Abstract
Key points
Dentate spikes are fast fluctuations of hilar local‐field potentials that take place during rest and are thought to reflect input arriving from the entorhinal cortex to the hippocampus.
During dentate spikes, neuronal firing in hippocampal input (dentate gyrus) and output (CA1/CA3) regions is uncoupled.
To date, the behavioural significance of dentate spikes is unknown.
Here, we provide evidence that disrupting the dentate spike‐related uncoupling of the dentate gyrus and the CA1/CA3 subregions for 1 h after training retards associative learning.
We suggest dentate spikes play a significant role in memory consolidation.
Abstract
Hippocampal electrophysiological oscillations, namely theta and ripples, have been implicated in encoding and consolidation of new memories, respectively. According to existing literature, hippocampal dentate spikes are prominent, short‐duration (<30 ms), large‐amplitude (∼2–4 mV) fluctuations in hilar local‐field potentials that take place during awake immobility and sleep. Interestingly, previous studies indicate that during dentate spikes dentate gyrus granule cells increase their firing while firing of CA1 pyramidal cells are suppressed, thus resulting in momentary uncoupling of the two hippocampal subregions. To date, the behavioural significance of dentate spikes is unknown. Here, to study the possible role of dentate spikes in learning, we trained adult male Sprague–Dawley rats in trace eyeblink classical conditioning. For 1 h immediately following each conditioning session, one group of animals received hippocampal stimulation via the ventral hippocampal commissure (vHC) contingent on dentate spikes to disrupt the uncoupling between the dentate gyrus and the CA1 subregions. A yoked control group was stimulated during immobility, irrespective of brain state, and another control group was not stimulated at all. As a result, learning was impaired only in the group where vHC stimulation was administered contingent on dentate spikes. Our results suggest dentate spikes and/or the associated uncoupling of the dentate gyrus and the CA1 play a significant role in memory consolidation. Dentate spikes could possibly reflect reactivation and refinement of a memory trace within the dentate gyrus triggered by input from the entorhinal cortex.
Keywords: dentate gyrus, hippocampus, learning
Key points
Dentate spikes are fast fluctuations of hilar local‐field potentials that take place during rest and are thought to reflect input arriving from the entorhinal cortex to the hippocampus.
During dentate spikes, neuronal firing in hippocampal input (dentate gyrus) and output (CA1/CA3) regions is uncoupled.
To date, the behavioural significance of dentate spikes is unknown.
Here, we provide evidence that disrupting the dentate spike‐related uncoupling of the dentate gyrus and the CA1/CA3 subregions for 1 h after training retards associative learning.
We suggest dentate spikes play a significant role in memory consolidation.
Abbreviations
- ANOVA‐RM
analysis of variance for repeated measures
- CS
conditioned stimulus
- DG
dentate gyrus
- DS
dentate spike
- EMG
electromyography
- EXP
experimental group
- LFP
local‐field potential
- NC
normal control group
- US
unconditioned stimulus
- vHC
ventral hippocampal commissure
- YC
yoked control group
Introduction
Normal hippocampal function is crucial for learning and memory (Scoville & Milner, 1957). Hippocampal electrophysiological activity is characterized by alternating epochs of regular slow activity called theta (3–12 Hz) and large‐amplitude irregular activity (Buzsáki, 2002, 2015). During voluntary movement, such as when a rat is running in a maze, or when attending to external stimuli, theta tends to dominate hippocampal local‐field potentials (LFPs) (Buzsáki, 2002). During periods of awake immobility, for example when a rat stops and sits still, theta subsides and sharp‐wave–ripple complexes take place at random intervals (Buzsáki, 2015). Both theta and ripples have been postulated to play a special role in learning: theta has been assigned to the encoding of new information ‘on‐line’ and ripples to the ‘off‐line’ consolidation of recently acquired memories into long‐term storage (Buzsáki, 1989).
In addition to ripples evident in the hippocampal CA3 and CA1 subregions, intermittent events called dentate spikes take place in the dentate gyrus during awake immobility and slow‐wave sleep (Bragin et al. 1995). Dentate spikes are short‐duration (<30 ms), large‐amplitude (∼2–4 mV) deflections in hilar LFPs suggested to be evoked by entorhinal cortical input to the hippocampus in the intact brain and take place virtually simultaneously in both ventral and dorsal as well as left and right hippocampi (Bragin et al. 1995). During dentate spikes, neuronal firing is narrowly limited to the dentate gyrus and hilus, where both GABAergic interneurons and granule cells fire in an organized manner, granule cell firing centred around the peak of the dentate spike (Bragin et al. 1995; Penttonen et al. 1997). In contrast, CA3 and CA1 pyramidal cells tend to decrease their firing rate during dentate spikes (Bragin et al. 1995; Penttonen et al. 1997). Electrical stimulation via the angular bundle at the peak of the dentate spike leads to enhanced responses of dentate gyrus granule cells (Bramham, 1998) suggesting that they might provide a narrow time window during which input from the neocortex could impose maximal effects on the hippocampal circuit. However, the role of dentate spikes in learning and memory has so far not been directly studied.
To examine whether dentate spikes might play a role in memory consolidation, we trained adult male Sprague–Dawley rats in trace eyeblink classical conditioning since hippocampus is required for the acquisition of this associative learning task (Kim et al. 1995; Takehara et al. 2002, 2003). Due to the very short nature of dentate spikes, it is impossible to detect and disturb them with a closed‐loop setup. Therefore, we took an alternative approach and specifically aimed to disrupt the uncoupling of the dentate gyrus (DG) and the CA3/CA1 that normally takes place during dentate spikes (Bragin et al. 1995). After each conditioning session, animals in our experimental group received hippocampal stimulation via the ventral hippocampal commissure (vHC) contingent on dentate spikes. A yoked control group was stimulated during immobility, irrespective of brain state, and yet another control group was not stimulated at all. vHC stimulation is known to elicit a brief burst of neuronal firing in the CA1 and CA3, followed by hyperpolarization of both interneurons and pyramidal cells up to several hundreds of milliseconds (Penttonen et al. 1998; Girardeau et al. 2009). As CA1 and CA3 cells do not normally fire during dentate spikes (Bragin et al. 1995; Penttonen et al. 1997), vHC stimulation in the experimental group should disrupt the normal course of events associated with dentate spikes (see Fig. 1). Our hypothesis was that if dentate spikes and the following information processing in the hippocampus are necessary for learning, acquisition of trace eyeblink classical conditioning would be impaired in the experimental group but not in the yoked control group or in the non‐stimulated control group.
Figure 1. Stimulation electrodes were placed in the ventral hippocampal commissure (vHC) (A and C) and recording electrodes were placed in the hilus (B and D) to study the role of dentate spikes (E and F) in learning with the help of electrical stimulation of the hippocampus via the vHC (G and H).
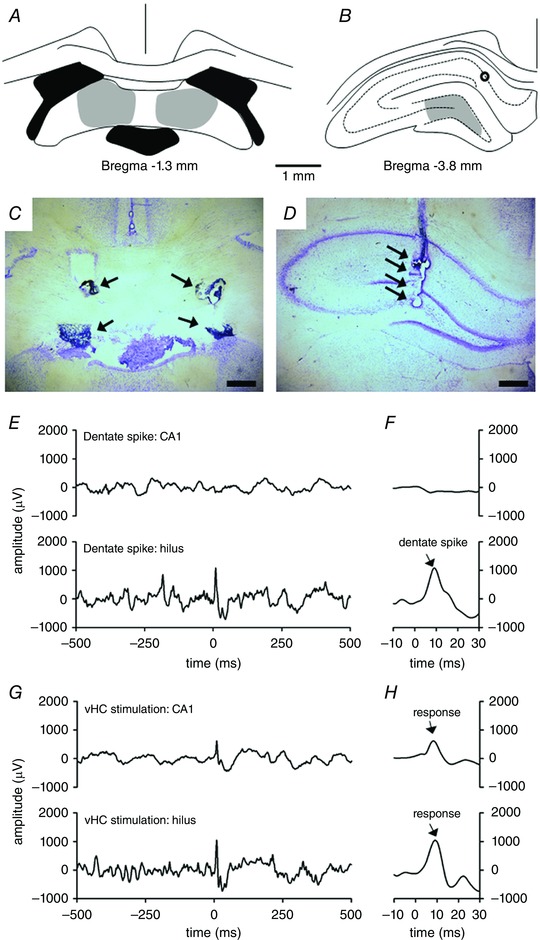
A, stimulation electrodes were placed in the vHC (grey shading). Black indicates ventricles. B, recording electrodes were targeted to the hilus (grey shading). Black circle indicates placement of a recording electrode in the CA1. C, example photomicrograph of stimulation electrode placement. Arrows point to electrode tips. D, example photomicrograph of recording electrode placement in the hilus. Arrows point to electrode tips. Horizontal scale bars in C and D are 500 μm in length. E and F, examples of spontaneous local‐field potentials (LFPs) illustrating a dentate spike (starting at time point 0) recorded from the CA1 (upper panel) and hilus (lower panel) in one rat belonging to the yoked control group. F is a magnification of E. Note the absence of a major deflection in CA1 during a dentate spike in hilus. G and H, LFPs recorded during vHC stimulation (at time point 0, bipolar pulse of 0.2 ms in total duration). H is a magnification of G. Note the clear response in CA1 when compared to the spontaneously generated dentate spike illustrated in E and F. [Color figure can be viewed at wileyonlinelibrary.com]
Methods
Ethical approval
All the experimental procedures were implemented in accordance with directive 2010/63/EU of the European Parliament and of the Council on the care and use of animals for research purposes. The animal experiments were approved by the Animal Experiment Board of Finland.
Subjects
The subjects were 39 adult male Sprague–Dawley rats (Harlan Laboratories/Envigo) weighing ∼300 g (∼10 weeks) at surgery. All animals were housed on the premises of the animal research unit at the University of Jyväskylä. Food and water were freely available, and room temperature and humidity were controlled at 21 ± 2°C and 50 ± 10%, respectively. All rats had aspen chips (Tapvei, Kaavi, Finland) at the bottom of the cage as bedding material. Rats were maintained on a 12 h–12 h light–dark cycle, with lights on at 08.00 h. All procedures were conducted during the light portion of the cycle.
Surgery
Chronic electrodes were implanted to record hippocampal local‐field potentials (LFPs) from the hilus and to stimulate the hippocampus via the ventral hippocampal commissure (see Fig. 1). Recording electrodes were made of Formvar‐insulated nichrome wire (bare diameter 50 μm, no. 762000, A‐M Systems Inc., Carlsborg, WA, USA) glued together using cyanoacrylate with a tip separation of 200–250 μm. Bipolar stimulation electrodes were made of Formvar‐coated stainless steel dual‐wire with a diameter of 100 μm and a tip separation of ∼500 μm. Rats were premedicated with carprofen (5 mg kg−1, s.c.; Rimadyl, 50 mg ml−1, Pfizer Oy, Helsinki, Finland) and buprenorphine (0.03 mg kg−1, s.c.; Temgesic, 0.3 mg ml−1, RB Pharmaceuticals Limited, Slough, UK). Half an hour later the rats were anaesthetized using an intraperitoneal (i.p.) injection of sodium pentobarbital (60 mg kg−1; Mebunat, 60 mg ml−1, Orion Oyj, Espoo, Finland). A booster injection of 15 mg kg−1 was given if needed to keep the level of anaesthesia steady throughout the surgery. Saline was injected s.c. every hour at a dose of 2 ml to prevent dehydration.
The head was shaved and the rat was secured to a stereotaxic device (Stoelting Co., Wood Dale, IL, USA) using blunt ear bars. An incision was made to reveal both the bregma and the lambda. Four skull screws were implanted and connected in pairs to serve as reference (11 mm posterior and 2 mm lateral to bregma) and earth (4 mm anterior and 2 mm lateral to bregma) for LFP recordings. Two bundles of four recording electrodes (tip separation 200–250 μm) were lowered either into the left hippocampus or at both left and right hippocampi aiming the tip of the longest electrode at the hilus (3.6/4.5 mm posterior, 1.5/2.2 mm lateral to bregma and 3.6/4.0 mm below bregma). Simultaneously, stimulation electrodes were lowered to the ventral hippocampal commissure (1.3 mm posterior, 1.1 mm lateral to bregma and 4.1 mm below bregma). The final depths for the recording and stimulation electrodes were determined by passing a bipolar 0.2 ms pulse of <160 μA through the stimulation electrode and examining the effects on LFPs recorded in the hippocampus. Once a large (≥1 mV) positive response at around 6–8 ms after stimulation was detected in the lowermost recording points, the electrodes were cemented in place using dental acrylic mass anchored to the skull via the skull screws. Lastly, two bipolar electrodes made of stainless steel wire insulated with Teflon (bare diameter 127 μm; no. 791500, A‐M Systems) were implanted through the upper right eyelid to record eyeblinks and deliver shock to the eyelid during eyeblink conditioning.
The rat was then placed in a heated cage until awake. The rat was returned to the home cage when it was moving normally and its welfare was monitored daily thereafter until the end of the experiment. Buprenorphine (0.03 mg kg−1 s.c.) was administered for postoperative analgesia and the surgery wound was cleaned with saline if needed.
Experimental procedure
The outline of the experiment is presented in Fig. 2 A. During training, electromyographic (EMG) signals from the upper right eyelid and LFPs from the hippocampus were recorded. The EMG signal was bandpass filtered between 100 and 300 Hz (Cyberamp380 or A‐M Systems 2100; A‐M Systems, Sequim, WA, USA). To acquire neural measures, a low‐noise pre‐amplifier (MPA8I, MultiChannel Systems (MCS), 10×, Reutlingen, Germany) was directly attached to the electrode connector anchored with dental acrylic to the rat's head. A flexible, insulated cable was used to connect the animal to the filter‐amplifier (FA64I, filter: 1–5000 Hz, 50×, MCS). All signals were digitized (USB‐ME‐64 System, MCS) and recorded with Mc_Rack software (MCS) using a 20 kHz sampling rate. Finally, all signals were digitally low‐pass‐filtered below 500 Hz (4th order Bessel) and stored using a 2 kHz sampling rate. LabVIEW (National Instruments Corporation, Austin, TX, USA) was used for online signal analysis and triggering of events.
Figure 2. Trace eyeblink conditioning in rats was used to study the effect of disrupting dentate spike‐related hippocampal neural activity on learning.
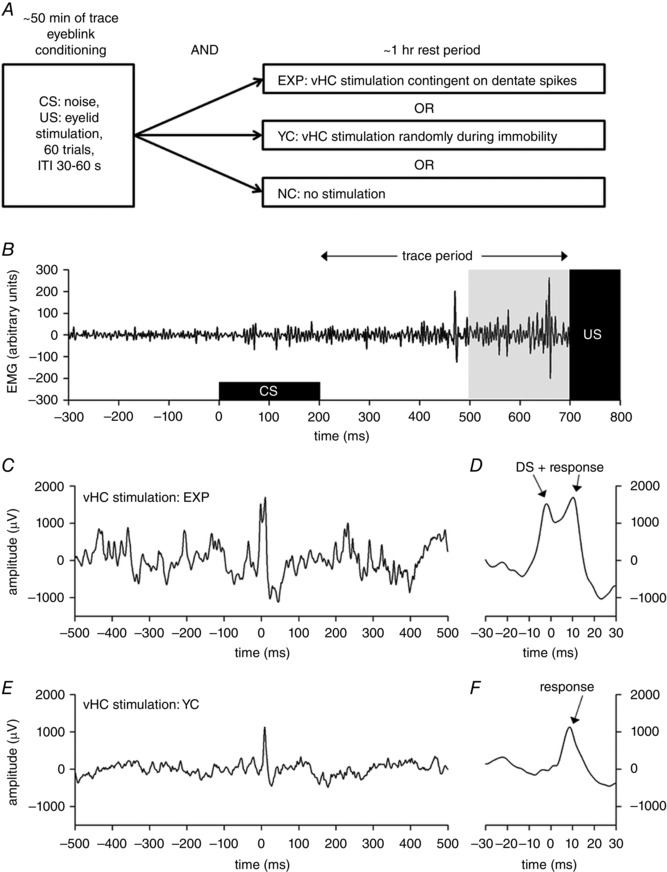
The outline of the experiment is presented in A and an example of a typical conditioned eyeblink response in B. Examples of hippocampal stimulation via the vHC are shown in panels C–F. A, rats were trained in trace eyeblink conditioning using a 500 ms trace period. The conditioned stimulus (CS) was a 200 ms, 75 dB white noise auditory stimulus. The unconditioned stimulus (US) was a 100 ms electrical stimulation of the eyelid that elicited a robust blink of the eye. A total of 60 trials were presented during each daily session, with an intertrial interval (ITI) of 30–60 s. Conditioning was followed immediately by a 1 h rest period. During the rest period, animals were either left alone (normal control, NC) or the hippocampus stimulated via the ventral hippocampal commissure (vHC) contingent on dentate spikes (EXP). For animals in the yoked control group (YC) vHC stimulation was administered to a random brain state but during immobility. B, an example of electromyogram (EMG) from the stimulated eyelid during a trial in which the animal emitted a typical conditioned response. The pale grey box indicates the time period during which blinking was categorized as a conditioned response. C, an example of hilar local‐field potential during hippocampal stimulation (at 0 ms) via the vHC, immediately following a dentate spike (DS). D, same data as in C but with a higher time resolution. E, an example of hilar local‐field potential during hippocampal stimulation (at 0 ms) via the vHC, to a random brain state but during immobility. F, same data as in E but with a higher time resolution. Note the ∼10 ms delay in the hippocampal‐evoked response to vHC stimulation.
Trace eyeblink classical conditioning
All recordings took place in grey plastic cylinders (floor 28 cm × 24 cm, height 53 cm) filled with a ∼3 cm‐thick layer of standard wooden bedding material. The recording cylinder was cleaned of visible dirt, and faeces were removed between each animal. The recordings were conducted inside a Faraday cage, in dimmed lighting, and always took place during the light phase of the day. First, each rat was habituated to the recording chamber for 30 min. During this habituation session the functioning of the recording electrodes were tested. Then, animals were further habituated to the recording in pairs for 1 h. The next day, animals started training in trace eyeblink conditioning in pairs.
The conditioning session always started with a 5 min recording of spontaneous LFPs. Conditioning was then started. A white noise (75 dB, 200 ms) was used as a conditioned stimulus (CS), and a 100 ms, 100 Hz burst of 0.5 ms bipolar pulses of periorbital shocks was used as an unconditioned stimulus (US). The amplitude of the shock varied between 0.1 and 1.5 mA and was adjusted individually for each rat on each day, starting from the amplitude used the previous day (from 0.3 mA on the first day). Shock amplitude was set to the minimum current needed to elicit a robust full closure of the eyelid. Each trace conditioning trial consisted of the 200 ms white noise CS followed by a 500 ms stimulus‐free trace interval that separated the CS from the presentation of the 100 ms shock US. A total of 60 trials per session were presented with an intertrial interval randomly varying between 30 and 60 s. A total of eight conditioning sessions were carried out (Tuesday–Friday and Monday–Thursday).
Hippocampal stimulation via ventral hippocampal commissure (vHC)
After each conditioning session the animals remained in the recording cylinder for another hour. Movement was detected by an accelerometer (EVAL‐ADXL335Z, Analog Devices Inc., Norwood, MA, USA) attached to the LFP preamplifier (MPA8I, MCS) cord. The animals were divided into three groups as follows. In the normal control (NC) group, no hippocampal stimulation was given. In the experimental group (EXP), the hippocampus was stimulated via stimulation electrodes placed in the vHC immediately (∼0–5 ms variable delay) following the peak of the dentate spike. The vHC mainly projects to the contralateral CA3 subregion of the hippocampus, thus connecting the two hippocampi (Shinohara et al. 2012). In addition, the vHC sends widespread connections to the DG and the CA1. The animal was included in the analyses only if the vHC stimulation produced a hilar response of >1 mV in amplitude peaking at <10 ms from stimulation onset. Note that stimulation even at a longer delay would probably have been efficient in disrupting the uncoupling between the DG and the CA3/CA1 as the silencing of the pyramidal cells induced by the dentate spike seems to last at least some tens of milliseconds (Bragin et al. 1995; Penttonen et al. 1997; Headley et al. 2017). Signal from a recording electrode showing clear dentate spikes and a large (>1 mV) positive response to the vHC stimulation was conveyed to LabVIEW for detecting spontaneous dentate spikes and triggering stimulation. A peak threshold (minimum ∼0.6 mV) and a rise threshold (minimum ∼0.7 mV in 10 ms) were set individually for each animal based on visual inspection of the LFP signal during the 1 h habituation session conducted prior to starting the conditioning. The signal from the accelerometer was also conveyed to LabVIEW. A set threshold for detecting movement of 0.001 m/s2 was used to only look for dentate spikes during immobility. The LabVIEW routine was run every 2 ms with a sampling rate of 1 kHz. Each animal in the experimental group was paired with a yoked control (YC) group animal. Animals in the YC group received vHC stimulation simultaneously with the EXP animal they were paired with, if they were immobile. Stimulations missed due to movement were compensated for by manually administering stimulations at random intervals as soon as the YC animal remained immobile for longer than 1 s. This ensured that the number of stimulations delivered to the YC animal at any given point in time during the recording was as close to that delivered to the EXP animal as possible. An 8‐channel general purpose stimulus generator (model STG4008, MCS) was used for delivering the vHC stimulation. A bipolar pulse of 0.2 ms in total duration was used. Stimulation amplitude (max. 160 μA) was adjusted for each animal in the EXP and the YC group so that the amplitude of the hippocampal response in the electrode showing spontaneous dentate spikes was 1–1.5 mV in amplitude. This is comparable to the amplitude of the spontaneous dentate spikes (range from 1 to 2.5 mV). This adjustment of stimulation amplitude was done during the very first 30 min habituation session.
Histology
After the experiment, rats were killed by exposure to a rising concentration of CO2, and death was verified by rapid decapitation using a guillotine. The locations of the electrode tips in the brain were marked by passing a DC anodal current (200 mA, 5 s) through them. The brain was then removed and stored in 4% paraformaldehyde solution (pH 7.4) for at least 48 h. The brain was coronally sectioned with a vibratome (Leica VT1000) into 40 μm slices. The slices were attached to slides, dried and then stained with Prussian blue and Cresyl violet. The electrode locations were determined with the aid of a microscope and a brain atlas (Paxinos & Watson, 1998).
Data analysis
Eyeblinks
MATLAB (The MathWorks Inc., Natick, MA, USA) was used for off‐line data analysis. The EMG signal was high‐pass filtered off‐line (100 Hz) and Hilbert‐transformed. An envelope curve following the peaks of the signal was calculated. Next the mean and the standard deviation (SD) of the signal during a 200 ms period immediately preceding the onset of the CS were obtained for each animal during each session separately. For each trial, the threshold for a learned response was set at mean + 3 SD. Responses had to occur during the last 200 ms of the trace period, and the EMG signal had to stay above the predetermined threshold for at least 5 ms for a blink to be classified as a learned response. An example of a learned response is shown in Fig. 2 B.
Dentate spikes
Dentate spikes were detected off‐line using MATLAB. A set threshold for movement was used (1000 arbitrary units) to determine periods of immobility. Next, the mean and SD of the hilar LFP signal during immobility was derived. A threshold was set at mean + 4 SD. Then, positive deflections in LFP amplitude that exceeded the threshold were detected using a 20 ms window split in half: both the maximum LFP amplitude during the latter half (peak) and the difference between the maximum LFP amplitudes during the former and latter halves (rise) had to exceed the threshold to qualify the event as a dentate spike. Further, a safety period of 100 ms was set after each vHC stimulation so that responses to stimulation would not be confused with dentate spikes.
Statistics
IBM SPSS Statistics 24 (IBM Corporation, Armonk, NY, USA) was used for statistics. Sigmaplot (Systat Software Inc., San Jose, CA, USA) was used for data visualization. Analysis of variance for repeated measures (ANOVA‐RM) was used to analyse changes across training. Whenever a significant interaction emerged, separate repeated measures ANOVAs were conducted for each group. Greenhouse–Geisser corrected P values are reported when the sphericity assumption was violated according to Mauchly's test. Bonferroni‐corrected P values were used for post hoc comparisons when appropriate. One‐way ANOVA was used for comparisons between groups. Paired samples Student's t test was used for comparing two related measures.
Results
Histology
To be included in the analysis, the animals had to have (1) successful EMG recordings and eyelid stimulation, (2) successful vHC stimulation (Fig. 1 A and C), and (3) succesful recordings from the hilus (Fig. 1 B and D) throughout the whole experiment. This yielded the following group sizes: normal control group (NC), n = 8; yoked control group (YC), n = 5; experimental group (EXP), n = 7. Representative examples of ventral hippocampal commissure (vHC) stimulation and recording (hilus) electrode placement are shown in Fig. 1 C and D. An example of hilar LFP with a dentate spike is illustrated in Fig. 1 E and F. In one animal, a recording electrode was also placed in the CA1 region (see Fig. 1 B for histology). An example LFP in CA1 recorded during a dentate spike is illustrated in Fig. 1 E and F.
Dentate spike rate did not differ between the groups at baseline or change across conditioning
To examine whether dentate spikes occurred at an equal rate in all groups at a naive state, we analysed the 1 h habituation session before any conditioning. The number of dentate spikes per immobile minute (DS rate) was determined for each rat. At baseline, the DS rate in the NC group was 2.9 ± 0.5 min−1 (mean (M) ± standard error of mean (SEM)), in the YC group 2.7 ± 0.7 min−1, and in the EXP group 2.8 ± 0.4 min−1 (one‐way ANOVA: F [2,17] = 0.05, P = 0.95). To study changes in DS rates across conditioning, we examined the 1 h rest recordings following each conditioning session: no change in the DS rate across conditioning was detected (ANOVA for repeated measures, 4 blocks of 2 sessions by 3 groups: interaction of session and group: F [6,51] = 0.77, P = 0.55; main effect of block: F [3,51] = 2.05, P = 0.15). In addition, DS rate did not differ between the baseline recording and the 1 h rest recording after the first conditioning session (paired samples t test: t (19) = 0.13, P = 0.90), or between the baseline recording and the average of all eight recordings conducted after conditioning (t (19) = 1.57, P = 0.13). However, throughout the experiment, there were significantly more dentate spikes detected in the NC group after the conditioning sessions compared to the other two groups (main effect of group: F [2,17] = 5.12, P = 0.02; post hoc comparisons: YC vs. NC: P = 0.04, YC vs. EXP: P = 1.00 and EXP vs. NC: P = 0.05). To conclude, the rate of occurrence of dentate spikes per se does not change as a function of learning and hence does not seem to directly relate to learning at the behavioural level (please see also next section).
Next, we analysed the vHC stimulation rate and accuracy across conditioning. On average 241 ± 19 (M ± SEM) vHC stimulations were administered during the 1 h rest period in the EXP (and the YC) group. There was no change in the stimulation rate across conditioning (ANOVA‐RM: F [6,36] = 1.93, P = 0.10). Off‐line detection of dentate spikes using MATLAB indicated that on average 67% (±2 percentage units, SEM) of dentate spikes in the animals assigned to the experimental group were also detected on‐line by LabVIEW and followed by stimulation. There was no change in the DS detection accuracy across conditioning (ANOVA‐RM: F [6,36] = 0.90, P = 0.51). In the YC group, less than 1% of dentate spikes detected off‐line were followed by vHC stimulation. Note that the NC group received no vHC stimulation.
Learning was impaired by vHC stimulation when administered immediately following dentate spikes
The behavioural results of the experiment are summarized in Fig. 3. For studying changes in learned responding across conditioning, we averaged measures from two consecutive sessions to yield learned response occurrence (%) per two‐session blocks (4). As there was no difference in learning between the two control groups (NC vs. YC, ANOVA‐RM: main effect of block: F [3,33] = 10.26, P < 0.001; main effect of group: F [1,11] = 0.69, P = 0.42; interaction: F [3,33] = 1.40, P = 0.26), the two control groups were pooled and compared to the EXP group (see Girardeau et al. 2009 for similar analysis). ANOVA‐RM revealed a significant difference in learning rate between the control rats and the rats in the EXP group (interaction of block and group: F [3,54] = 8.47, P = 0.001). Separate analyses for control and EXP rats confirmed that animals in the control groups learned the trace eyeblink conditioning (ANOVA‐RM: F [3,36] = 11.48, P < 0.001) whereas animals in the EXP group showed no increase in learned responses across training (ANOVA‐RM: F [3,18] = 1.41, P = 0.27). The highest percentage of learned responses (per session, within last 4 sessions) was significantly higher in the control groups compared to the experimental group (one‐way ANOVA: F [1,18] = 7.27, P = 0.02). To summarize, as a function of trace eyeblink conditioning, rats in the control groups learned to blink in response to the white noise prior to the eyelid stimulation whereas animals in the experimental group did not.
Figure 3. Hippocampal stimulation via the ventral hippocampal commissure impaired learning if it took place contingent on hippocampal dentate spikes.
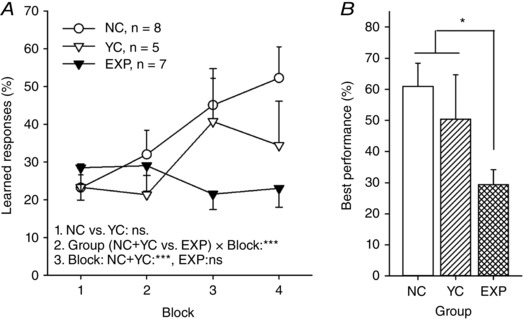
A, learned responses across two‐session blocks of trace eyeblink classical conditioning. Only animals in the non‐stimulated normal control (NC) and the stimulated yoked control (YC) groups learned. Animals in the experimental group (EXP) did not show an increase in learned responding. Line 1. refers to the result of a repeated measures ANOVA comparing the NC and the YC groups. Line 2. refers to the result of repeated measures ANOVA comparing the control groups (NC + YC) to the EXP group. Line 3. refers to results of separate repeated measures ANOVAs conducted on control (NC + YC) and experimental groups to study the change in learned responding across conditioning. B, highest percentage of learned responding per session (last 4 sessions). At best, animals in the control groups performed more learned responses than animals in the experimental group. The difference was significant according to a one‐way ANOVA. Asterisks refer to P values: *** P ≤ 0.001, * P < 0.05. Vertical lines indicate standard error of mean.
Discussion
Hippocampal dentate spikes are large, short‐duration deflections evident in hilar LFPs during still alertness and slow‐wave sleep (Bragin et al. 1995). Here, we examined the possible role of dentate spikes in memory consolidation in adult male Sprague–Dawley rats using trace eyeblink conditioning as a model of learning. Our results indicate that disrupting hippocampal function via ventral hippocampal commissural stimulation immediately after dentate spikes during a 1 h rest period following the daily learning session disrupts learning of a hippocampus‐dependent associative task. This suggests dentate spikes play a role in memory consolidation.
According to Buzsáki's two‐stage theory (Buzsáki, 1989), during epochs of theta, granule cells in the dentate gyrus encode incoming information about external events arriving from the entorhinal cortex into weak representations in the CA3. Interestingly, the phase of theta seems to modulate hippocampal responses to external stimuli and learning about those stimuli (Hasselmo et al. 2002; Nokia et al. 2015). Further, according to Buzsaki's theory (Buzsáki, 1989), during a rest period following the initial learning event, CA3 neurons potentiated most recently initiate ripples, that is, induce firing of the larger ensemble of previously activated CA3 neurons and their target CA1 pyramidal cells. From these cells activation spreads to connected cells thus strengthening the synaptic connection between the CA3 and the CA1 and the representation of the encoded event within the hippocampus (Buzsáki, 2015). It has also been suggested that contingent on ripples, information is transferred from the hippocampus to the neocortex for long‐term storage (Sirota et al. 2003). Previous findings suggest that ripple occurrence rate is affected by the slow oscillations in the entorhinal cortex and the dentate gyrus (Sullivan et al. 2011): fewer ripples are detected in vivo during the entorhinal cortical down‐state compared to the up‐state. Interestingly, increasing synchronization of activity between the hippocampus and the medial prefrontal cortex immediately following ripples enhances learning (Maingret et al. 2016). On the other hand, disrupting ripples (Girardeau et al. 2009; Jadhav et al. 2012) or the following information flow from the hippocampus to the neocortex (Nokia et al. 2012; Maingret et al. 2016) both impair learning. Taken together, Buzsaki's two‐stage theory (Buzsáki, 1989) is supported by experimental evidence. How dentate spikes fit into this model is yet to be determined.
To put dentate spikes in perspective one must start by considering the nature of and differences between neocortical and hippocampal neuronal activity. Compared to cortical principal cells, dentate granule cells tend to fire very rarely (Jung & McNaughton, 1993), an observation that has inspired a commonly held view of the dentate gyrus as the core structure in pattern separation (McClelland et al. 1995). It has been suggested that cortical signals corresponding to dense representations of recent events arriving via the perforant path are transformed – within the dentate gyrus – to a sparse hippocampal code forming the base of episodic memory in the hippocampus (Acsady & Kali, 2007). This transformation is likely to involve firing of dentate granule cells and interneurons in an organized fashion – and here is where dentate spikes might come into play: the results of the initial study by Bragin et al. (1995) suggest that in the intact brain, dentate spikes are evoked by entorhinal cortical layer II input to the hippocampus. As a response to this input arriving via the perforant path, hilar interneurons (Bragin et al. 1995), as well as principal cells and interneurons in the dentate gyrus, tend to fire whereas pyramidal cells in the CA3 and CA1 decrease their firing rate (Penttonen et al. 1997). That is, neuronal firing in the dentate gyrus/hilus and the CA3/CA1 subregions becomes momentarily highly uncoupled during dentate spikes. This could allow the formation of neuronal ensembles (perhaps corresponding to, for example, episodic memories) within the dentate gyrus that are independent of CA3/CA1 contributions.
Recently, the occurrence of ripples and dentate spikes was examined in freely moving rats (Headley et al. 2017). The results indicate that the occurrence of a dentate spike is 3 times more likely 50–80 ms after a ripple. That is, dentate spikes seem to follow ripples. In addition, if a dentate spike took place during a ripple, that ripple was significantly smaller in amplitude than ripples occurring outside of dentate spikes (Headley et al. 2017), suggesting that dentate spikes might suppress ripples. This is to be expected as pyramidal cell firing in CA3 and CA1 is suppressed during dentate spikes (Bragin et al. 1995; Penttonen et al. 1997). On the other hand, dentate spikes that took place immediately prior to a ripple were also significantly smaller in amplitude than those occurring without a ripple (Headley et al. 2017). Taken together, earlier findings support the notion that the learning deficit observed in our current study results from disrupted dentate spike‐related processing and not from disrupting ripples. If anything, stimulation targeted at dentate spikes (EXP group) should have been less likely to disrupt ripples than that administered at random during awake immobility (YC group). Note that the yoked control group that received vHC stimulations in the absence of movement during quiet waking learned as usual. In addition, as we found (1) no increase in the occurrence of dentate spikes across conditioning in the normal control group, and (2) a lower occurrence of dentate spikes in both the EXP and the YC group compared to the NC group throughout conditioning, the number of dentate spikes per se does not seem to be crucial for learning. Rather, it is possible that the neuronal firing sequences (or suppression of firing in the CA3/CA1 pyramidal cells) that take place during dentate spikes are important for learning. These possibilities should be addressed in further studies.
In the present study, when we disrupted the dentate spike‐related ‘uncoupling’ of dentate and CA3/CA1 networks via electrical stimulation of the ventral hippocampal commissure, learning was retarded. Considering that due to technical constraints only on average 2/3 of the dentate spikes in the experimental group were stimulated, learning could have been virtually abolished if all of the dentate spikes were captured by the brain–computer interface. Note that vHC stimulation elicits a burst of cell firing in the CA3/CA1 subregions followed by hyperpolarization and silencing of cells that can last for up to hundreds of milliseconds (Penttonen et al. 1998; Girardeau et al. 2009). The co‐occurrence of dentate spikes and vHC stimulation‐induced CA3/CA1 pyramidal cell and interneuron firing (and the possibly following hyperpolarization) is in direct contrast to the normal state where CA3/CA1 cells remain silent during dentate spikes. vHC stimulation may also increase the resonant firing of some depolarized pyramidal neurons at fixed gamma frequency (Penttonen et al. 1998). In sum, vHc stimulation contingent on dentate spikes could prevent not only the formation of neuronal ensembles within the dentate gyrus but could also disrupt information transfer through the trisynaptic loop and from cortical areas back to the hippocampus.
Related to the above, the study by Headley and colleagues (2017) also reveals that neocortical inter‐regional coupling at the gamma (35–100 Hz) band is increased specifically following dentate spikes in comparison to ripples. Moreover, peaking at around 40 ms after the dentate spike, gamma power is increased especially in the medial prefrontal cortex which is considered important for trace eyeblink conditioning as evidenced by both lesion and recording studies (for a recent review, please see Takehara‐Nishiuchi, 2016). Together, these findings suggest that the deficit in learning observed in the EXP group in our current study might be due, at least in part, not only to a disruption of processing within the hippocampus but also to a disruption in larger, cortical networks that are normally tuned or orchestrated by the occurrence of hippocampal dentate spikes. It is possible that the vHC stimulation used in our study affected, for example, medial prefrontal cortical activity either directly or indirectly, via effects on hippocampal activity.
The observed appearance of single dentate spikes at a rate of ∼3 min−1 in our current data suggest that dentate spikes might provide an efficient mechanism for repeated consolidation of a memory trace, with further refinements at each successive occurrence. Interestingly, 15–20 s is usually considered the minimum intertrial interval required for efficient trace eyeblink conditioning. This suggests that both during conditioning and subsequent rest the wide range neuronal networks should have at least some tens of seconds of ‘idle’ time to support memory formation at the cellular and molecular level. Note that compared to dentate spikes, ripples take place some 2–4 times more frequently, roughly every ∼5–10 s (see for example Nokia et al. 2012). Considering our current findings in light of the two‐stage model of memory formation (Buzsáki, 1989), it seems that the theory should be extended to include a role for the dentate gyrus also in off‐line memory consolidation, not only in on‐line encoding of events related to hippocampal theta and gamma oscillations.
Dentate spikes were first identified 20 years ago (Bragin et al. 1995; Penttonen et al. 1997; Bramham, 1998) but have since then been largely ignored. To our knowledge, our current study is the first attempt to unveil the behavioural significance of dentate spikes. Our results suggest that dentate spikes might actually play a significant role in memory consolidation. It is possible that dentate spikes reflect the formation and refining of a sparse code of neocortical representations within the dentate gyrus, a process crucial for the formation of episodic memories. Therefore, the relation of dentate spikes to behaviour warrants further studies.
Additional information
Competing interests
The authors of this manuscript declare no competing interests.
Author contributions
The experiments were performed in the Laboratory centre of the University of Jyväskylä, Jyväskylä, Finland. M.S.N. contributed to the conception and design of the work, acquired and analysed the data, interpreted the results and wrote the manuscript. I.G. and T.W. contributed to the design of the work, acquired and analysed the data, and revised the manuscript. H.T. and M.P. contributed to the conception and design of the work, analysed and interpreted the data and wrote the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by the Academy of Finland (grant no. 139767 to M.P. and grant nos. 275954, 284155 and 286384 to M.S.N.).
Acknowledgements
The authors would like to thank Arto Lipponen, Jarno Mikkonen and Lauri Viljanto for technical help and Jan Wikgren for comments on the experimental plan.
Linked articles This article is highlighted by a Perspective by Ewell. To read this Perspective, visit https://doi.org/10.1113/JP274502.
References
- Acsady L & Kali S (2007). Models, structure, function: the transformation of cortical signals in the dentate gyrus. Prog Brain Res 163, 577–599. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, van Landeghem M & Buzsaki G (1995). Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol 73, 1691–1705. [DOI] [PubMed] [Google Scholar]
- Bramham CR (1998). Phasic boosting of medial perforant path‐evoked granule cell output time‐locked to spontaneous dentate EEG spikes in awake rats. J Neurophysiol 79, 2825–2832. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (1989). Two‐stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2002). Theta oscillations in the hippocampus. Neuron 33, 325–340. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2015). Hippocampal sharp wave‐ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G & Zugaro MB (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12, 1222–1223. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C & Wyble BP (2002). A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 14, 793–817. [DOI] [PubMed] [Google Scholar]
- Headley DB, Kanta V & Pare D (2017). Intra‐ and interregional cortical interactions related to sharp‐wave ripples and dentate spikes. J Neurophysiol 117, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW & Frank LM (2012). Awake hippocampal sharp‐wave ripples support spatial memory. Science 336, 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW & McNaughton BL (1993). Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3, 165–182. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE & Thompson RF (1995). Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci 109, 195–203. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL & O'Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102, 419–457. [DOI] [PubMed] [Google Scholar]
- Maingret N, Girardeau G, Todorova R, Goutierre M & Zugaro M (2016). Hippocampo‐cortical coupling mediates memory consolidation during sleep. Nat Neurosci 19, 959–964. [DOI] [PubMed] [Google Scholar]
- Nokia MS, Mikkonen JE, Penttonen M & Wikgren J (2012). Disrupting neural activity related to awake‐state sharp wave‐ripple complexes prevents hippocampal learning. Front Behav Neurosci 6, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Waselius T, Mikkonen JE, Wikgren J & Penttonen M (2015). Phase matters: responding to and learning about peripheral stimuli depends on hippocampal θ phase at stimulus onset. Learn Mem 22, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press, Inc, San Diego, CA, USA. [Google Scholar]
- Penttonen M, Kamondi A, Acsady L & Buzsaki G (1998). Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci 10, 718–728. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Sik A, Acsady L & Buzsaki G (1997). Feed‐forward and feed‐back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus 7, 437–450. [DOI] [PubMed] [Google Scholar]
- Scoville WB & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Hosoya A, Yahagi K, Ferecsko AS, Yaguchi K, Sik A, Itakura M, Takahashi M & Hirase H (2012). Hippocampal CA3 and CA2 have distinct bilateral innervation patterns to CA1 in rodents. Eur J Neurosci 35, 702–710. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D & Buzsaki G (2003). Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA 100, 2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K & Buzsaki G (2011). Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J Neurosci 31, 8605–8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S & Kirino Y (2003). Time‐dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23, 9897–9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Takatsuki K & Kirino Y (2002). Time‐limited role of the hippocampus in the memory for trace eyeblink conditioning in mice. Brain Res 951, 183–190. [DOI] [PubMed] [Google Scholar]
- Takehara‐Nishiuchi K (2016). The anatomy and physiology of eyeblink classical conditioning. Curr Top Behav Neurosci (in press; https://doi.org/10.1007/7854_2016_455). [DOI] [PubMed] [Google Scholar]


