Abstract
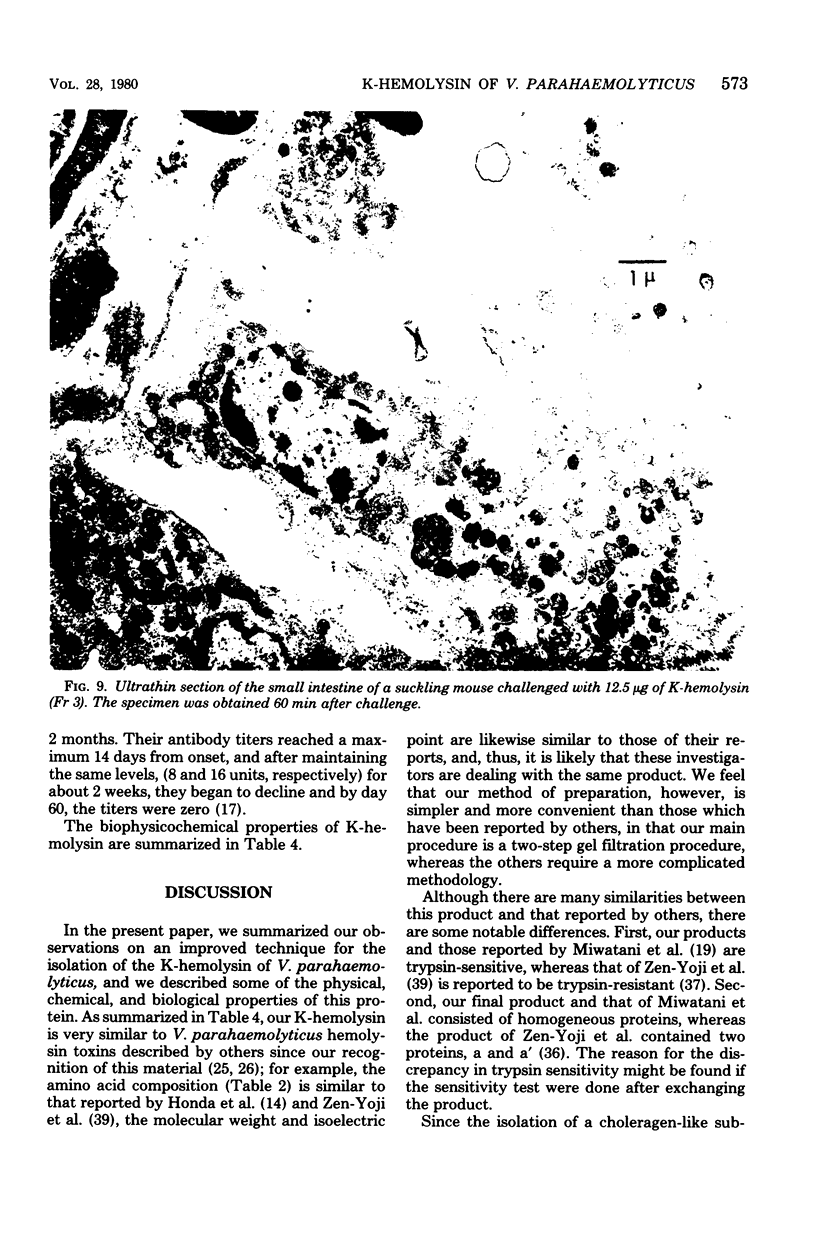
Kanagawa phenomenon-associated hemolysin (K-hemolysin) was purified by Sephadex gel and ion-exchange column chromatography, after the culture supernatant had been adsorbed on and eluted from diethylaminoethyl-Sepharose CL-6B, and acid precipitated. K-hemolysin was a heat-stable and trypsin-susceptible protein with an apparent molecular weight of 44,000, the subunit of which was 22,000. The isoelectric point was 4.9. The minimum hemolytic dose was 0.1 μg/ml. The fifty percent lethal dose by intravenous injection was 1.4 μg. Electron microscopy of the small intestine of suckling mice orally challenged with the highest dose (50 μg) not only showed disappearance of epithelial cell microvilli, but also structural disturbances of the endoplasmic reticulum and mitochondrial swelling. One blueing dose representing permeability factor activity was 0.3 μg, and positive reaction in the rabbit ileal loop appeared at above 125 μg. Besides these data in experimental models, we discovered the appearance of an antibody in patients which neutralizes K-hemolysin during the course of the disease. This finding reinforces our view that K-hemolysin plays a most significant role in the pathogenesis of this enteric human disease.
Full text
PDF


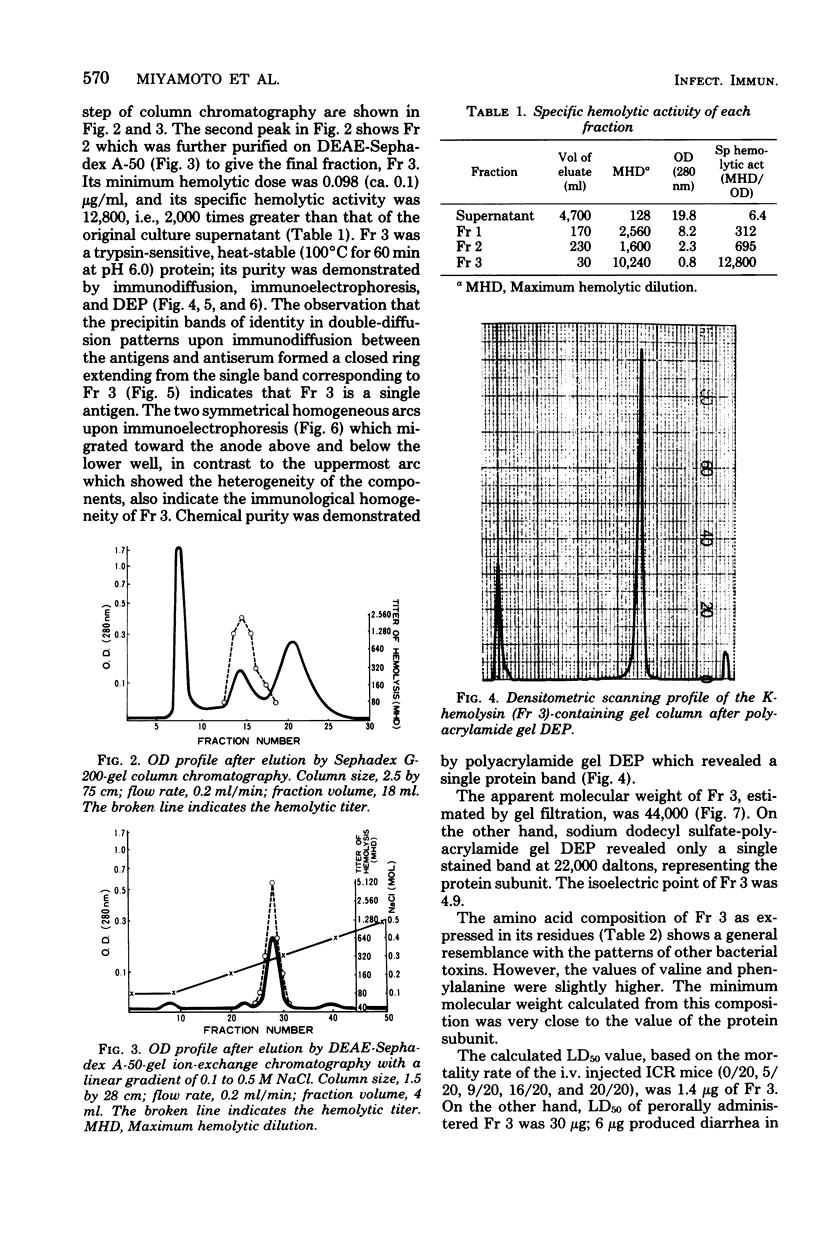





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DE S. N. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959 May 30;183(4674):1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Goshima K., Takeda Y., Sugino Y., Miwatani T. Demonstration of the cardiotoxicity of the thermostable direct hemolysin (lethal toxin) produced by Vibrio parahaemolyticus. Infect Immun. 1976 Jan;13(1):163–171. doi: 10.1128/iai.13.1.163-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Shimizu M., Takeda Y., Miwatani T. Isolation of a factor causing morphological changes of chinese hamster ovary cells from the culture filtrate of Vibrio parahaemolyticus. Infect Immun. 1976 Oct;14(4):1028–1033. doi: 10.1128/iai.14.4.1028-1033.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda T., Hasibuan M. A., Takeda Y., Miwatani T. Identification of lethal toxin with the thermostable direct hemolysin produced by Vibrio parahaemolyticus, and some physicochemical properties of the purified toxin. Infect Immun. 1976 Jan;13(1):133–139. doi: 10.1128/iai.13.1.133-139.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miyamoto Y., Kato T., Obara Y., Akiyama S., Takizawa K., Yamai S. In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J Bacteriol. 1969 Nov;100(2):1147–1149. doi: 10.1128/jb.100.2.1147-1149.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Obara Y., Nikkawa T., Yamai S., Kato T. Proceedings: Extraction, purification, and biophysico-chemical characteristics of a "Kanagawa phenomenon"-associated hemolytic factor of Vibrio parahaemolyticus. Jpn J Med Sci Biol. 1975 Feb;28(1):87–90. [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Niikawa T., Obara Y., Yamai S., Miyamoto Y. Purification of a hemolysin from Vibrio parahaemolyticus. Jpn J Med Sci Biol. 1972 Jun;25(3):197–200. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Oashi M., Shimada T., Fukumi H. In vitro production of enterotoxin and hemorrhagic principle by Vibrio cholerae, NAG. Jpn J Med Sci Biol. 1972 Jun;25(3):179–194. doi: 10.7883/yoken1952.25.179. [DOI] [PubMed] [Google Scholar]
- Obara Y. [Hemolytic factors of vibrio parahemolyticus. 1. Hemolytic activity of the supernatant fluid of its culture]. Kansenshogaku Zasshi. 1971 Sep;45(9):385–391. doi: 10.11150/kansenshogakuzasshi1970.45.385. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Sakurai J., Matsuzaki A., Miwatani T. Purification and characterization of thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1973 Nov;8(5):775–780. doi: 10.1128/iai.8.5.775-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON E. O. Modification of tyrosine during performic acid oxidation. Biochim Biophys Acta. 1954 Nov;15(3):440–441. doi: 10.1016/0006-3002(54)90052-9. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yanagase Y., Inoue K., Ozaki M., Ochi T., Amano T. Hemolysins and related enzymes of Vibrio parahaemolyticus. I. Identification and partial purification of enzymes. Biken J. 1970 Jun;13(2):77–92. [PubMed] [Google Scholar]







