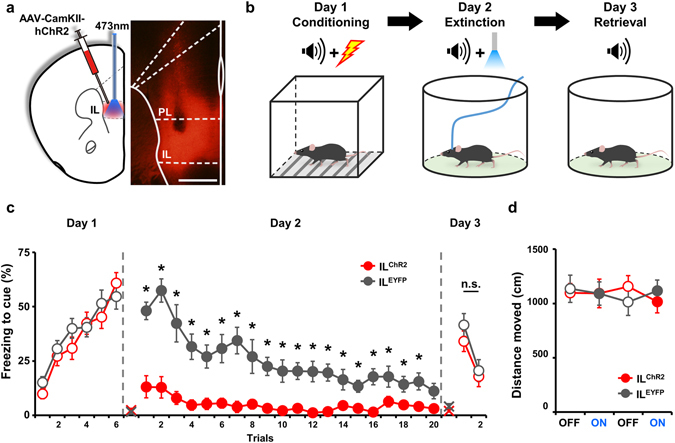
Stimulation of the IL during extinction training decreases freezing without affecting retrieval of extinction memory. (a) Schematic representation of AAV2/9-CamKIIa-hChR2(H143R)-mCherry infection and optic fiber placement in the IL. The schematic of the mouse brain is drawn based on the Franklin and Paxinos mouse brain atlas. Scale bar: 500 μm. (b) Experimental procedure for extinction with optic stimulation. (c) ChR2 stimulation of the IL during extinction training decreased freezing to tone on day 2, but did not facilitate extinction retrieval tested on day 3 (ILChR2, n = 12; ILEYFP, n = 10). (d) ChR2 stimulation of the IL did not change the locomotor activity of mice in an open field (ILChR2, n = 9; ILEYFP, n = 9). Data are presented as means ± s.e.m. Empty circle, absence of optic stimulation; filled circle, presence of optic stimulation; X, baseline freezing level.
Next, we tested the hypothesis that strengthening of extinction retrieval depends on a presynaptic mechanism, focusing on inputs from the BLA, since this region is known to be involved in fear extinction13, 14. To stimulate axonal projections from the BLA to the IL, we delivered a viral vector expressing ChR2 (BLAChR2) or EYFP (BLAEYFP) to the BLA (Fig. 2a) and placed an optic fiber in the IL (Supplementary Fig. S1b). To determine whether simulation of projections from the BLA could induce neural activity in the IL, we recorded IL activity in vivo following light stimulation in mice expressing ChR2 or EYFP in the BLA (Supplementary Fig. S2a). Blue laser stimulation under the same conditions used in behavioral experiments (10 Hz, 20 ms) successfully induced action potentials in the BLAChR2 group, but failed to affect neural activity in the BLAEYFP group (Supplementary Fig. S2b,c). An analysis of multiunit activity (MUA) showed that the neural activity of the IL was significantly increased by light stimulation in the BLAChR2 group (Supplementary Fig. S2d,f; Wilcoxon Signed Rank test, *P < 0.0001), whereas there was no laser stimulation-dependent change in MUA in the BLAEYFP group (Supplementary Fig. S2e,g; Paired t-test, t 6 = 0.503, P = 0.633). During extinction training on day 2, we delivered photostimuli to the IL of BLAChR2 mice to specifically stimulate BLA input to the IL (Fig. 2a). BLAChR2 mice showed no difference in freezing responses to CS compared with BLAEYFP mice in all trials during extinction training (Fig. 2b; ANOVA group effect: F 1,19 = 0.413, P = 0.528). Interestingly, however, when fear extinction was tested 24 h after extinction, BLAChR2 mice showed significantly decreased freezing responses to CS compared with BLAEYFP mice (Fig. 2b; ANOVA group effect: F 1,19 = 5.948, *P = 0.025; post hoc tests: *P = 0.019 for trial 1, and P = 0.073 for trial 2). These results support the interpretation that BLA inputs to the IL are critical for the facilitation of extinction memory, which results in stronger retrieval of extinction on the next day.
Figure 2.
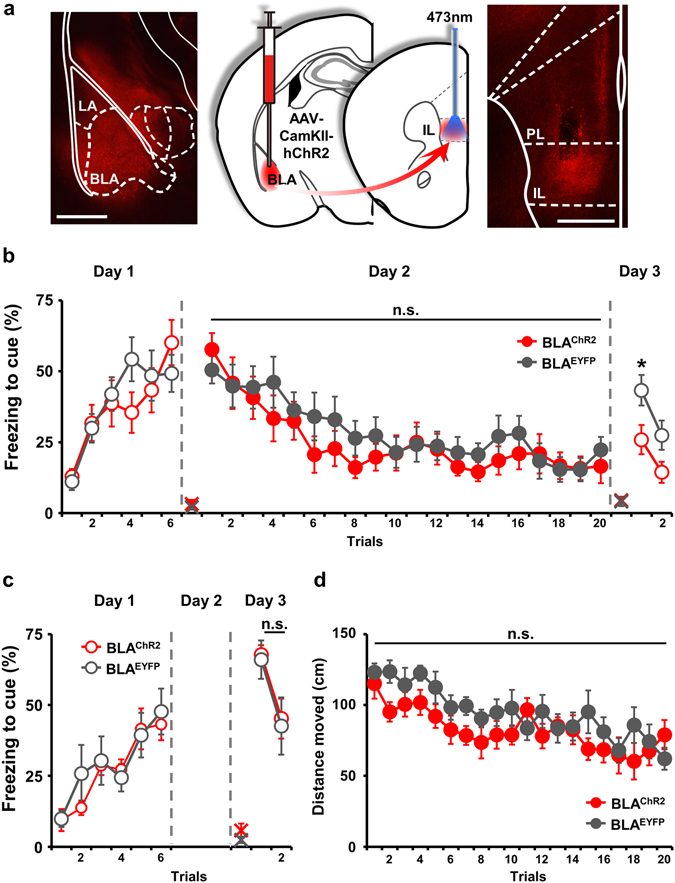
Stimulation of presynaptic input of the BLA during extinction training facilitates extinction memory without affecting freezing responses during extinction training. (a) Schematic representation of AAV2/9-CamKIIa-hChR2(H143R)-mCherry infection in the BLA and optic fiber placement in the IL. Scale bar: 500 μm. (b) ChR2 stimulation of presynaptic input from the BLA to the IL during extinction training did not affect freezing responses to a tone on day 2, but facilitated extinction retrieval, measured as freezing responses on day 3 (BLAChR2, n = 10; BLAEYRP, n = 11). (c) Optic stimulation of presynaptic input from the BLA to the IL without extinction training did not affect freezing in retrieval tests (BLAChR2, n = 7; BLAEYFP, n = 7). (d) Optic stimulation of afferent projections from the BLA to the IL did not alter locomotion (BLAChR2, n = 7; BLAEYFP, n = 7). Data are presented as means ± s.e.m. Empty circle, absence of optic stimulation; filled circle, presence of optic stimulation; X, baseline freezing level.
To test whether stimulation of BLA inputs to the IL actually facilitate extinction memory in a manner that depends on a new association of CS with a ‘safe’ signal, we applied optic stimulation of BLA-IL axonal projection to conditioned mice without tone presentation during day 2 (Supplementary Fig. S1c and Fig. 2c). We found that this CS-free photostimulation caused no differences in fear retrieval between BLAChR2 and BLAEYFP groups (Fig. 2c; ANOVA group effect: F 1,12 = 0.0505, P = 0.826). Moreover, an analysis of moving distance during laser illumination showed that stimulation of afferent projections from the BLA to the IL had no effect on the locomotor activity of mice (Fig. 2d; ANOVA group effect: F 1,12 = 1.828, P = 0.201). These results strongly suggest that BLA-to-IL input facilitates extinction memory without affecting post-synaptic modulation of freezing circuits.
Discussion
Extinction memory is known to be independent of the mechanism that modulates fear memory itself2. However, studies have shown that IL neurons modulate extinction memory through their post-synaptic impacts on the amygdala involved in fear memory processing1, 10. Our study demonstrates that presynaptic input and postsynaptic output of IL neurons function in a dissociable way: photostimulation of presynaptic inputs from the BLA to the IL facilitates extinction memory (Fig. 2), whereas somatic activation of IL neurons decreases freezing without affecting extinction memory (Fig. 1). These findings strongly suggest that extinction memory can be formed in the IL without affecting post-synaptic modulation of freezing circuits. We confirmed that under our photostimulation conditions, mice showed no difference in locomotor activity compared with control mice in response to CS (Fig. 2b,d) or in an open field test (Fig. 1d), excluding the possibility that the freezing response is affected by a photostimulation-induced change in locomotor behavior.
Our conclusion is consistent with previous reports that extinction of conditioned fear is accompanied by increased field potential responses in the BLA-IL pathway26, and that electrical stimulation facilitates extinction memory through enhancement of this projection5. In addition, a specific population of BLA neurons known to be activated during acquisition of extinction memory13 sends projections to the IL, and optogenetic inhibition of BLA neurons projecting to IL during extinction has been shown to impair retrieval of extinction memory without affecting freezing during illumination14, findings that are consistent with our demonstration that stimulation induces facilitation of extinction memory without affecting freezing.
In contrast to previous reports and the conclusions reached here, a recent study reported that the induction of LTD in the BLA-IL synapse by high-frequency photostimulation of the ChR2 variant, ChETA, before extinction learning facilitates the formation of extinction memory, suggesting an inhibitory role of this input19. In these latter experiments, however, LTD pretreatment was given before extinction learning and decreased the CS-induced freezing response19 before acquisition of extinction memory, suggesting any impact on the freezing circuit mechanism that reduces the fear response itself. However, in our gain-of-function experiment, photostimulation of the BLA-IL synapse was coupled to CS during extinction learning, and this facilitated extinction memory without affecting the freezing-circuit mechanism (Fig. 2b). Thus, the discrepancy between our conclusions and this previous report may reflect differences in experimental conditions, including the timing of optogenetic treatment and the consequent impact on the freezing circuit.
We also demonstrated a post-synaptic impact of IL neurons on the freezing response to CS, even before or during extinction memory learning (Fig. 1c), probably through the well-known projections from the IL to the intercalated nuclei (ICN)10, 27, clusters of GABAergic neurons that inhibit freezing outputs from the central amygdala28, 29. In a similar vein, high-frequency photostimulation of the BLA-IL synapse to induce LTD30 could cause a reduction in freezing through potentiation of this IL-ICN connection, as seen in electrical stimulation of the IL. A previous study reported that the post-synaptic impact of IL neurons is only functional after extinction memory acquisition, as evidenced by the observation that photostimulation of IL neurons has no effect on CS-induced freezing before extinction learning31. This discrepancy may be caused by using different mouse strain which can affect fear behavior32, however, it remains unclear how the conditions of optogenetic stimulation of IL neurons used in this previous report could bypass the existing post-synaptic impact on the freezing circuit. Consistent with the anatomical connection of the IL to the freezing circuit as well as our hypothesis, previous studies have shown that activation of the IL through electrical4, 33, pharmacological34, 35, or optogenetic36 approaches leads to a significant reduction in freezing responses to CS.
Our results clearly show that extinction memory formation in the IL can occur independent of the postsynaptic effects of the IL on freezing circuits (Fig. 1b). A recent study, however, showed that bilateral photostimulation of ChR2-expressing IL neurons produces the same effect as unilateral electrical stimulation, reducing freezing during extinction memory training and strengthening retrieval of extinction36. Our findings suggest that this facilitation of extinction memory may result from activation of presynaptic areas of the IL through activation of callosal inputs from the contralateral IL18. Optogenetic stimulation of interhemispheric projections can induce excitatory post-synaptic potentials (EPSPs) in mPFC pyramidal neurons projecting to subcortical regions37; thus, simultaneous stimulation of these presynaptic inputs and postsynaptic IL neurons may potentiate the IL38 and affect the formation of extinction memory. In addition, a stronger photostimulation (5 mW) was used in the former study than was used here (1 mW), a potentially important difference given that neural and behavioral responses can differ depending on the intensity of light stimulation39, 40. Accordingly, stronger photostimulation of IL neurons may induce changes in a broader range of target neurons associated with extinction memory.
The freezing circuit-independent facilitation of extinction memory in the IL may contribute to the fine modulation of extinction memory expression through the freezing circuit. Studies have shown that expression of extinction memory is modulated according to contextual information3 encoded by the hippocampus41, 42. Given that IL neurons receive inputs from the hippocampus and the amygdala18, the integration of various streams of information in the IL would appear to be critical for the expression of extinction memory. Finally, this gating mechanism for extinction memory in the IL could be a relevant target for understanding and treating fear and anxiety disorders, such as post-traumatic stress disorder.
Materials and Methods
Subjects
Adult male C57BL/6J mice (10–13 wk old) were used for all experiments. Food and water was available ad libitum under a 12:12 hour light:dark cycle (lights on, 06:00) throughout all experimental procedures. All animal care and experimental procedures were performed in accordance with protocols approved by the Animal Care and Use Committee of Korea Advanced Institute of Science and Technology (KAIST).
Stereotaxic surgery
Mice were anesthetized with Avertin (2, 2, 2-tribromoethanol; Sigma, USA) and placed on a stereotaxic frame (Neurostar, Germany) for viral infection and implantation of fiber optic cannulae. Two AAV constructs were used for viral expression: AAV2/9-CamKIIa-hChR2(H134R)-mCherry-WPRE-hGH, purchased from University of Pennsylvania Vector Core (https://www.med.upenn.edu/gtp/vectorcore), was used for expression of hChR2 in excitatory pyramidal neurons21, 22; and AAV2/5-CamKIIa-eYFP, from the University of North Carolina Vector Core (https://www.med.unc.edu/genetherapy/vectorcore), was used as a control. AAVs were delivered unilaterally to the IL (anteroposterior [AP], + 1.90 mm; mediolateral [ML], ± 0.30 mm; dorsoventral [DV], −2.40 mm) or BLA (AP, −1.30 mm; ML, ± 3.40 mm; DV, −4.10 mm) using a Nanofil syringe with a 33-gauge injection needle (World Precision Instruments, Inc., USA). A monofiber optic cannula (200 μm diameter, 2.5 mm length; Doric Lenses, Canada) was implanted into the IL by affixing it to the skull with Super-Bond (Sun Medical, Japan). After surgery, mice were singly housed to prevent damage of intracranial implants and were allowed 2 wk for recovery and viral expression before behavioral testing.
Histology
After completion of experiments, the brain was removed, fixed by placing in phosphate-buffered 4% formaldehyde solution for 1–2 d, cut into 60-μm-thick coronal sections using vibratome (Leica VT1200S, Germany), and mounted on slides. Virally mediated expression of fluorescent proteins and the location of optic fibers were confirmed by imaging sections with a fluorescence microscope (Olympus IX41; Olympus, Japan), guided by the Franklin and Paxinos mouse brain atlas43. Animals showing off-target optic fiber location or absence of viral expression at the end of the optic fiber were excluded from the analysis.
Auditory fear conditioning
For auditory fear conditioning (day 1), mice were placed in a standard conditioning chamber (Coulbourn Instruments, USA). After a 3-min habituation, mice received six tones (3000 Hz, 80 dB, 30 s) at 2-min intervals that co-terminated with an electrical shock (0.5 mA, 1 s). On day 2, mice were positioned in an acrylic cylinder (diameter, 23 cm). After a 3-min acclimation period, mice received 20 tones at 40-s intervals to acquire fear extinction. Each tone was coupled with stimulation by blue laser (wavelength, 473 nm) illumination, delivered as 10-Hz, 20-ms pulses. Laser power (~1 mW for IL somata stimulation and ~4 mW for BLA projection stimulation) was measured at the tip of the fiberoptic cannula. For experiments employing stimulation without extinction training, optic stimulation was presented without tones. The next day, mice were placed in the same context as used for extinction training, and retrieval of extinction was tested. After 3 min, two tones were presented at 40-s intervals without optic stimulation. The behavior of mice throughout all procedures was recorded as a video file and analyzed for freezing behavior and locomotor activity using FreezeFrame 3 (Actimetrics, USA) and Ethovision XT (Noldus, Netherlands) software, respectively.
Open field with optic stimulation
Mice were positioned in the center of 42 × 42 cm acrylic box with white walls and floor. After 15-min acclimation period and a 5-min light-off session, mice received blue laser stimulation (473 nm wavelength, 10 Hz, 20 ms) for 5 min (light-on session). These light off/on sessions were repeated one more time. All mouse behavior was recorded as a video file and analyzed using Ethovision (Noldus, Netherlands).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by a grant from the National Leading Research Laboratory Program of the Korea Ministry of Science, ICT and Future Planning (2016015167 to D.K). We thank Dr. K. Deisseroth (Stanford University) for providing optogenetic viral vectors.
Author Contributions
J.H. performed behavioral analyses and optogenetic experiments. J.H. and D.K. designed the project and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04335-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 3.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 6.Morawska MM, Fendt M. The effects of muscimol and AMN082 injections into the medial prefrontal cortex on the expression and extinction of conditioned fear in mice. J Exp Biol. 2012;215:1394–1398. doi: 10.1242/jeb.068213. [DOI] [PubMed] [Google Scholar]
- 7.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 9.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience. 2012;205:112–124. doi: 10.1016/j.neuroscience.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Bacon SJ, Headlam AJN, Gabbott PLA, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: A light and electron microscope study. Brain Research. 1996;720:211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- 13.Herry C, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 14.Senn V, et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matterII. Evidence from selective inactivation of cell bodies and axon initial segments. Experimental Brain Research. 1998;118:489–500. doi: 10.1007/s002210050305. [DOI] [PubMed] [Google Scholar]
- 16.Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 17.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 18.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 19.Klavir O, Prigge M, Sarel A, Paz R, Yizhar O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci. 2017 doi: 10.1038/nn.4523. [DOI] [PubMed] [Google Scholar]
- 20.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Liu XB, Jones EG. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Natl Acad Sci USA. 1996;93:7332–7336. doi: 10.1073/pnas.93.14.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van den Oever MC, et al. Ventromedial Prefrontal Cortex Pyramidal Cells Have a Temporal Dynamic Role in Recall and Extinction of Cocaine-Associated Memory. The Journal of Neuroscience. 2013;33:18225–18233. doi: 10.1523/JNEUROSCI.2412-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen JP, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proceedings of the National Academy of Sciences. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goshen I, et al. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Lee E, et al. Left brain cortical activity modulates stress effects on social behavior. Sci Rep. 2015;5:13342. doi: 10.1038/srep13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vouimba RM, Maroun M. Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology. 2011;36:2276–2285. doi: 10.1038/npp.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, ParÉ D. infralimbic cortex activation increases c-fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paré, D. & Smith, Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience57, 1077–1090, doi:http://dx.doi.org/10.1016/0306-4522(93)90050-P (1993). [DOI] [PubMed]
- 29.Likhtik, E., Popa, D., Apergis-Schoute, J., Fidacaro, G. A. & Pare, D. Amygdala intercalated neurons are required for expression of fear extinction. Nature454, 642–645, doi:http://www.nature.com/nature/journal/v454/n7204/suppinfo/nature07167_S1.html (2008). [DOI] [PMC free article] [PubMed]
- 30.Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. Journal of Neurophysiology. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Cho HY, Augustine GJ, Han JH. Selective Control of Fear Expression by Optogenetic Manipulation of Infralimbic Cortex after Extinction. Neuropsychopharmacology. 2016;41:1261–1273. doi: 10.1038/npp.2015.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camp M, et al. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Genes, Brain and Behavior. 2009;8:744–752. doi: 10.1111/j.1601-183X.2009.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 34.Thompson BM, et al. Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn Mem. 2010;17:591–599. doi: 10.1101/lm.1920810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang C-h, Maren S. Medial prefrontal cortex activation facilitates re-extinction of fear in rats. Learning & Memory. 2011;18:221–225. doi: 10.1101/lm.2070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, A T. et al. Pyramidal Neurons in Prefrontal Cortex Receive Subtype-Specific Forms of Excitation and Inhibition. Neuron81, 61-68, doi:http://dx.doi.org/10.1016/j.neuron.2013.10.031 (2014). [DOI] [PMC free article] [PubMed]
- 38.Feldman, D E. The Spike-Timing Dependence of Plasticity. Neuron75, 556–571, doi:http://dx.doi.org/10.1016/j.neuron.2012.08.001 (2012). [DOI] [PMC free article] [PubMed]
- 39.Lee H, et al. Scalable control of mounting and attack by Esr1 + neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature470, 221–226, doi:http://www.nature.com/nature/journal/v470/n7333/abs/10.1038-nature09736-unlocked.html#supplementary-information (2011). [DOI] [PMC free article] [PubMed]
- 41.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 42.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paxinos, G. & Franklin, K. B. The mouse brain in stereotaxic coordinates. (Gulf Professional Publishing, 2004).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


