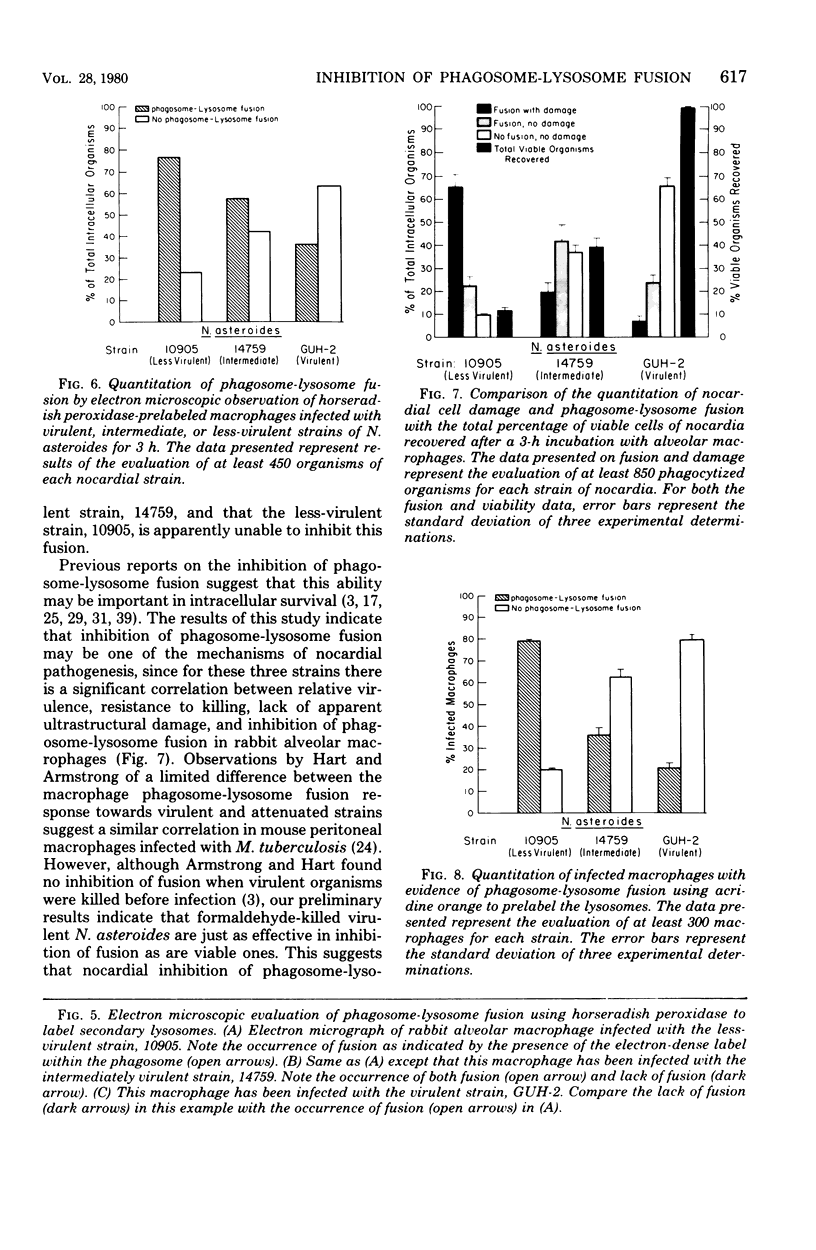
Abstract
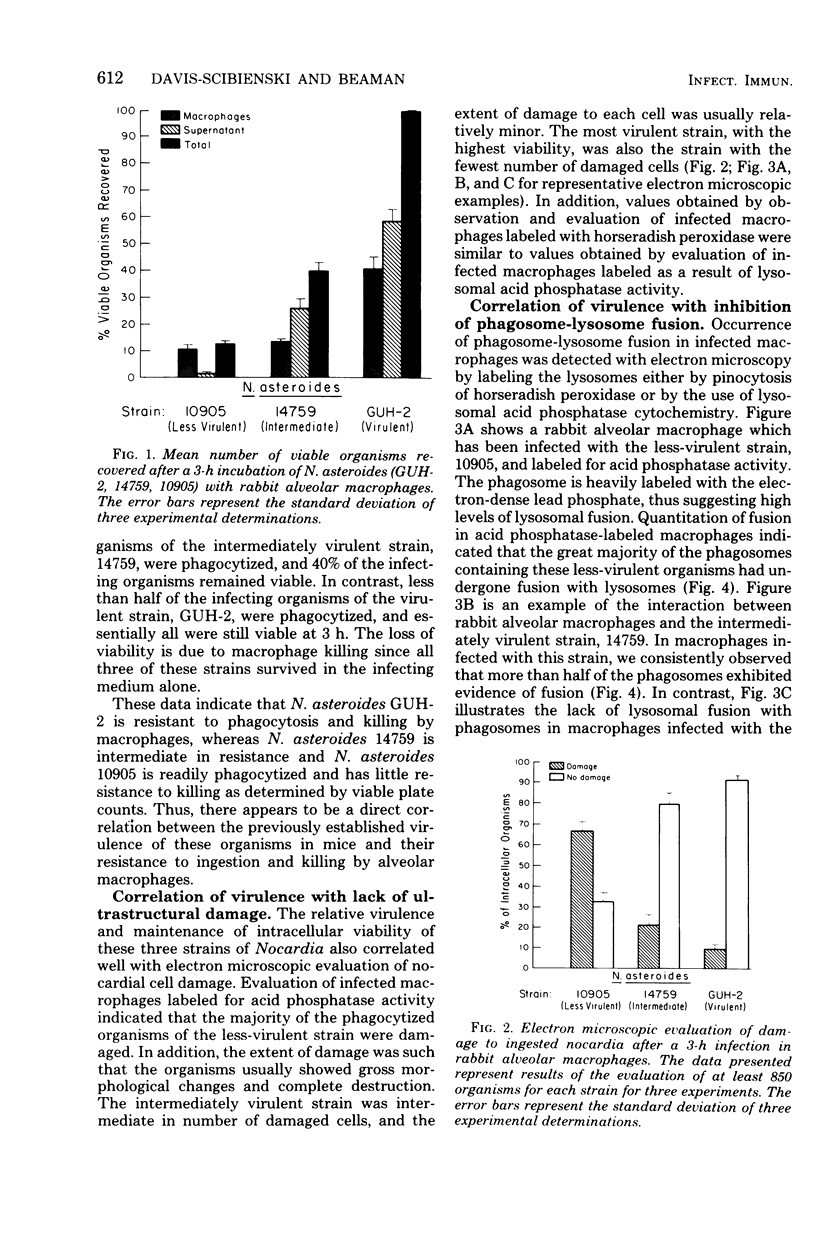
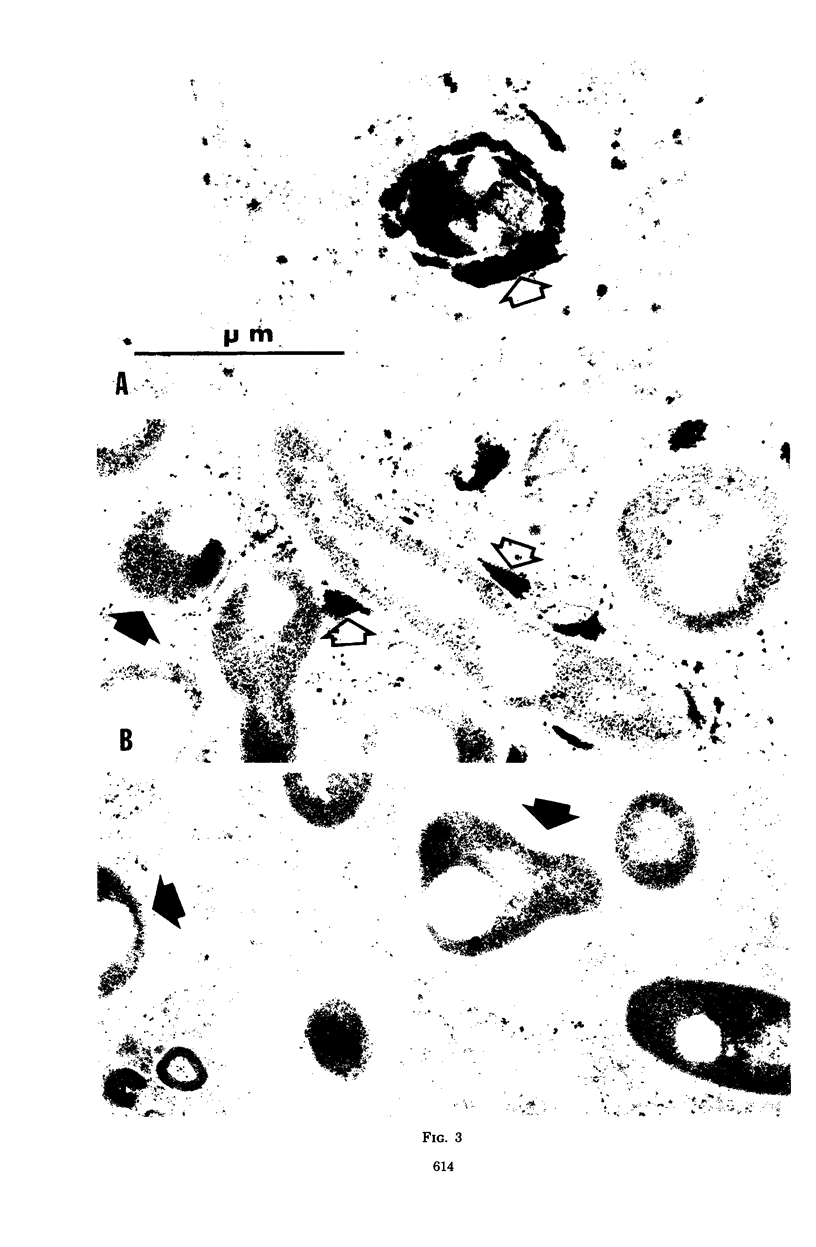
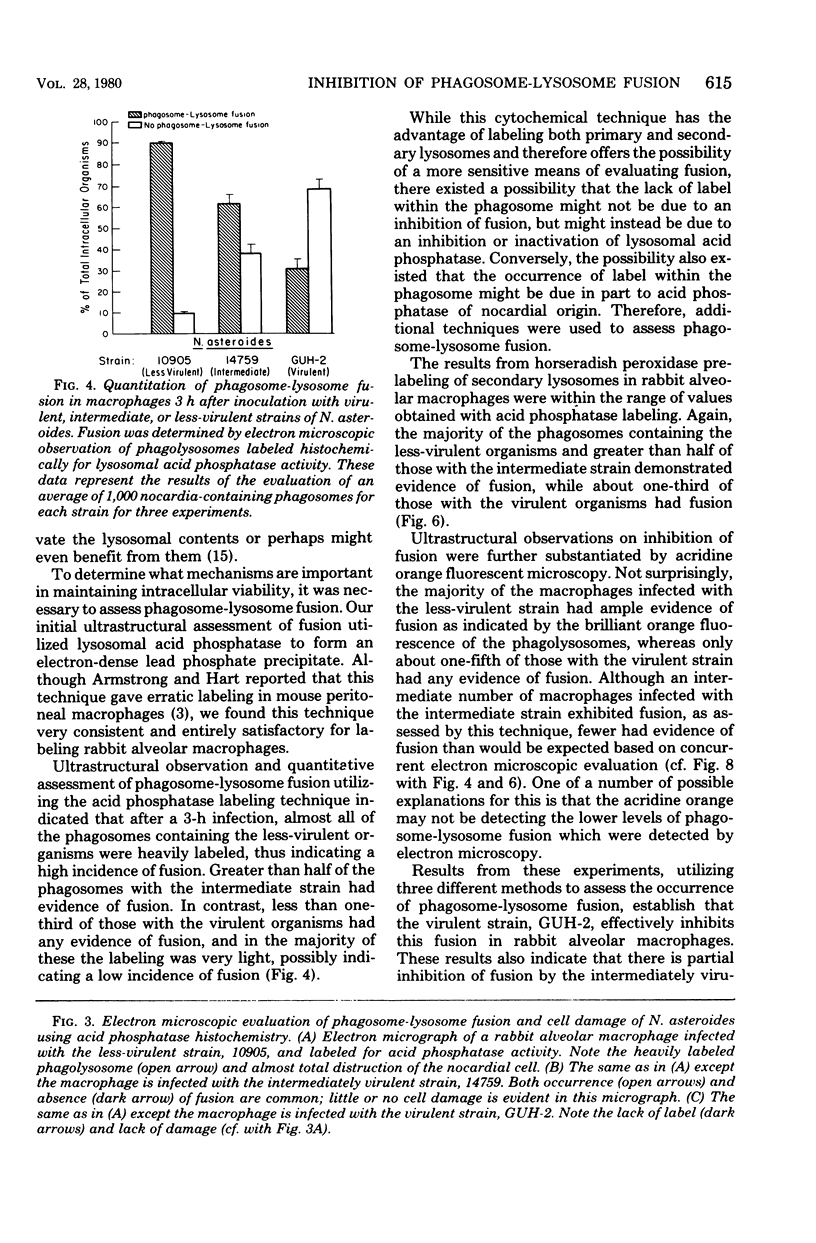
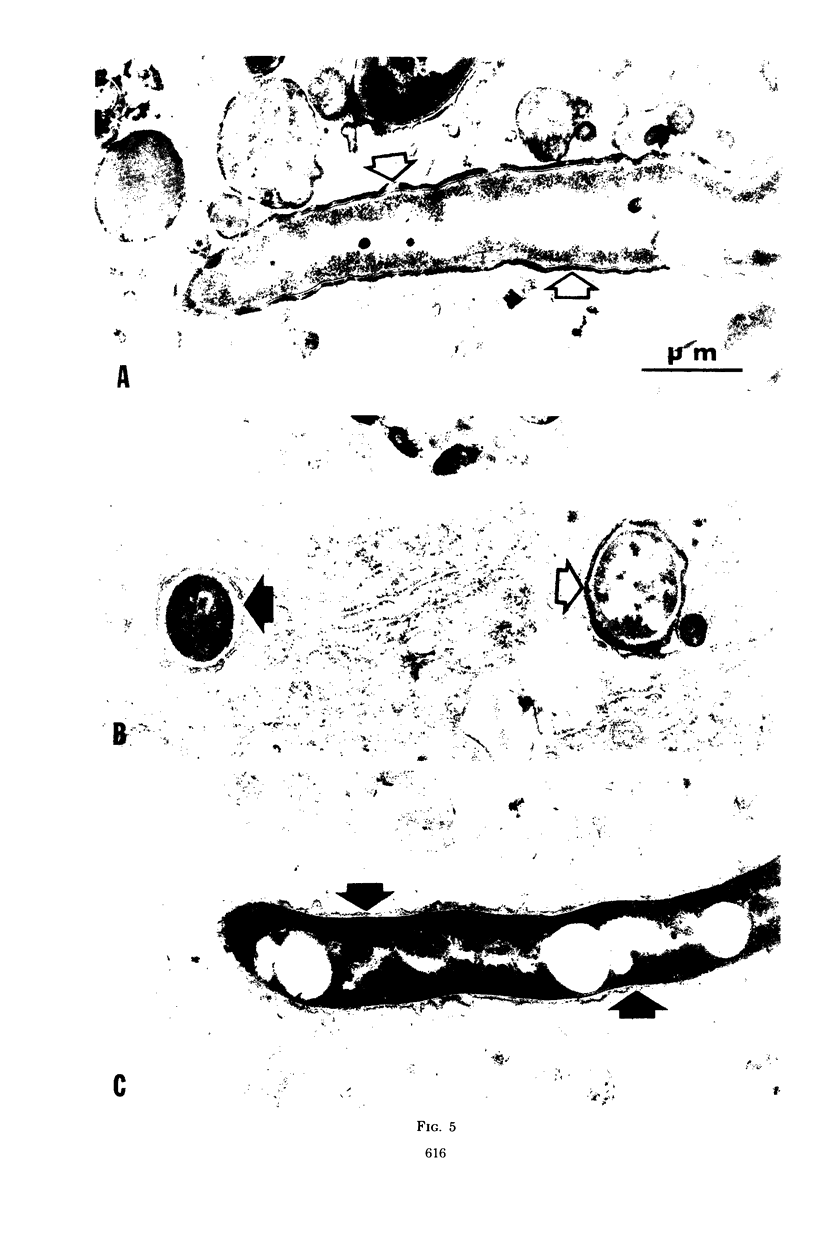
In vitro-maintained rabbit alveolar macrophages were infected with three strains of Nocardia asteroides. It was found that N. asteroides GUH-2 was resistant to macrophage killing, while N. asteroides 14759 was intermediate in resistance, and N. asteroides 10905 had little resistance to killing by macrophages. These observations correlated well with the data on relative virulence previously determined in mice. To establish the intracellular events leading to these differences, we determined the occurrence of phagosome-lysosome fusion in infected macrophages by both electron and fluorescent microscopic methods. It was found that the virulent strain GUH-2 inhibited phagosome-lysosome fusion; the intermediately virulent strain, 14759, partially inhibited fusion; and the less-virulent strain, 10905, was unable to inhibit fusion. In addition, electron microscopy of infected macrophages demonstrated that cells of the virulent strain, GUH-2, were not damaged, and only some of the cells of the intermediately virulent strain, 14759, were damaged, while most of the cells of the less virulent strain, 10905, exhibited considerable cellular destruction. These data indicated a direct correlation between the virulence of these organisms and their resistance to killing by alveolar macrophages, their lack of macrophage-induced ultrastructural damage, and their ability to inhibit phagosome-lysosome fusion. Thus, it appears that inhibition of phagosome-lysosome fusion in alveolar macrophages may be one of the mechanisms of pathogenicity of virulent strains of N. asteroides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana-infected macrophages. J Protozool. 1975 Nov;22(4):502–508. doi: 10.1111/j.1550-7408.1975.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Anderson D. R., Hopps H. E., Barile M. F., Bernheim B. C. Comparison of the ultrastructure of several rickettsiae, ornithosis virus, and Mycoplasma in tissue culture. J Bacteriol. 1965 Nov;90(5):1387–1404. doi: 10.1128/jb.90.5.1387-1404.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbotin J. N., Thomas D. Electron microscopic and kinetic studies dealing with an artificial enzyme membrane. Application to a cytochemical model with the horseradish peroxidase-3,3'-diaminobenzidine system. J Histochem Cytochem. 1974 Nov;22(11):1048–1059. doi: 10.1177/22.11.1048. [DOI] [PubMed] [Google Scholar]
- Beaman B. L. An ultrastructural analysis of Nocardia during experimental infections in mice. Infect Immun. 1973 Nov;8(5):828–840. doi: 10.1128/iai.8.5.828-840.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Burnside J., Edwards B., Causey W. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976 Sep;134(3):286–289. doi: 10.1093/infdis/134.3.286. [DOI] [PubMed] [Google Scholar]
- Beaman B. L. In vitro response of rabbit alveolar macrophages to infection with Nocardia asteroides. Infect Immun. 1977 Mar;15(3):925–937. doi: 10.1128/iai.15.3.925-937.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Interaction of Nocardia asteroides at different phases of growth with in vitro-maintained macrophages obtained from the lungs of normal and immunized rabbits. Infect Immun. 1979 Oct;26(1):355–361. doi: 10.1128/iai.26.1.355-361.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect Immun. 1977 Jun;16(3):995–1004. doi: 10.1128/iai.16.3.995-1004.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois L., Beaman B. L. Probable L-forms of Nocardia asteroides induced in cultured mouse peritoneal macrophages. Infect Immun. 1974 Mar;9(3):576–590. doi: 10.1128/iai.9.3.576-590.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. A., Draper P. An electron-microscope study of rat fibroblasts infected with Mycobacterium lepraemurium. J Pathol. 1970 Sep;102(1):21–26. doi: 10.1002/path.1711020105. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Draper P., Hart P. D. Mycobacteria and lysosomes: a paradox. Nature. 1969 Feb 15;221(5181):658–660. doi: 10.1038/221658a0. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Dumont A., Robert A. Electron microscopic study of phagocytosis of Histoplasma capsulatum by hamster peritoneal macrophages. Lab Invest. 1970 Sep;23(3):278–286. [PubMed] [Google Scholar]
- Evans M. J., Levy L. Ultrastructural changes in cells of the mouse footpad infected with Mycobacterium leprae. Infect Immun. 1972 Feb;5(2):238–247. doi: 10.1128/iai.5.2.238-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN B. A., KROSS D. J., CIRCO R. Host-parasite relationships in brucellosis. II. Destruction of macrophage cultures by Brucella of different virulence. J. J Infect Dis. 1961 May-Jun;108:333–338. doi: 10.1093/infdis/108.3.333. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Green G. M. Lung defense mechanisms. Med Clin North Am. 1973 May;57(3):547–562. doi: 10.1016/s0025-7125(16)32259-3. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A. Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1974 Oct;10(4):742–746. doi: 10.1128/iai.10.4.742-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M., Blyth W. A., Taverne J. Interactions of TRIC agents with macrophages and BHK-21 cells observed by electron microscopy. J Hyg (Lond) 1973 Sep;71(3):515–528. doi: 10.1017/s0022172400046507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY J. F., FINEGOLD S. M., FROMAN S., WILL D. W. The changing spectrum of nocardiosis. A review and presentation of nine cases. Am Rev Respir Dis. 1961 Mar;83:315–330. doi: 10.1164/arrd.1961.83.3.315. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975 Aug 21;47(1):1–9. doi: 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]
- Wilder M. S., Edberg J. C. Interaction of virulent and avirulent Listeria monocytogenes with cultured mouse peritoneal macrophages. Infect Immun. 1973 Mar;7(3):409–415. doi: 10.1128/iai.7.3.409-415.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]




