Abstract
Purpose
To review our experience with a screening program that included sequential cervical length measurements in our large population of triplet pregnancies.
Methods
Seventy-eight triplet pregnancies were retrospectively included. Cervical length measurements were performed by transvaginal ultrasound in 2-week intervals from week 16 + 0 onwards in a tertiary-care center in Vienna. The main outcome measurement was preterm delivery prior to 32 + 0 weeks of gestation. Statistical analyses were performed using paired and unpaired t tests and a stepwise linear regression model.
Results
There were 26 cases of preterm delivery (33.3%). Women with preterm delivery revealed significant cervical length shortening from week 22 + 0 (median 33 mm, interquartile range, IQR 17–39) to 24 + 0 (median 21 mm, IQR 7–30; p = 0.005). This was not observed in women without preterm delivery. From week 22 + 0 onwards, both groups showed further significant 2-week differences in cervical length (p < 0.05). Univariate analysis of cervical length in weeks 20 + 0, 22 + 0, and 24 + 0 as well as cervical length dynamics from 22 + 0 to 24 + 0 predicted preterm delivery.
Conclusions
In triplet pregnancies, a decrease in cervical length seems physiological from week 22 + 0 onwards. A sharp decrease in cervical length from the 22 + 0 to the 24 + 0 week as well as the smaller cervical length in weeks 20 + 0, 22 + 0, and 24 + 0 increase the risk of preterm delivery.
Keywords: Cervical insufficiency, Cervical length, Preterm delivery, Multiple pregnancy, Triplet pregnancy
Introduction
Over the past decades, the incidence of multifetal gestations has increased dramatically which also includes triplet pregnancies [1]. In these pregnancies, the average pregnancy age at delivery ranges from 31 to 33 completed weeks of gestation, with a majority of triplets being born before the 35th week [2–5]. It is evident that this prematurity is accompanied by high rates of neonatal complications which include both neonatal mortality and morbidity [6]. Although gestational hypertension and its complications, first and foremost preeclampsia, also contribute to the risk of preterm delivery in triplet pregnancies, the majority of cases is complicated by preterm labor and cervical shortening [5]. Notably, chorionicity has also been proven to be a risk factor for earlier delivery in triplets with dichorionic pregnancies having been reported to deliver more likely <30 weeks of gestation than trichorionic pregnancies [7]. Moreover, one could also hypothesize that chorionicity and/or the amount of amniotic fluid might influence cervical length (CL) in triplet pregnancies, probably even without having a major impact on gestational length. However, the efforts of identifying those women at risk for preterm triplet delivery have also focused on CL measurement [2, 8–16]. Despite the fact that these studies measured the CL at different pregnancy stages and resulted in different cut-off levels, they demonstrated that the CL can be used as a more or less reliable predictive tool. At the Medical University of Vienna, serial CL measurements of women at risk of preterm delivery (PTD) have been part of the clinical routine for a long time [17, 18]. This also applies to women with triplet pregnancies. We thus intended to critically review our experience with this diagnostic regimen. We aimed to evaluate the kinetics of CL and to test the predictive value of the CL at different gestational ages for the prediction of preterm triplet delivery before 32 + 0 weeks of gestation. In addition, we also focused on the influence of chorionicity on CL dynamics.
Materials and methods
As reported previously [17, 18], a screening program for pregnant women at perceived risk of PTD has been established for many years at the Department of Maternal-Fetal Medicine of the Medical University of Vienna, Austria. The department is the national reference center for maternal-fetal medicine in eastern Austria and the annual number of deliveries was at least 2500 during the study period. The screening program included CL measurement by transvaginal ultrasound every 2 weeks from 16 + 0 until 30 + 0. All ultrasound examinations were performed by highly experienced operators (either obstetricians or certified medical technical assistants). All CL measurements (Fig. 1) were carried out according to the guidelines of the Fetal Medicine Foundation (available online at http://www.fetalmedicine.com/fmf/training-certification/certificates-of-competence/cervical-assessment/). The shortest of at least three measurements was documented [18].
Fig. 1.
Sonographic cervical length measurement
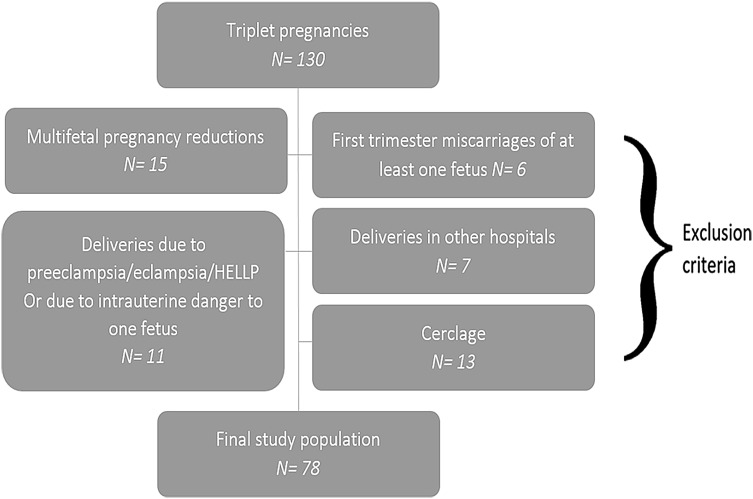
In this retrospective analysis, we studied 130 women who were diagnosed with a triplet pregnancy at the time of the first trimester screening from January 2003 to August 2015 (n = 130). The following patients were then excluded from the study: Seven women did not deliver at the department and had no follow-up (n = 7). Eleven cases with PTD before 32 + 0 weeks of gestation due to other reasons, namely preeclampsia/eclampsia, the HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), and an imminent intrauterine danger to one of the fetuses’ life (n = 11) were also excluded. Thirteen women had undergone cerclage and were, thus, also excluded from the analysis. Moreover, we excluded all women who underwent multifetal pregnancy reduction (n = 15) or had a first trimester miscarriage of at least one fetus (n = 6). This means that only cases that started with triplets and gave birth to triplets were included. The study examines spontaneous preterm deliveries as opposed to iatrogenic ones. This resulted in a final study population of 78 triplet pregnancies for this analysis of serial CL measurements. A flow chart is depicted in Fig. 2.
Fig. 2.
Study flow chart
Relevant data were acquired retrospectively using the Viewpoint® software (GE Healthcare, Wessling, Germany). This is the basic perinatologic database used at the department. CL measurements were performed every 2 weeks from 16 + 0 until 30 + 0. In addition, the following parameters were included: history of previous conization procedures, age at delivery, body mass index (BMI) at the initial visit, parity, previous preterm birth due to cervical insufficiency, preterm labor, or preterm premature rupture of membranes, previous second trimester miscarriage, pregnancies after in vitro fertilization (IVF), cigarette smoking, and chorionicity (pregnancies were divided into mono-/dichorionic and trichorionic).
This study was approved by the Institutional Review Board of the Medical University of Vienna (IRB Number: 1602/2015) on April 14th 2016. Neither written nor verbal informed consent is necessary in retrospective studies according to the Ethics Committee of the Medical University of Vienna and was thus not obtained. The data were de-identified for statistical analysis.
Nominal variables are reported as numbers and frequencies, and continuous variables as medians and interquartile ranges (IQR). Paired t tests were applied to test for differences between subsequent CL measurements within one group. An unpaired t test was used to test for the differences in initial CL between women with and without early PTD. For t tests, data had to be normally distributed as evaluated by the Kolmogorov–Smirnov test. This was the case for all of these analyses. For t tests, the t value and the degree of freedom (df) are provided. In a stepwise linear regression model for prediction of PTD <32 + 0, we included the following parameters: basic patient characteristics, pregnancy-related parameters including chorionicity and previous conization procedures, and CL measurements as chosen according to the analyses of CL dynamics and to clinical considerations (see below). These analyses were performed to allow an early prediction of PTD <32 + 0. The optimal cut-off for CL was calculated as the threshold value with the highest specificity and sensitivity based on the receiver-operating characteristics (ROC) curve as a sensitivity versus (1 − specificity) plot. This is associated with the highest discriminatory ability. The discriminatory ability of the investigated parameters is described as the correlation between specificity and sensitivity, and was measured by the area under the receiver-operating curve (AUC). Where appropriate, values are given with a 95% confidence interval (95% CI). Statistical analysis was performed using SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, USA). Differences were considered statistically significant if p < 0.05.
Results
The median gestational age at delivery was 32 completed weeks (IQR 29–33). 16 (20.5%) women, delivered between 23 + 0 and 32 + 0 weeks of gestation. Four women (5.1%) suffered a late miscarriage (17 + 0 to 18 + 6 weeks) and 6 women (7.7%) delivered a premature infant with lifesigns between weeks 19 + 0 and 23 + 0. This resulted in an overall rate of 33.3% (26/78) for early PTD <32 + 0 weeks used for the following analyses. Details on patient characteristics are shown in Table 1.
Table 1.
Basic patient characteristics
| Age (years)a | 31 (27; 35) |
| Body mass index (kg/m2)a | 22.8 (21.0; 25.7) |
| Previous second trimester miscarriage or preterm deliveryb | 7 (9.0) |
| Previous conizationb | 3 (3.8) |
| Pregnancy after IVF treatmentb | 53 (67.9%) |
| Parityb | |
| 0 | 55 (70.5) |
| 1 | 18 (23.1) |
| ≥2 | 5 (6.4) |
| Cigarette smoking during pregnancyb | 9 (11.5) |
| Chorionicityb | |
| 1 | 1 (1.3) |
| 2 | 22 (28.2) |
| 3 | 55 (70.5) |
| Amniocityb | |
| 1 | 0 |
| 2 | 0 |
| 3 | 78 (100) |
Data are presented as a median (interquartile range) or b numbers (frequencies)
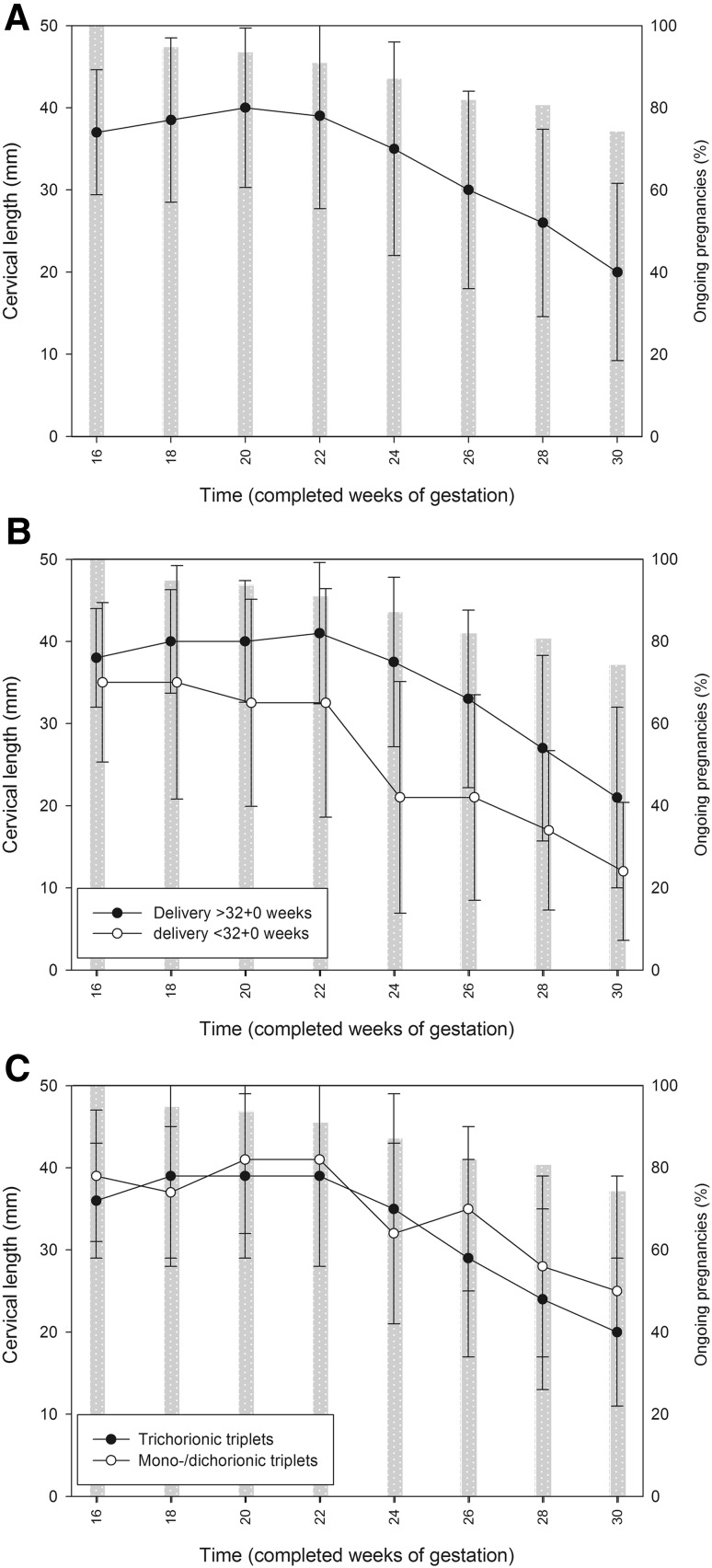
Dynamics in CL are plotted in Fig. 3. Due to the second trimester miscarriages and the preterm deliveries, only those with an ongoing pregnancy were available for CL measurement at follow-up examinations. In detail, in week 16 + 0, all women underwent CL measurements, 74 (94.9%) in week 18 + 0, 73 (93.6%) in week 20 + 0, 71 (91.0%) in week 22 + 0, 68 (87.2%) in week 24 + 0, 64 (82.1%) in week 26 + 0, and 63 (80.8%) in week 28 + 0. For the whole study population, a stable CL course was seen from week 16 + 0 (median 37 mm, IQR 34–40) to week 22 + 0 (median 39 mm, IQR 33–43; p = 0.773, t = 0.291, df = 39). Thereafter, significant declines in CL were noted with median differences of 4–5 mm every 2 weeks: median CL was 35 mm (IQR 26–42; p < 0.001, t = 4.090; df = 42) in week 24 + 0 and declined to 30 mm (IQR 20–36; p < 0.001, t = 3.943; df = 38) in week 26 + 0; in week 28 + 0, it had further decreased to a median 26 mm (IQR 16–32; p < 0.001, t = 4.868; df = 45) and to a median of 20 mm (IQR 14–26; p = 0.009, t = 2.752; df = 40) in week 30 + 0 (Fig. 3a).
Fig. 3.
Dynamics in cervical lengths in the course of routine screening in triplet pregnancies (data provided as median and standard deviation). a Complete study population. b Women with (n = 37, white dots) and without preterm delivery <32 + 0 weeks of gestation (n = 54, black dots). The group of women with preterm delivery includes those with a second trimester miscarriage. c Women with mono-/dichorionic triplets (n = 23, white dots) and trichorionic triplets (n = 55, black dots). The gray bars indicate how many pregnancies were ongoing at this time of measurement (provided in %). Gestational age is plotted on the x-axis
The comparison of CL measurements between women with (n = 26) and without PTD <32 + 0 weeks (n = 52) is shown in Fig. 3b. In week 16 + 0, groups did not differ in CL (35 mm, IQR 28–39, vs. 38 mm, IQR 35–41, respectively; p = 0.058, t = −1.935, df = 53). When focusing on women with PTD <32 + 0 weeks, a significant difference in CL was found only between weeks 22 + 0 and 24 + 0 (median 33 mm, IQR 17–39 vs. 21 mm, IQR 7–30; p = 0.005, t = 3.440, df = 12). Women without PTD <32 + 0, showed significant 2-week differences from week 22 + 0 on (p < 0.05).
A logistic regression model was computed to analyze predictive factors for PTD <32 + 0 (Table 2). In addition to the general patient and pregnancy characteristics, we chose to include CL at weeks 16 + 0, 18 + 0, 20 + 0, 22 + 0, and 24 + 0 as well as CL dynamics between weeks 22 + 0 and 24 + 0, since the latter revealed a significant CL decline also for women with PTD <32 + 0 (see above). The logistic regression models’ univariate analyses revealed that all CL parameters differed significantly between women with and without PTD <32 + 0 except CL in weeks 16 + 0 and 18 + 0. In multivariate analyses, all parameters that had been significant in the univariate models were included except CL dynamics between weeks 22 + 0 and 24 + 0 due to redundancy. However, none reached statistical significance (Table 2).
Table 2.
Prediction of early preterm delivery in women with a triplet pregnancy
| Delivery <32 + 0 week (n = 37) | Delivery ≥32 + 0 week (n = 54) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI)a | Wald’s test | p (LR test)b | Adjusted OR (95% CI)a | Wald’s test | p (LR test)b | |||
| Age (years)c | 31.5 (26.8; 37.0) | 31.0 (27.0; 33.0) | 1.04 (0.95; 1.13) | 0.644 | 0.422 | – | – | – |
| Body mass index (kg/m2)c | 23.9 (20.6; 26.7) | 22.5 (21.0; 25.4) | 1.03 (0.91; 1.17) | 0.278 | 0.598 | – | – | – |
| Chorionicity (3 versus 1 or 2)d | 21 (80.8) | 35 (67.3) | 2.04 (0.66; 6.35) | 1.517 | 0.218 | |||
| Previous preterm delivery or second trimester miscarriaged | 4 (15.4) | 3 (5.8) | 2.97 (0.61; 14.41) | 1.825 | 0.177 | – | – | – |
| Previous conizationd | 1 (3.8) | 2 (3.8) | 1.00 (0.09; 11.57) | 0.000 | 1.000 | – | – | – |
| Pregnancy after IVF treatmentd | 16 (61.5) | 37 (71.2) | 0.65 (0.24; 1.75) | 0.731 | 0.392 | – | – | – |
| Parityd | ||||||||
| 0 | 18 (69.2) | 37 (71.2) | Reference | 1.706 | – | – | – | |
| 1 | 5 (19.2) | 13 (25.0) | 0.79 (0.24; 2.56) | 0.154 | 0.426 | – | – | – |
| ≥2 | 3 (11.5) | 2 (3.8) | 3.08 (0.47; 20.1) | 1.384 | – | – | – | |
| Cigarette smokingd | 3 (11.5) | 6 (11.5) | 1.00 (0.87; 1.15) | 0.000 | 1.000 | – | – | – |
| CLe at week 16 + 0 (mm)c | 35 (28; 39) | 38 (35; 41) | 0.93 (0.85; 1.01) | 3.146 | 0.058 | – | – | – |
| CLe at week 18 + 0 (mm)c | 35 (29; 41) | 40 (35; 44) | 0.93 (0.87; 1.01) | 3.381 | 0.066 | – | – | – |
| CLe at week 20 + 0 (mm)c | 33 (28; 42) | 40 (36; 45) | 0.90 (0.82; 0.98) | 6.276 | 0.012 f | 1.04 (0.84; 1.29) | 0.131 | 0.717 |
| CLe at week 22 + 0 (mm)c | 33 (17; 39) | 41 (36; 43) | 0.92 (0.68; 0.98) | 6.958 | 0.008 f | 1.01 (0.76; 1.36) | 0.006 | 0.937 |
| CLe at week 24 + 0 (mm)c | 21 (7; 30) | 38 (31; 42) | 0.91 (0.86; 0.97) | 9.873 | 0.002 f | 0.84 (0.68; 1.03) | 7.262 | 0.097 |
| CLe dynamics: week 22 + 0 to 24 + 0 (mm)c | −10.5 (−16.0; −1.6) | −2.5 (−7.0; 1.0) | 0.88 (0.79; 0.97) | 6.043 | 0.014 f | Excluded for redundancy | ||
Results of the univariate and multivariate analysis
aOR (95% CI) = odds ratio (95% confidence interval)
bLR test = likelihood ratio test
cContinuous variable, provided in median (interquartile range)
dNominal variable, provided in n (%)
eCL = cervical length
fItalic letters indicate statistical significance
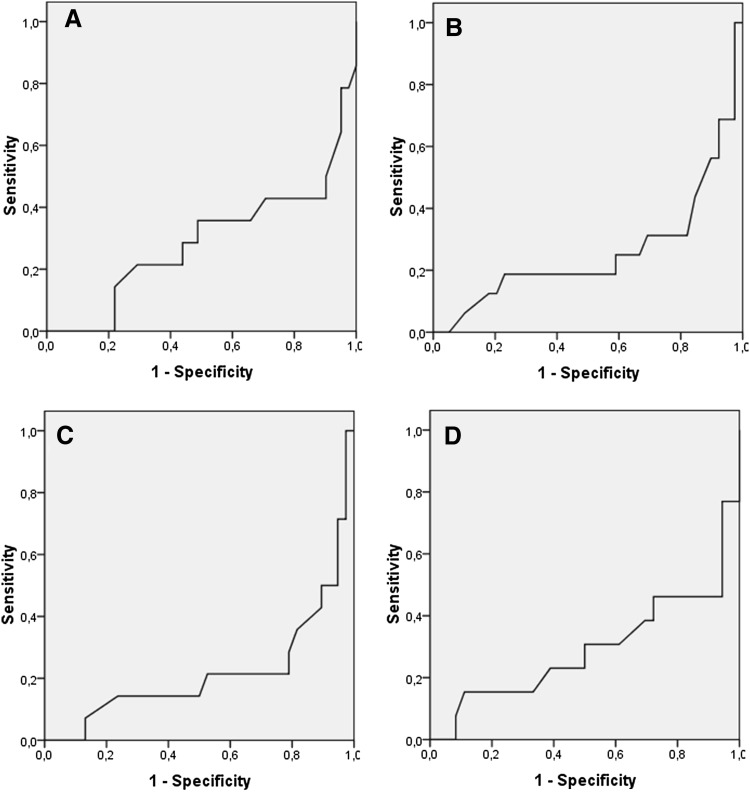
For all significant predictors (Table 2), the following optimized cut-off values for the prediction of PTD <32 + 0 were calculated (see Fig. 4 for receiver-operating characteristic curves): <34 mm for week 20 + 0 (sensitivity 0.57, 95% CI 0.29–0.82; specificity 0.90, 95% CI 0.77–0.97; positive predictive value 0.67, 95% CI 0.35–0.90; negative predictive value 0.86, 95% CI 0.72–0.95), <34 mm for week 22 + 0 (sensitivity 0.69, 95% CI 0.41–0.89; specificity 0.82, 95% CI 0.66–0.92; positive predictive value 0.61, 95% CI 0.36–0.83; negative predictive value 0.86, 95% CI 0.71–0.95), <35 mm for week 24 + 0 (sensitivity 0.79, 95% CI 0.49–0.95; specificity 0.79, 95% CI 0.63–0.90; positive predictive value 0.58, 95% CI 0.34–0.80; negative predictive value 0.91, 95% CI 0.76–0.98), and a decrease between weeks 22 + 0 and 24 + 0 exceeding 10 mm (sensitivity 0.54, 95% CI 0.25–0.81; specificity 0.94, 95% CI 0.81–0.99; positive predictive value 0.78, 95% CI 0.40–0.97; negative predictive value 0.85, 95% CI 0.70–0.94).
Fig. 4.
Receiver-operating characteristic curves for the prediction of preterm delivery <32 + 0 weeks using the following cervical length cut-off values: a <34 mm for week 20 + 0; b < 34 mm for week 22 + 0; c <35 mm for week 24 + 0; and d a decrease between weeks 22 + 0 and 24 + 0 exceeding 10 mm
In addition, the influence of chorionicity on CL was evaluated. The according graphs are plotted in Fig. 3c. No differences in CL between trichorionic and mono-/dichorionic triplets were found at any gestational age (p > 0.05). In mono-/dichorionic pregnancies (n = 23), significant declines in CL were found between weeks 26 + 0 and 28 + 0 (35 mm, IQR 22–38, vs. 28 mm, IQR 16–32; p = 0.024, t = 2.528, df = 14) and 28 + 0 and 30 + 0 (28 mm, IQR 16–32, vs. 25 mm, IQR 10–33; p = 0.042, t = 2.261, df = 13). In trichorionic pregnancies (n = 55), CL shortened in a statistically significant manner between weeks 22 + 0 and 24 + 0 (39 mm, IQR 33–42, vs. 35 mm, IQR 25–41; p = 0.001, t = 3.784, df = 30), 24 + 0 and 26 + 0 (35 mm, IQR 25–41, vs. 29 mm, IQR 19–36; p = 0.001, t = 3.590, df = 27), and 26 + 0 and 28 + 0 (29 mm, IQR 19–36, vs. 24 mm, IQR 16–32; p < 0.001, t = 4.111, df = 30).
Comment
This retrospective analysis on CL in triplet pregnancies provided three major results: (1) from week 22 + 0 onwards, a decrease of CL in the overall study population could be observed; (2) triplet pregnancies resulting in second trimester miscarriages and PTD <32 + 0 weeks showed a pronounced decrease in CL between weeks 22 + 0 and 24 + 0; and (3) significant predictors of PTD <32 + 0 are the CL in the 20th, 22nd, and 24th week of gestation, as well as the dynamics of CL from the 22 + 0 to the 24 + 0 week.
In our dataset, triplet pregnancies resulted in PTD from weeks 23 + 0 to 31 + 6 in 20.5% and in second trimester miscarriage in 12% with all of these cases due to cervical, preterm labor, or preterm premature rupture of membranes only. This is comparable to the literature reporting rates of 13–64.7% [4, 15] with the majority of incidences ranging from 16 to 31.4% [10, 11, 13, 14].
As demonstrated in Fig. 3, a certain decline of CL appears to be physiological in triplet pregnancies. From week 22 + 0 on, significant 2-week declines in CL were found for the whole study population as well as for women without PTD <32 + 0. This finding is in accordance with previous studies [15, 19]. Ramin et al. also reported that progressive cervical shortening was visible only after week 20 + 0 [15]. Hiersch et al. observed a more rapid cervical shortening during gestation in triplets compared to twin pregnancies [18]. However, a sharp decline in cervical length from week 22 + 0 to 24 + 0 seems to be specific for women with ongoing triplet pregnancies at risk for PTD between 24 + 0 and 32 + 0 weeks (Table 2). The predictive value of CL on PTD has already been demonstrated in previous studies [2, 4, 8, 9, 11–16, 19]. Notably, optimized cut-off values for absolute CL were 34–35 mm in our study which resulted in sensitivity and specificity values of 60–80 and 70–90%, respectively. Previous studies on triplet pregnancies often used a lower cut-off value of 25 mm [14, 20] or calculated similar optimized cut-off values [11–13]. We find it hard to comment on these differences. In some studies, the lower cut-off level is likely related to the fact that standardized measurement was performed in a higher week of gestation and, thus, related to the physiological decline in CL [15, 19]. Notably, the optimized cut-off values in our study provide quite high negative predictive values (nearly 90% in weeks 20 + 0, 22 + 0, and 24 + 0) which are reassuring for the patient. Only van den Mheen et al. used a higher CL cut-off of 30 mm for the prediction of PTD <32 + 0 weeks [4]. Applying this criterion, they achieved a similar negative predictive value of 87.4%. It seems reasonable that a negative predictive value of 100% would be desirable since it would provide maximum stress relief for the pregnant woman. In a previous study, this could be reached with a cut-off level of 20 mm in weeks 25–28 [14]. Unfortunately, our results do not prove these findings.
None of the CL parameters mentioned above remained significant predictors in the multivariate model (Table 2). It seems reasonable to assume a certain redundancy of values. Moreover, the analysis is influenced by the fact that women with a shorter CL might have already experienced second trimester miscarriage or PTD and could therefore not be included in the subsequent CL measurements. However, each of these CL parameters seems to have its relevance in clinical routine and none of them should be considered more accurate and meaningful than the others.
Concerning the analysis of CL dynamics in women with mono-/dichorionic versus trichorionic triplets (Fig. 3c), no striking differences between these two groups were found. The 2-week CL decreases from week 22 + 0 on reached statistical significance in the trichorionic group more often which is likely due to the smaller patient population with mono-/dichorionic triplets. Moreover, chorionicity was not a predictive parameter for PTD <32 + 0 in the logistic regression model (Table 2) which somehow is in contrast to a recent report [7]. Thus, our data suggest that chorionicity is of only minor impact on CL. However, larger studies need to clarify this topic.
The main strength of our study is the large sample size. To the best of our knowledge, this is the largest study on CL in triplet pregnancies so far. Moreover, CL screening was rigorous and evaluation of CL dynamics with 2-week intervals might have improved data validity. The limitations of the study are its retrospective design and the lack of fetal fibronectin testing. Since this method is now being routinely applied, more complete datasets may be available in the future. This is due to the historical population and the fact that some of these procedures had been performed outside of the center.
In conclusion, in triplet pregnancies, a decrease in CL seems physiological from week 22 + 0 onwards. A sharp decline in CL from the 22 + 0 to the 24 + 0 week increases the risk of PTD <32 + 0 weeks. Optimized cut-off values for CL in weeks 20 + 0, 22 + 0, and 24 + 0 were 34–35 mm. Further study on CL dynamics in triplet pregnancies is recommended in order to develop multivariate predictive models with higher reliability.
Acknowledgements
Open access funding provided by Medical University of Vienna.
Authors contribution
SP: the project’s and the manuscript’s conception and design, acquisition of data, statistical analyses, drafting the article and revising it for intellectual content, final approval of the version to be published. SS: the project’s and the manuscript’s conception and design, drafting the article and revising it for intellectual content, final approval of the version to be published. VW and KC: the project’s and the manuscript’s conception and design, acquisition of data, drafting the article and revising it for intellectual content, final approval of the version to be published. JO: the project’s and the manuscript’s conception and design, statistical analyses, drafting the article and revising it for intellectual content, final approval of the version to be published.
Compliance with ethical standards
Funding
No supplemental funding was provided for this research.
Conflict of interest
The authors report no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board of the Medical University of Vienna (IRB Number: 1602/2015). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Neither written nor verbal informed consent is necessary in retrospective studies according to the Ethics Committee of the Medical University of Vienna and was thus not obtained.
References
- 1.American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine ACOG practice bulletin no. 144: multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol. 2014;123(5):1118–1132. doi: 10.1097/01.AOG.0000446856.51061.3e. [DOI] [PubMed] [Google Scholar]
- 2.Maslovitz S, Hartoov J, Wolman I, Jaffa A, Lessing JB, Fait G. Cervical length in the early second trimester for detection of triplet pregnancies at risk for preterm birth. J Ultrasound Med. 2004;23(9):1187–1191. doi: 10.7863/jum.2004.23.9.1187. [DOI] [PubMed] [Google Scholar]
- 3.Kiely JL. What is the population-based risk of preterm birth among twins and other multiples? Clin Obstet Gynecol. 1998;41(1):3–11. doi: 10.1097/00003081-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 4.van de Mheen L, Ravelli AC, Oudijk MA, et al. Prediction of time to delivery week-by-week in women with a triplet pregnancy. Am J Perinatol. 2016;33(14):1394–1400. doi: 10.1055/s-0036-1583190. [DOI] [PubMed] [Google Scholar]
- 5.Chibber R, Fouda M, Shishtawy W, Al-Dossary M, Al-Hijji J, Amen A, Mohammed AT. Maternal and neonatal outcome in triplet, quadruplet and quintuplet gestations following ART: a 11-year study. Arch Gynecol Obstet. 2013;288(4):759–767. doi: 10.1007/s00404-013-2796-x. [DOI] [PubMed] [Google Scholar]
- 6.Blickstein I, Keith LG. Outcome of triplets and high-order multiple pregnancies. Curr Opin Obstet Gynecol. 2003;15(2):113–117. doi: 10.1097/00001703-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Lopes Perdigao J, Straub H, Zhou Y, Gonzalez A, Ismail M, Ouyang DW. Perinatal and obstetric outcomes of dichorionic vs trichorionic triplet pregnancies. Am J Obstet Gynecol. 2016;214(5):659.e1–659.e5. doi: 10.1016/j.ajog.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Meath AJ, Ramsey PS, Mulholland TA, Rosenquist RG, Lesnick T, Ramin KD. Comparative longitudinal study of cervical length and induced shortening changes among singleton, twin, and triplet pregnancies. Am J Obstet Gynecol. 2005;192(5):1410–1415. doi: 10.1016/j.ajog.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 9.van de Mheen L, Schuit E, Lim AC, Porath MM, Papatsonis D, Erwich JJ. Prediction of preterm birth in multiple pregnancies: development of a multivariable model including cervical length measurement at 16 to 21 weeks’ gestation. J Obstet Gynaecol Can. 2014;36(4):309–319. doi: 10.1016/S1701-2163(15)30606-X. [DOI] [PubMed] [Google Scholar]
- 10.Fox NS, Rebarber A, Roman AS, Klauser CK, Peress D, Saltzman DH. Combined fetal fibronectin and cervical length and spontaneous preterm birth in asymptomatic triplet pregnancies. J Matern Fetal Neonatal Med. 2012;25(11):2308–2311. doi: 10.3109/14767058.2012.691579. [DOI] [PubMed] [Google Scholar]
- 11.To MS, Skentou C, Cicero S, Liao AW, Nicolaides KH. Cervical length at 23 weeks in triplets: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2000;16(6):515–518. doi: 10.1046/j.1469-0705.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 12.Maymon R, Herman A, Jauniaux E, Frenkel J, Ariely S, Sherman D. Transvaginal sonographic assessment of cervical length changes during triplet gestation. Hum Reprod. 2001;16(5):956–960. doi: 10.1093/humrep/16.5.956. [DOI] [PubMed] [Google Scholar]
- 13.Poggi SH, Ghidini A, Landy HJ, McLaren R, Oyelese Y, Alvarez M, Pezzullo JC, Collea JV. Role of transvaginal sonographic cervical length in predicting spontaneous preterm delivery in triplet pregnancies with therapeutic cerclage. J Reprod Med. 2003;48(10):785–788. [PubMed] [Google Scholar]
- 14.Guzman ER, Walters C, O’reilly-Green C, Meirowitz NB, Gipson K, Nigam J, Vintzileos AM. Use of cervical ultrasonography in prediction of spontaneous preterm birth in triplet gestations. Am J Obstet Gynecol. 2000;183(5):1108–1113. doi: 10.1067/mob.2000.108875. [DOI] [PubMed] [Google Scholar]
- 15.Ramin KD, Ogburn PL, Jr, Mulholland TA, Breckle RJ, Ramsey PS. Ultrasonographic assessment of cervical length in triplet pregnancies. Am J Obstet Gynecol. 1999;180(6 Pt 1):1442–1445. doi: 10.1016/S0002-9378(99)70033-5. [DOI] [PubMed] [Google Scholar]
- 16.Fait G, Har-Toov J, Gull I, Lessing JB, Jaffa A, Wolman I. Cervical length, multifetal pregnancy reduction, and prediction of preterm birth. J Clin Ultrasound. 2005;33(7):329–332. doi: 10.1002/jcu.20159. [DOI] [PubMed] [Google Scholar]
- 17.Pils S, Eppel W, Seemann R, Natter C, Ott J. Sequential cervical length screening in pregnancies after loop excision of the transformation zone conisation: a retrospective analysis. BJOG. 2014;121(4):457–462. doi: 10.1111/1471-0528.12390. [DOI] [PubMed] [Google Scholar]
- 18.Pils S, Eppel W, Promberger R, Winter MP, Seemann R, Ott J. The predictive value of sequential cervical length screening in singleton pregnancies after cerclage: a retrospective cohort study. BMC Pregnancy Childbirth. 2016;16(1):79. doi: 10.1186/s12884-016-0866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiersch L, Rosen H, Okby R, Freeman H, Barrett J, Melamed N. The greater risk of preterm birth in triplets is mirrored by a more rapid cervical shortening along gestation. Am J Obstet Gynecol. 2016;215(3):357.e1–357.e6. doi: 10.1016/j.ajog.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Moragianni VA, Aronis KN, Craparo FJ. Biweekly ultrasound assessment of cervical shortening in triplet pregnancies and the effect of cerclage placement. Ultrasound Obstet Gynecol. 2011;37(5):617–618. doi: 10.1002/uog.8905. [DOI] [PubMed] [Google Scholar]