Abstract
Background
A cancer diagnosis leads to increased psychological and emotional distress. However, in the aftermath of a traumatic event, such as being diagnosed with breast cancer, an individual may also experience beneficial changes in life perspective, relationships with others, and more. These changes are collectively known as posttraumatic growth (PTG). Studies have demonstrated that cortisol levels have been linked with cancer survival, yet an investigation of the relationship between PTG and cortisol has yet to be conducted among cancer patients.
Methods
The relationship of PTG to cortisol levels was examined among 99 metastatic breast cancer patients.
Results
We found a significant correlation between PTG and diurnal cortisol slope (Spearman’s rho = −0.21, p < 0.05), indicating a link between positive psychological changes and healthier endocrine functioning in cancer patients.
Conclusions
PTG in response to the stress of cancer was related to more normal (i.e., steeper) diurnal cortisol patterns. Longitudinal studies are recommended to investigate these mechanisms in relationship to cancer survival.
Keywords: breast cancer, posttraumatic growth, diurnal cortisol, endocrine function
Introduction
Cancer diagnosis and treatment is an extremely stressful and even traumatic experience that can result in poor psychological adjustment. Although extensive research has shown psychologically damaging effects of cancer, it can also stimulate positive psychological changes, identified as posttraumatic growth (PTG). In 1995, American psychologists Richard Tedeschi and Lawrence Calhoun coined the term “posttraumatic growth” (PTG) to describe the struggle with adversity that ultimately leads to positive life changes (Tedeschi and Calhoun, 1995). A substantial literature has documented PTG in a wide range of people, but our study focused on its implications in breast cancer patients (see Cordova, 2008). With an overall lifetime prevalence of 12.3% in American females (Howlader et al., 2013), we found that focusing on breast cancer would provide meaningful results for many individuals who might be struggling with the non-biological consequences of cancer. Further, metastatic breast cancer’s prognosis remains low, with a survival rate of 24.3% over a five-year period (Howlader et al., 2013). This threat to one’s mortality can be considered a traumatic event, and the growth that often occurs in the aftermath is a prime example of PTG.
While many studies have found that negative psychological outcomes of cancer disrupt cortisol rhythms (Bower et al., 2005; Giese-Davis et al., 2006), it is unclear if individuals who are better able to cope with cancer and its sequelae have healthier cortisol patterns. Since flatter cortisol slopes predict worse survival in breast and lung cancer patients (Sephton et al., 2000; Sephton et al., 2012), a relationship between PTG and cortisol levels is of great interest for determining the potential protective effects of positive adjustment to cancer. This study sought to link PTG with biological benefits in the form of healthier endocrine function as defined by a steeper decline from morning to evening—negative cortisol slope.
Cortisol levels typically fluctuate throughout the day, peaking just before awakening and declining over the rest of the day, resulting in the 24-hour cycle known as the diurnal cortisol rhythm (Edwards et al., 2001). To date, there have been relatively few studies examining psychosocial variables that might predict a more normal (i.e., negative) cortisol slope in metastatic breast cancer patients. Cruess and colleagues found that cognitive-behavioral stress management enhanced benefit finding in breast cancer patients and reduced serum cortisol levels (Cruess et al., 2000). Other studies have also revealed the potential biological benefits of PTG in cancer populations, identifying improved immune function in participants who reported higher levels of PTG (Dunigan et al., 2007; McGregor et al., 2004).
The current literature has emphasized that negative psychological effects of cancer are related to dysregulation of the HPA axis, but there is relatively little examination of the potential biological benefits of PTG. We hypothesized that higher scores on the Posttraumatic Growth Inventory would be related to more negative cortisol slope (greater decline from morning to evening), implying a healthier endocrine response. Such a finding would emphasize not just the negative impact of a cancer diagnosis, but also the potential for growth in the aftermath of such a traumatic event accompanied by stabilization of HPA stress response.
Methods
The study was a secondary analysis from a previous study on HPA axis dysregulation in metastatic breast cancer (Spiegel et al., 2006). In order to participate in the study, patients must have had documented metastatic or recurrent breast cancer, live in the Greater San Francisco Bay Area, have proficiency in English, and have a Karnofsky rating of at least 70% to indicate performance status (Spiegel et al., 2006). From a total pool of 221 metastatic breast cancer patients, 111 were eligible and consented to participate. These patients became aware of the study through referrals from oncologists at Stanford and other nearby hospitals, newspaper advertisements, and word of mouth. Of the 111 patients who consented and were eligible, eight dropped out and four subjects were later excluded due to steroid use during baseline assessment. The final study sample consisted of 99 female metastatic breast cancer patients.
Assessments
Collection of baseline saliva to monitor cortisol levels was obtained after participants consented to the study (Range = 7–20 days). Saliva samples were obtained on two consecutive days at five intervals throughout the day in their homes (at waking, 30 minutes after waking, noon, 1700h, and 2100h). After collecting cortisol samples from participants, they were stored at −70 °C until laboratory centrifugation and duplicate assays for salivary cortisol were obtained using luminescence immunoassay (LIA) reagents from Immuno-Biological Laboratories, Inc. in Hamburg, Germany. Assay sensitivity was measured to be 0.015 μg/dL (Spiegel et al., 2006).
Cortisol levels were computed in three different ways. 1) The slope of diurnal cortisol was measured. Samples were taken at waking, noon, 5 and 9 PM (excluding 30 minutes post-waking) for two consecutive days. To provide a better fit for the data, a logarithmic transformation was applied to the cortisol levels. The two-day baseline log cortisol slope measured the overall trend in cortisol levels, with a greater negative number indicating an adaptive slope, declining through the day (M = −0.15626, SD = 0.0724). This was chosen as the primary cortisol measure to be examined in relationship to PTG because in prior studies it proved to be an independent predictor of subsequent survival time (Sephton et al., 2000; Sephton et al., 2012). 2) A second measurement was the total concentrations of cortisol upon waking (M = −0.7228, SD = 0.580). 3) Finally, cortisol levels were compared at waking and 30 minutes after waking to determine this waking rise (M = 0.3209, SD = 0.571). The rise in cortisol 30 minutes after waking is a measure of adrenal responsiveness to stimulation by ACTH, (Schmidt-Reinwald et al., 1999) and is associated with flatter diurnal cortisol slopes (Giese-Davis et al., 2006) and is steeper on weekdays than weekends, independent of time of awakening (Thorn et al., 2006). We included these two secondary measures to examine HPA activity in relation to morning arousal, and because they are sensitive to depression in this population (Giese-Davis et al., 2006).
The Posttraumatic Growth Inventory (PTGI) is a 21-item self-report measure that asks participants to quantify the extent to which they have experienced certain positive life changes as a result of a traumatic experience. Items are rated on a six-point Likert scale, from zero (“I did not experience this change as a result of the event”) to five (“I experienced this change to a very great degree as a result of the event”). The items are further divided into five subscales, which measure different aspects of growth: “New Possibilities,” “Relating to Others,” “Personal Strength,” “Spiritual Change,” and “Appreciation of Life.” The scale has high internal consistency (Cronbach’s alpha = 0.90), test-retest reliability, and validity (Tedeschi and Calhoun, 1996). Participants completed this questionnaire a median of three days after consenting to participate (M = 59.87, SD = 20.4).
Statistical Analysis
The Shapiro-Wilk test for normality was performed to assess the distribution of the data. While the PTGI-Total was normally distributed, the cortisol measures were not, so Spearman’s rho correlation coefficients were used to examine the relationship between the PTGI and salivary cortisol levels. We used hierarchical regression to examine the relationship of significant covariates with PTGI and cortisol slope. SPSS (version 21 for Windows) was used for data analysis.
Results
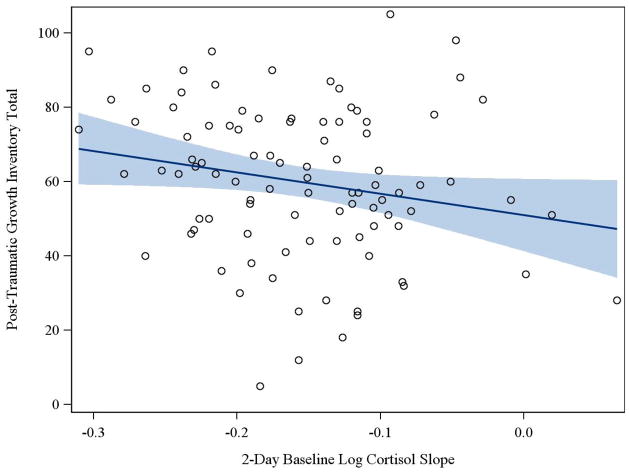
A simple correlation was computed to test for an association between PTGI scores and two-day baseline log cortisol slope. As seen in Table 2, the total score on the PTGI was significantly associated with cortisol slope in the predicted direction. In order to determine which components of the PTGI were driving this relationship, Spearman rank order correlation coefficients for each subscale of the instrument and cortisol slope were examined. It was found that the “Relating to Others” subscale was the only subscale of the inventory, other than the total score, that was significantly related to cortisol slope (p = 0.039). Figure 1 demonstrates that high scores on the PTGI are linearly associated with more negative (normal) cortisol slopes.
Table 2.
Correlation between PTG and diurnal cortisol slope
| Psychosocial Variables | 2-Day Baseline Log Cort Slope (N = 95) | |
|---|---|---|
| Spearman’s Rho | p-Value | |
| Posttraumatic growth | ||
| PTGI-Total | −0.213 | 0.039 |
| PTGI-Relating to Others | −0.213 | 0.039 |
| PTGI-New Possibilities | −0.114 | 0.264 |
| PTGI-Personal Strength | −0.166 | 0.101 |
| PTGI-Appreciation of Life | −0.136 | 0.180 |
| PTGI-Spiritual Changes | −0.133 | 0.190 |
Figure I.
Higher scores on the Posttraumatic Growth Inventory are associated with more negative cortisol slopes (r = −0.213, p = 0.039). Cortisol slopes were quantified based on a logarithmic transformation of 8 data points taken over the course of two days.
Covariate analyses
In our preliminary analysis, we found that income level, education, marital status, age, time since recurrence, progesterone receptor status, and metastatic site were neither related to PTGI scores nor cortisol slope (p > 0.05). Time since chemotherapy and estrogen receptor status, however, were related to PTGI scores (p = 0.011 and p = 0.017, respectively). After performing hierarchical regression with the inclusion of these two covariates in the first block and PTGI in the second block, we still found the relationship between the PTGI total score and cortisol slope to be significant (β = −.222, p = .05). Similarly, the PTGI “Relating to Others” subscale remained significantly associated with cortisol slope (β = −.255, p = .022).
The other two secondary measures of cortisol (waking and 30-minutes post-waking) were not related to any of the subscales of the PTGI nor the PTGI total scores.
Discussion
The results of the study highlight a significant relationship between PTG and more negative cortisol slopes among metastatic breast cancer patients. Given evidence that loss of normal diurnal variation in cortisol predicts shorter survival with breast cancer (Sephton et al., 2000), an association between PTG and cortisol levels could indicate a link between positive psychological response to the existential threat involved in breast cancer, its treatment, and disease outcome.
This study has several limitations. For example, the data were collected ten years ago, so it is unclear if the results are still applicable today. With advances in cancer treatment, patients today might think differently about cancer as compared to ten years ago (e.g., chronic vs. fatal disease). Additionally, these data were cross-sectional, so we cannot infer causality of the emergent relationships. Future studies could usefully investigate these relationships over time to determine the potential causal role that PTG has on improved endocrine function in patients with advanced cancer. Our analysis was also limited by the size of the sample. Despite having 99 participants in the study, a larger sample size would likely have yielded results with greater generalizability.
We found a significant relationship between PTG and cortisol slopes. If therapy is able to target the specific mechanisms that lead to the experience of PTG, health care professionals should strive to help their patients find benefits amidst the discomforts and existential concerns that emerge in the aftermath of the diagnosis and treatment of breast cancer.
Table I.
Descriptive Statistics for 99 Women with Metastatic Breast Cancer.
| Measure | 25th | Percentile Median (50th) or % | 75th |
|---|---|---|---|
| Demographics | |||
| Age at Baseline (years) | 47 | 54 | 62 |
| Age at Initial Diagnosis | 42 | 47 | 53 |
| Age at Metastasis/Recurrence | 45 | 51 | 58 |
| Ethnicity (% Caucasian) | 85.7 | ||
| Education | |||
| High School Diploma or Below | 2.0 | ||
| Trade School or Some College | 34.3 | ||
| Bachelor’s Degree | 20.2 | ||
| Some Graduate School or Master’s | 39.4 | ||
| Ph.D., M.D. or J.D. | 4.0 | ||
| Income (%) | |||
| < $20,000 | 5.6 | ||
| $20,000–$39,999 | 20.2 | ||
| $40,000–$59,999 | 12.4 | ||
| $60,000–$79,999 | 18.0 | ||
| $80,000–$99,999 | 13.5 | ||
| $100,000 and Above | 30.3 | ||
| Marital Status (%) | |||
| Single/Never Married | 7.1 | ||
| Married/Living as Married | 67.7 | ||
| Separated, Divorced, or Widowed | 25.3 | ||
| Employment Status (%) | |||
| Full Time | 22.2 | ||
| Part Time | 12.1 | ||
| Retired/Not Employed | 65.7 | ||
| Medical Status | |||
| Disease-Free Interval | 14 | 37 | 84 |
| Time DX to Study Entry (Months) | 30 | 77 | 116 |
| Time Metastasis to Study Entry (Months) | 7 | 13 | 36 |
| Estrogen Receptor Status (% Negative) | 29.3 | ||
| Progesterone Receptor Status (% Negative) | 31.5 | ||
| Site of Metastasis/Recurrence | |||
| Chestwall | 35.5 | ||
| Bone | 22.6 | ||
| Viscera | 41.9 | ||
| Treatment (% Yes) | |||
| Surgery | 93.8 | ||
| Reconstruction | 25.8 | ||
| Chemotherapy | |||
| Within 2 months of baseline | 14.3 | ||
| Radiation | |||
| Within 2 months of baseline | 12.2 | ||
| Hormone Therapy | |||
| Within 2 months of baseline | 29.6 | ||
| Antidepressant Use at Baseline | 40.2 | ||
| History of: | |||
| Diabetes | 3.1 | ||
| Stroke | 1.0 | ||
| Hypertension | 17.3 | ||
| Heart Disease | 3.1 | ||
| Smoking | 5.1 | ||
Acknowledgments
FUNDING SOURCE
The study was funded by the National Cancer Institute (RO1CA118567, David Spiegel, M.D., Principal Investigator).
The study involved the analysis of a portion of data collected in a study of Sleep and Survival with Metastatic Breast Cancer funded by the National Cancer Institute (RO1CA118567, David Spiegel, M.D., Principal Investigator).
Footnotes
Contributors
Michael Diaz conducted the literature review, performed the statistical analysis, and wrote the first draft of the manuscript. Arianna Aldridge-Gerry provided editorial support, statistical consultation, and contributed to the overall design of the article. David Spiegel designed the original study, wrote its protocol, and provided necessary edits and revisions to the article.
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Cordova MJ. Facilitating posttraumatic growth following cancer. Trauma Recovery Growth Posit Psychol -Pectives Posttraumatic Stress. 2008:185–205. [Google Scholar]
- Cruess DG, Antoni MH, McGregor BA, Kilbourn KM, Boyers AE, Alferi SM, Carver CS, Kumar M. Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosom Med. 2000;62:304–308. doi: 10.1097/00006842-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Dunigan J, Carr B, Steel J. Posttraumatic Growth, Immunity and Survival in Patients with Hepatoma. Dig Dis Sci. 2007;52:2452–2459. doi: 10.1007/s10620-006-9477-6. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Yutsis M, Neri E, Taylor CB, Kraemer HC, Spiegel D. Depression and Stress Reactivity in Metastatic Breast Cancer. Psychosom Med. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: Apr, 2013. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive–behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. n.d doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Giese-Davis J, Taylor CB, Kraemer H. Stress sensitivity in metastatic breast cancer: analysis of hypothalamic–pituitary–adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–1244. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi RG, Calhoun LG. Trauma & transformation: Growing in the aftermath of suffering. Sage Publications, Inc; Thousand Oaks, CA, US: 1995. [Google Scholar]
- Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]