Abstract
Key points
Small transmembrane proteins such as FXYDs, which interact with Na+,K+‐ATPase, and the micropeptides that interact with sarco/endoplasmic reticulum Ca2+‐ATPase play fundamental roles in regulation of ion transport in vertebrates.
Uncertain evolutionary origins and phylogenetic relationships among these regulators of ion transport have led to inconsistencies in their classification across vertebrate species, thus hampering comparative studies of their functions.
We discovered the first FXYD homologue in sea lamprey, a basal jawless vertebrate, which suggests small transmembrane regulators of ion transport emerged early in the vertebrate lineage.
We also identified 13 gene subfamilies of FXYDs and propose a revised, phylogeny‐based FXYD classification that is consistent across vertebrate species.
These findings provide an improved framework for investigating physiological and pathophysiological functions of small transmembrane regulators of ion transport.
Abstract
Small transmembrane proteins are important for regulation of cellular ion transport. The most prominent among these are members of the FXYD family (FXYD1–12), which regulate Na+,K+‐ATPase, and phospholamban, sarcolipin, myoregulin and DWORF, which regulate the sarco/endoplasmic reticulum Ca2+‐ATPase (SERCA). FXYDs and regulators of SERCA are present in fishes, as well as terrestrial vertebrates; however, their evolutionary origins and phylogenetic relationships are obscure, thus hampering comparative physiological studies. Here we discovered that sea lamprey (Petromyzon marinus), a representative of extant jawless vertebrates (Cyclostomata), expresses an FXYD homologue, which strongly suggests that FXYDs predate the emergence of fishes and other jawed vertebrates (Gnathostomata). Using a combination of sequence‐based phylogenetic analysis and conservation of local chromosome context, we determined that FXYDs markedly diversified in the lineages leading to cartilaginous fishes (Chondrichthyes) and bony vertebrates (Euteleostomi). Diversification of SERCA regulators was much less extensive, indicating they operate under different evolutionary constraints. Finally, we found that FXYDs in extant vertebrates can be classified into 13 gene subfamilies, which do not always correspond to the established FXYD classification. We therefore propose a revised classification that is based on evolutionary history of FXYDs and that is consistent across vertebrate species. Collectively, our findings provide an improved framework for investigating the function of ion transport in health and disease.
Keywords: FXYD proteins, micropeptides, Na+−K+−ATPase, phospholemman, protein phosphorylation, SERCA, vertebrate evolution
Key points
Small transmembrane proteins such as FXYDs, which interact with Na+,K+‐ATPase, and the micropeptides that interact with sarco/endoplasmic reticulum Ca2+‐ATPase play fundamental roles in regulation of ion transport in vertebrates.
Uncertain evolutionary origins and phylogenetic relationships among these regulators of ion transport have led to inconsistencies in their classification across vertebrate species, thus hampering comparative studies of their functions.
We discovered the first FXYD homologue in sea lamprey, a basal jawless vertebrate, which suggests small transmembrane regulators of ion transport emerged early in the vertebrate lineage.
We also identified 13 gene subfamilies of FXYDs and propose a revised, phylogeny‐based FXYD classification that is consistent across vertebrate species.
These findings provide an improved framework for investigating physiological and pathophysiological functions of small transmembrane regulators of ion transport.
Abbreviations
- CDS
coding DNA sequence
- DWORF
dwarf open reading frame
- EST
expressed sequence tag
- mya
million years ago
- SERCA
sarco/endoplasmic reticulum Ca2+‐ATPase
Introduction
Ion transporters play key roles in cell biology. Ion transporter‐mediated ion gradients and ion fluxes (Skou, 1957) are involved in a broad array of functions in all living organisms, such as energy production (Mitchell, 1961), coupled transmembrane transport of various substrates (Crane, 1960), intracellular signalling (Heilbrunn & Wiercinski, 1947), as well as active movement (Manson et al. 1977; Matsura et al. 1977). Ion fluxes also underlie intercellular communication (Cole & Curtis, 1939; Hodgkin et al. 1952), thus enabling coordination of biological responses between diverse cells and tissues in multicellular organisms. Genes encoding ion transporters are abundant in genomes of all living organisms (Lander et al. 2001; Venter et al. 2001). In addition to ion transporters, vertebrates have multiple families of small transmembrane proteins that are important for regulation of ion transport. The most prominent among these are phospholemman (FXYD1) (Palmer et al. 1991; Crambert et al. 2002) and other members of the FXYD family (Sweadner & Rael, 2000), as well as phospholamban (Tada et al. 1979; Simmerman et al. 1986), sarcolipin (Odermatt et al. 1997; Odermatt et al. 1998), myoregulin (Anderson et al. 2015) and dwarf open reading frame (DWORF) (Nelson et al. 2016).
The FXYD family, characterized by the eponymous Phe‐Xaa‐Tyr‐Asp (FXYD) motif (Sweadner & Rael, 2000), currently comprises seven mammalian FXYDs (FXYD1–7) (Sweadner & Rael, 2000) and at least nine fish FXYDs (FXYD2 and FXYD5–12) (Cornelius et al. 2005; Tipsmark, 2008). Their major function is regulation of Na+,K+‐ATPase in excitable and osmoregulatory tissues (Mahmmoud et al. 2003; Geering, 2008; Tipsmark et al. 2011). Other functions include regulation of the Na+/Ca2+ exchanger (Zhang et al. 2003) and the L‐type Ca2+ channels (Zhang et al. 2015) in cardiac myocytes, regulation of permeability in kidney tubules and intestinal epithelia (Okuda et al. 2010; Lubarski et al. 2011), and, possibly, regulation of the gastric‐type H+,K+‐ATPase (Crambert et al. 2005). Unlike the FXYDs, which are expressed in the plasma membrane of various cells, phospholamban, sarcolipin, myoregulin and DWORF are expressed in the membrane of the sarcoplasmic reticulum of muscle cells, where they regulate the sarco/endoplasmic reticulum Ca2+‐ATPase (SERCA). Phospholamban, sarcolipin and myoregulin inhibit SERCA, while DWORF stimulates it by displacing these inhibitory proteins (Nelson et al. 2016). Clearly, small transmembrane regulators of ion transport have diversified extensively in vertebrates. In contrast, their number is restricted in invertebrates (Navarre et al. 1992; Magny et al. 2013), suggesting their diversification was important for vertebrate evolution.
The evolutionary history of FXYDs has been subjected to different interpretations (Tipsmark, 2008; Studer et al. 2011), which has left two major questions unanswered. Firstly, the timing of the emergence of FXYDs in the vertebrate lineage is uncertain. The most distant orthologue (see Box 1 for a glossary of terms) of mammalian FXYDs was found in the spiny dogfish (Mahmmoud et al. 2000; Cornelius et al. 2005), a modern representative of Chondrichthyes (cartilaginous fishes). Thus, FXYDs might be specific to Gnathostomata (jawed vertebrates), although their expression in Cyclostomata (jawless vertebrates) cannot be excluded with certainty (Studer et al. 2011). Conversely, phospholamban and sarcolipin are likely to have been generated by a duplication of a pre‐vertebrate ancestral gene (Magny et al. 2013), suggesting Cyclostomata had at least one regulator of SERCA. Consistent with this view, sequence alignments suggest DWORF is present in Cylostomata (Nelson et al. 2016). These data suggest that regulators of SERCA emerged earlier than FXYDs; however, the ancestral pre‐vertebrate gene and the timing of its duplications in the vertebrate lineage have not been determined.
Box 1. Glossary.
CDS: coding DNA sequence; part of the genomic DNA that is translated.
Clade: a taxon that includes all species descending from a common ancestor.
Conserved synteny: synteny means on the same strand, while conserved synteny refers to conservation of gene order on the chromosomes of different, but related, species.
ESTs: expressed sequence tags are short reads from the cDNA and represent genes that are expressed in a given tissue and/or developmental stage.
Homologues (homologous genes): Genes that originate from a common ancestor.
Orthologues (orthologous genes): Genes in different species originating from the same ancestral gene. For instance, FXYD2 is a deeply ancestral FXYD gene, from which all FXYD2 genes in vertebrates are derived.
Paralogues (paralogous genes): Genes originating from the duplication of the same ancestral gene. For instance, duplication of FXYD2 in Actinopterygii produced fish‐specific paralogue of FXYD2 (FXYD2f).
Secondly, evolutionary relationships between the fish and the mammalian FXYDs are unclear (Sweadner & Rael, 2000; Mahmmoud et al. 2003; Tipsmark, 2008). Some of the supposedly fish‐specific FXYDs (FXYD8–12) (Yang et al. 2013) could be orthologous to the mammalian FXYDs (FXYD1–7). Such uncertainties in the current FXYD classification hamper comparative studies of FXYD function in fishes and other lower vertebrates. This is an important obstacle – since lower vertebrate models have been used to study human diseases (Cai et al. 2013) – to developing new drugs (Asnani & Peterson, 2014; MacRae & Peterson, 2015) and to uncovering fundamental physiological mechanisms (Donovan et al. 2000). Therefore, gaps in the evolutionary history of FXYDs and regulators of SERCA have practical ramifications that go beyond pure academic research.
Here we provide evidence of early vertebrate origins and diversification of FXYDs and regulators of SERCA. In addition, we propose a revised FXYD classification, which is based on the likely evolutionary history of the FXYD family and is consistent across the vertebrate species.
Methods
Ethical approval
All experimental procedures for Petromyzon marinus were approved (ethical approval no. 523/12) by the institutional Ethics Committee (Norra Djurförsöksetiska Nämnden, Stockholm, Sweden) and conform to the principles and regulations of The Journal of Physiology.
Petromyzon marinus
Petromyzon marinus (sea lamprey) was captured in Lake Michigan, USA, and delivered by Acme Lamprey (Harrison, ME, USA). Lampreys (fasted) were kept in an aerated freshwater aquarium at 5°C, with a 12 h light–12 h dark cycle. They were killed by immersion in cold water (4–8°C) containing an overdose of tricaine methane sulphonate anaesthetic (MS‐222, 200–300 mg l−1; Sigma‐Aldrich, St Louis, MO, USA). After killing, lampries were immediately dissected. Tissues obtained during dissection were snap‐frozen in liquid nitrogen and then kept at –80°C until further analysis.
Identification and sequencing of FXYD homologue in Petromyzon marinus
P. marinus expressed sequence tags (ESTs) CO551377.1 and FD714523.1 were mapped to Lethenteron camtschaticum contig040605, which is deposited in GenBank under the accession number APJL01055902.1. Based on the sequence of four predicted exons and Lethenteron camtschaticum, contig040605 PCR primers were designed with the Primer3 algorithm (Table 1). Genomic DNA was extracted from a representative section of the P. marinus and isolated using the DNeasy Blood and Tissue Kit from Qiagen (Hilden, Germany). PCR was performed using GoTaq Green master mix (Promega, Madison, WI, USA) at an annealing temperature of 52°C and PCR products were gel purified using the MiniElute kit from Qiagen. Purified PCR products were Sanger sequenced (GATC Biotech, Konstanz, Germany) using the forward PCR primer.
Table 1.
PCR primers binding to genomic and complementary DNA
| Primer name | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| PCR primers binding to genomic DNA | ||
| Ex1–Intr2 | 5′‐TGATAACTCCTCATAAACCTGCCTC‐3′ | 5′‐GTGTGTGTCTTTGTGTACACATGTA‐3′ |
| Intr2–3 | 5′‐ACACACAATCTCAACAGGAACCTAT‐3′ | 5′‐ACTCAGCCTCAAATTAGTAACTGGT‐3′ |
| Intr4 | 5′‐ACCAGTTACTAATTTGAGGCTGAGT‐3′ | 5′‐TGAATGCGTTGATGAAGGAATGAAT‐3′ |
| Upstream | 5′‐CTGCCCTCTCTGTTTCAGTCG‐3′ | 5′‐AGGCAGGTTTATGAGGAGTTATCAA‐3′ |
| Downstream | 5′‐CTACAGCGACCCAGAGAATATCTAC‐3′ | 5′‐ATCGCCCACTAAATTATGAACAACC‐3′ |
| qPCR primers binding to complementary DNA | ||
| lcFXYD_cDNA | 5′‐CTCATAAAGCAGGAAATCCCAAACA‐3′ | 5′‐TGTTTACTGAACACGATGAGGAGTC‐3′ |
| lc_18s_cDNA | 5′‐CGTTATCGGAATGAACCAGACAAAT‐3′ | 5′‐AGGATTGACAGATTGAGAGCTCTTT‐3′ |
RNA extraction and quantitative real‐time PCR
RNA was extracted from the brain, eye, gills, liver, gut, heart, muscle and tail of P. marinus using the miRNeasy Kit from Qiagen. cDNA was synthetized from 1.5 μg RNA per tissue using the high capacity reverse transcription kit from Life Technologies (Carlsbad, CA, USA). qPCR primers were designed using Primer3 (Table 1). qPCR including a dissociation curve was performed in duplicate on the StepOnePlus machine using SYBR Green gene expression master mix (Life Technologies). After amplification the PCR product was gel purified (MiniElute Kit, Qiagen) and analysed by Sanger sequencing (GATC Biotech) using the reverse qPCR primer. Gene expression was normalized to the expression of 18S rRNA and expressed as fold change relative to the expression in brain using the ΔΔC t method. We performed all standard quality controls in accordance with the Minimum Information for Publication of Quantitative Real‐Time PCR Experiments (MIQE) guidelines (Bustin et al. 2009). Specificity of the PCR reaction was assessed by analysing the melt curves and by electrophoresis of the PCR products on a 1% agarose gel using 6× loading buffer, GeneRuler 100 bp DNA Ladder (both from Thermo Fisher Scientific, Waltham, MA, USA, #SM0241) and GelRed nucleic acid stain (Biotium, CA, USA, #41003). qPCR products were excised from the gel and purified using the MiniElute gel extraction kit (Qiagen). To confirm the presence of the LTYD motif, the purified qPCR products from the heart and liver were sent to GATC Biotech for Sanger‐sequencing analysis using the forward and reverse qPCR primers (Table 1).
Computational analysis of FXYD family members and other small transmembrane regulators of ion transport
Sequences of known FXYD genes and genes for SERCA regulators phospholamban, sarcolipin and myoregulin from Homo sapiens, Mus musculus, Danio rerio and Salmo salar were used in PSI‐BLAST (Altschul et al. 1997) searches against the GenBank database. NCBI RefSeq (Pruitt et al. 2014) sequences were used where available. TBLASTN (Camacho et al. 2009) searches against the NCBI EST database were performed to add protein sequences not annotated in GenBank. Preliminary alignments of FXYD core domain were obtained using the MUSCLE program (Edgar, 2004); sequences were grouped into subfamilies based on the clades in the trees reconstructed using the FastTree program (Price et al. 2009). Subfamilies were refined iteratively by collecting and realigning clade‐specific sequences with subsequent tree reconstruction. FXYD core domain sequences were extracted from the subfamily‐specific alignments and aligned together; tree was reconstructed using the FastTree program (Abascal et al. 2005). The optimal evolutionary model (JTT with gamma‐distributed site rates) was determined using the ProtTest program; a phylogenetic tree for the RefSeq subset of the sequences was reconstructed using the RAxML program to verify the subfamily assignments. Additionally, all pairs of subfamily‐specific alignments were aligned using the HHALIGN program (Soding, 2005) with scores recorded. A matrix of the relative pairwise distances was constructed using the D(A,B) = –log[S(A,B)/min(S(A,A),S(B,B))] formula, where D(A,B) is the distance between subfamilies A and B and S(A,B) is the HHALIGN score of the corresponding alignment between the subfamily alignments. A neighbour‐joining tree was reconstructed from this matrix using the NEIGHBOR program of the PHYLIP package (Felsenstein, 1996).
Sequences available in RefSeq were associated with the corresponding chromosomes and contigs in the NCBI Genome database; local genomic context was recorded. Conservation of the local context was used to verify the subfamily assignment and to infer the evolutionary scenario. Specifically, orthology relationships were inferred between genes, located in the clearly similar chromosomal neighbourhoods (as evidenced by reciprocal similarity between flanking genes) even if the sequence‐based similarity did not show such relationships unambiguously.
Dating of evolutionary events is reported according to the timetree.org database (using the median value of published estimates or expert estimates where available) (Hedges et al. 2015).
Identification and evaluation of phosphorylation sites in FXYDs
To identify putative phosphorylation sites in FXYD proteins, ScanSite (http://scansite.mit.edu/) and PhosphoSite (http://www.phosphosite.org/) were used. Predicted phosphorylation sites were evaluated against experimentally determined phosphorylation sites. Based on the scores generated by PhosphoSite, predicted phosphorylation sites were graded on a scale from 0 (the least reliable) to 9 (the most reliable).
Results
Search for small transmembrane regulators of ion transport in sequence databases
Annotated Cyclostomata proteins in GenBank or Ensembl database do not contain any reliable FXYD homologues. BLAST searches against Cyclostomata‐related expressed sequence tags (ESTs) (see Box 1) revealed two potentially related ESTs in P. marinus (CO551377.1 and FD714523.1) and three in hagfishes (FY411797.1, BJ653733.1 and BJ648843.1). FXYD homologues were not identified outside of the Craniata clade.
Phospholamban homologues are readily identified in the majority of the genomes of Craniata lineage, including Cyclostomata ESTs (FD715866), but was not found outside this clade. While most vertebrate species possess a single phospholamban homologue, bony fishes usually have two phospholamban genes. These two copies are surrounded by matching genome context, suggesting that the duplication involved a large segment of the ancestral chromosome. Possibly, these copies and the surrounding segments were retained since the whole genome duplication in fish (Amores et al. 1998; Amores et al. 2004; Jaillon et al. 2004). Myoregulin, a recently described regulatory peptide (Anderson et al. 2015), is annotated in several mammalian genomes and its coding DNA sequence (CDS) (see Box 1) is confidently recognized across all placental mammals.
Identification and sequencing of FXYD homologue in Petromyzon marinus
To validate the existence of an FXYD homologue in modern Cyclostomata, genomic DNA from a representative section of P. marinus was extracted, amplified and subsequently sequenced. Four sequencing products were aligned to the L. camtschaticum contig040605 APJL01055902.1 to create a hybrid sequence, where missing parts of the sequences were replaced by L. camtschaticum DNA. When this alignment was mapped to the predicted L. camtschaticum CDS sequences and a piece of P. marinus cDNA, an A–G mismatch was detected between P. marinus CDS cDNA and L. camtschaticum genomic DNA (APJL01055902.1). P. marinus cDNA sequence was used to adjust the hybrid sequence of P. marinus FXYD homologue. This FXYD homologue aligns with the published P. marinus GL488371 whole genome shotgun sequence. Comparison with P. marinus CDS cDNA confirmed that the gene of this FXYD homologue has four exons. The Phe in the signature motif of the FXYD family is changed to Leu. The LTYD motif spans the exon 2–3 boundary (Fig. 1) and is followed by another Tyr (LTYDY), which is a typical characteristic of the FXYD family (Sweadner & Rael, 2000).
Figure 1. Schematic overview of the coding sequence for FXYD homologue in Petromyzon marinus .

A, grey boxes represent the predicted 4 exons of the proto‐FXYD5 in P. marinus. Hatched boxes below the exons indicate the qPCR‐primer binding sites that span the exon 1 to exon 2 (forward primer) and exon 3 to exon 4 (reverse primer) junction. The sequence between the black arrows are the results of the qPCR‐product Sanger sequencing and reveal the genomic cDNA sequence that encodes the LTYD protein motif (marked as black boxes above exons 2 and 3). The dotted line within the LTYD coding sequence indicates the unknown sequence of intron 2 that was spliced from the cDNA. B, the predicted coding sequence of proto‐FXYD5 including the predicted amino acid translation.
Expression of FXYD homologue in Petromyzon marinus
To determine the expression pattern of the FXYD homologue in P. marinus, mRNA in samples from various tissues and organs was measured by qPCR using SYBR Green chemistry (Fig. 2). The FXYD homologue was most prominently expressed in liver, but was expressed across all tissues and organs tested (Fig. 2 A and B). Analysis of the melt curve indicated qPCR‐primers generated only specific PCR products (data not shown). This analysis was supported by electrophoresis of PCR products from gills, liver, gut and heart (Fig. 2 C) on a 1% agarose gel, which produced one clear band at the expected size (127 bp). The PCR products from liver and heart were excised, purified, and Sanger‐sequenced using the forward and reverse qPCR primers (Table 1). Obtained sequences were aligned to L. camtschaticum contig040605 APJL01055902.1, which confirmed the presence of the LTYD motif in PCR products from liver and heart of P. marinus. These data demonstrate that modern Cyclostomata inherited at least one FXYD gene.
Figure 2. Tissue distribution of FXYD homologue in P. marinus .

Expression of FXYD homologue (LTYD) mRNA was measured in different tissues by real‐time PCR. 18S rRNA was used as the endogenous control. A, C t values for LTYD and 18S rRNA. B, relative expression levels of LTYD. Gene expression was normalized to the expression of 18S rRNA and expressed as fold change relative to the expression in brain using the ΔΔC t method. C, agarose gel electrophoresis of real‐time PCR products from gills, liver, gut and heart.
Gene neighbourhoods of small transmembrane regulators of ion transport
The surrounding genome context of the FXYD family gene loci tends to be at least partially conserved across all Gnathostomata (Fig. 3). The most persistent of these is the pair of tetrapod FXYD6 and FXYD2 and their homologues in fishes that are flanked by TMPRSS13a and DSCAML1 genes. Conservation of the chromosomal synteny (see Box 1), if only lineage specific, supplements sequence similarity for identification of orthologous genes and helps to establish the likely evolutionary history of the family. Phospholamban is present in only one copy in the majority of Gnathostomata genomes in a conserved chromosomal location next to MCM9 gene (Fig. 4). Mammalian myoregulin is flanked by CCDC6 and SLC16A9 genes and is coded by a small open reading frame in what was originally annotated as a long non‐coding RNA (Fig. 4). This pair of genes is present in genomes of Actinopterygii and Reptilia, with a putative long non‐coding RNA annotated between them. Distant homologues of both CCDC6 and SLC16A9 are present in Chondrichthyes, but in different chromosomal neighbourhoods.
Figure 3. Gene neighbourhoods of FXYDs.

Neighbourhoods are presented schematically (i.e. not to scale). Black boxes denote FXYD genes and grey boxes denote conserved flanking genes. Pseudogenes are denoted by boxes with vertical lines and non‐coding RNAs by boxes with dots.
Figure 4. Gene neighbourhoods of phospholamban, sarcolipin and myoregulin.

Neighbourhoods are presented schematically (i.e. not to scale). Black boxes denote genes for phospholamban (PLN), sarcolipin (SLN), or myoregulin (MRLN), while grey boxes denote conserved flanking genes. Pseudogenes are denoted by boxes with vertical lines and non‐coding RNAs by boxes with dots. Two phospholamban genes are shown for Astyanax mexicanus, a representative of bony fishes.
Relationships between the FXYD family genes
Establishing orthology of FXYDs across different species has been challenging (Sweadner & Rael, 2000; Mahmmoud et al. 2003; Cornelius et al. 2005; Tipsmark, 2008). The FXYD classification in tetrapods and fishes is therefore not always congruent. Our protein sequence‐based phylogenetic reconstruction revealed 13 FXYD gene subfamilies (Fig. 5). Sequence similarity confidently groups these genes into several clades that mostly correspond to established classification of the FXYD family; however, there are also important exceptions. One exception are the primate FXYD8 genes, which branch inside the main FXYD6 clade according to our analysis. Another exception is the complex history of the bony fish and amphibian FXYD2 and FXYD6 genes, which include genes previously referred to as fxyd12 and fxyd8 (Tipsmark, 2008), respectively. Additionally, our analysis suggests that tetrapod FXYD3 and FXYD4 are co‐orthologous to fish fxyd9 and fxyd11 (Tipsmark, 2008), as well as fxyd10 (Mahmmoud et al. 2000; Cornelius et al. 2005). All other relationships involve very deep branches of the trees that are reconstructed from relatively short and compositionally biased sequences and, therefore, cannot be analysed with certainty.
Figure 5. Neighbour‐joining tree of 13 FXYD gene subfamilies.
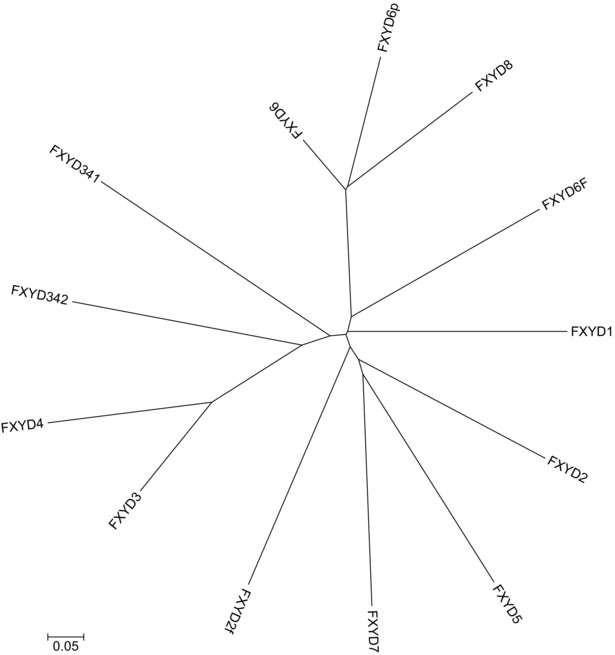
Combined phylogenetic and chromosome context analysis resolves the orthology relationships between the FXYD family clades (Table 2) and allows us to propose a classification that is based on the likely evolutionary history of the family and is consistent between the fish and tetrapod genes. Specifically, we propose the names FXYD2f and FXYD2a for the fish and amphibian, respectively, paralogues derived from FXYD2 and the names FXYD6f and FXYD6p for the fish and primate, respectively, paralogues derived from FXYD6. We also propose the names FXYD3/4, FXYD3/4‐1, and FXYD3/4‐2 to indicate the co‐orthologous relationship between the fish‐specific genes, which are currently known as FXYD10, FXYD11 and FXYD9, respectively, and the tetrapod genes coding for FXYD3 and FXYD4 (Table 2).
Table 2.
Overview of the new FXYD classification
| Gene | Cyclostomata | Chondrichthyes | Actinopterygii | Amphibia | Amniota | Primates | Other names | Comment |
|---|---|---|---|---|---|---|---|---|
| FXYD1 | + | + | + | Probably emerged in the tetrapod ancestor | ||||
| FXYD2 | ? | + | + | + | + | + | A deeply ancestral FXYD gene | |
| FXYD2f | + | + | fxyd12 | FXYD2 paralogue in cartilaginous and ray‐finned fishes | ||||
| FXYD2a | + | FXYD2 paralogue in amphibians | ||||||
| FXYD3/4 | ? | + | fxyd10 | Probably the ancestral form of the FXYD3–FXYD4 subfamily | ||||
| FXYD3/4‐1 | + | fxyd11 | One of the two FXYD‐3/4 paralogues in ray‐finned fishes | |||||
| FXYD3/4‐2 | + | fxyd9 | Another of the two FXYD‐3/4 paralogues in ray‐finned fishes | |||||
| FXYD3 | + | + | + | One of the two FXYD‐3/4 paralogues in tetrapods | ||||
| FXYD4 | ? | + | + | Another of the two FXYD‐3/4 paralogues in tetrapods; presence in amphibians in not confirmed, but likely | ||||
| FXYD5 | + | + | + | + | + | + | The only FXYD family member confirmed in Cyclostomata, tentatively identified as FXYD5 | |
| FXYD6 | ? | + | + | + | + | + | A deeply ancestral FXYD gene; presence in Cyclostomata is possible, but not confirmed | |
| FXYD6f | + | fxyd8 | FXYD6 paralogue in ray‐finned fishes | |||||
| FXYD6p | + | FXYD6 paralogue in primates | ||||||
| FXYD8 | + | FXYD6 paralogue in primates, pseudogenized in the Homo–Pan lineage | ||||||
| FXYD7 | ? | + | + | + | + | + | A deeply ancestral FXYD gene; presence in Cyclostomata is possible, but not confirmed |
Suffix ‘f’ denotes fish, suffix ‘a’ denotes amphibian, and suffix ‘p’ denotes primate.
Phosphorylation sites in FXYDs
Phosphorylation of cytoplasmic segments is a major regulatory mechanism that controls the interaction of FXYDs with Na+,K+‐ATPase (Mahmmoud et al. 2000; Despa et al. 2005; Bibert et al. 2008), as well as phospholamban and sarcolipin with SERCA (Simmerman et al. 1986; James et al. 1989; Gramolini et al. 2006; Bhupathy et al. 2009). Phosphorylation sites in phospholamban and sarcolipin are relatively well conserved across the vertebrate species (Gramolini et al. 2006; Gorski et al. 2013, 2015). Similarly, the C‐terminal phosphorylation motif of FXYD1 [–S62SIR(L/M)S(T/S)69–] (Palmer et al. 1991) that contains the three key phosphorylation sites (Ser63, Ser68 and Thr69 or Ser69) is well conserved across the FXYD1 proteins in Tetrapoda (Fig. 6). To establish whether other FXYD clades contain conserved phosphorylation motifs, putative phosphorylation sites were determined in orthologous and paralogous FXYDs using Scansite and PhosphoSite.
Figure 6. Phosphorylation sites in subfamilies FXYD1, FXYD2, FXYD2f, FXYD3/4, FXYD3 and FXYD4.

Phosphorylation sites, are graded on a scale from 0 (the least reliable) to 9 (the most reliable). [Color figure can be viewed at wileyonlinelibrary.com]
FXYD2 proteins in Tetrapoda and fishes, including the fish‐specific paralogue, designated by us as FXYD2f (aka FXYD12) (Table 2), have no predicted phosphorylation sites, but putative phosphorylation sites were found in all other FXYDs (Figs 6 and 7), including the newly described FXYD3/4 and FXYD6 clades (Table 2). Phosphorylation motif [–K71RTRSNS77–] of FXYD3/4 (FXYD10, aka PLMS) (Mahmmoud et al. 2003), which contains two phosphorylation sites (Ser75 and Ser77), resembles predicted phosphorylation motifs in C‐terminal domain of its fish paralogue FXYD3/4‐2 (aka FXYD9) (Tipsmark, 2008) (Fig. 6). FXYD3/4‐1 (aka FXYD11) (Tipsmark, 2008), the second fish paralogue of FXYD3/4, also contains predicted phosphorylation sites in its C‐terminal domain; however, the sequence surrounding these sites is dissimilar from the FXYD3/4 phosphorylation motif. FXYD3, a tetrapod paralogue of FXYD3/4, has a predicted phosphorylation motif [–L136ITPGS141–] that is well‐conserved across the Tetrapoda. This motif also appears in tetrapod FXYD4, but its predicted phosphorylation sites were graded as less reliable than those in FXYD3 (Fig. 6).
Figure 7. Phosphorylation sites in subfamilies FXYD5, FXYD6, FXYD6f, FXYD7 and FXYD8.

Phosphorylation sites, are graded on a scale from 0 (the least reliable) to 9 (the most reliable). [Color figure can be viewed at wileyonlinelibrary.com]
Primate FXYD6 and its annotated primate paralogue (a possible pseudogene) FXYD8 (Table 2) have almost the same predicted phosphorylation motif [–RCKCSFNQKP–] (Fig. 7). The phosphorylatable Ser residue is not present in all fish FXYD6 and FXYD6f proteins, but when present it is preceded by the two Cys residues as well as two or three basic Arg or Lys residues, which is reminiscent of the phosphorylation motif in primate FXYD6/FXYD8 proteins.
Discussion
Regulation of ion transport by small transmembrane proteins is likely to be an ancient fundamental mechanism and a recurring theme in metazoan evolution. FXYDs and regulators of SERCA are among the most important and diverse regulators of ion transport in vertebrates. However, their evolutionary history has been obscure. Here we provide evidence of early vertebrate origins and diversification of FXYDs and regulators of SERCA. Furthermore, we propose a revised FXYD classification, which reflects the evolutionary history and phylogenetic proximity of FXYDs across different vertebrate species and is consistent between the ray‐finned and cartilaginous fishes, as well as the tetrapods.
Small transmembrane regulators of ion transport in basal vertebrates
Our analyses indicate that FXYDs and regulators of SERCA (phospholamban and sarcolipin) first emerged in the Chordata lineage (Fig. 8). Our genetic analyses were validated by identification of a new FXYD homologue in sea lamprey, P. marinus, a modern representative of Cyclostomata. Due to sequence‐inferred affinity to FXYD5, we have named it proto‐FXYD5. The ubiquitous tissue distribution of FXYD homologue in P. marinus is broadly consistent with the expression profile of FXYD5 in Actinopterygii and mammals (Sweadner & Rael, 2000; Ino et al. 2002; Lubarski et al. 2005; Tipsmark, 2008), thus indirectly supporting its annotation as FXYD5. In lamprey, the FXYD motif is mutated to LTYD. A similar form of the motif (LSYD) is detected in hagfish ESTs (FY411797.1, BJ653733.1 and BJ648843.1). Several similar mutations in the key motif are also present in Actinopterygii, e.g. the Asp is mutated to Asn (FVYN) in salmon and zebrafish FXYD3/4‐1 (fxyd11) (Tipsmark, 2008; Saito et al. 2010; Hu et al. 2014) (Fig. 6), while Tyr is mutated to Phe (FXFD) in FXYD3/4‐2 (fxyd9) of different Actinopterygii species (Tipsmark, 2008; Yang et al. 2013; Fig. 6).
Figure 8. Major events in the evolutionary history of small transmembrane regulators of ion transport.

Proto‐FXYD5 is composed of four exons (Fig. 1). This resembles the structure of other FXYD genes, which usually have five or more exons (Sweadner & Rael, 2000). In contrast, genes for regulators of SERCA have two or three exons, with the entire coding sequence in exon 2 (phospholamban and sarcolipin) (Fujii et al. 1991; Odermatt et al. 1997) or exon 3 (myoregulin) (Anderson et al. 2015). Moreover, the LTYD motif in proto‐FXYD5 spans the boundary of exon 2/3, which is another structural similarity between proto‐FXYD5 and other FXYDs. Indeed, the FXYD motif is typically positioned at the exon–exon boundaries of FXYD proteins across the vertebrate species.
While proto‐FXYD5 was the only member of the FXYD family detected in extant Cyclostomata, our data indicate that the common ancestor of Gnathostomata had at least six FXYDs (Fig. 9). At the face value this suggests that most of the diversity of the FXYD family emerged the Gnathostomata lineage after the split from Cyclostomata. Alternatively, duplicated FXYD genes may have been already present in the Cyclostomata ancestor, but were subsequently lost in extant Cyclostomata. Another intriguing possibility is that extant Cyclostomata, lampreys and hagfishes have more than one FXYD family gene, but only one of them is retained in somatic cells of the tissues studied here. Indeed, other FXYD genes might be missing in adult somatic cells of lampreys due to developmentally programmed genome rearrangement that might lead to losses in somatic cells, amounting to ∼20% of germline DNA, including entire genes (Smith et al. 2009). A similar mechanism operates in hagfish cells (Kohno et al. 1986).
Figure 9. Schematic representation of the evolutionary history of FXYD genes.

See the text for explanation of the evolutionary history of FXYDs.
Evolutionary history of the FXYD family
The evolutionary history of the FXYD family remains largely unresolved largely due to the relatively short sequence of FXYDs, their ancient divergence, and low conservation outside the FXYD motif. However a combination of sequence‐based phylogenetic analysis, the conservation of local chromosome context, and the distinct gene expression profiles in various vertebrate clades allowed us to infer the following evolutionary scenario (Figs 8 and 9). The FXYD family likely emerged ca 600–800 million years ago (mya) in the Chordata lineage, leading to the Craniata common ancestor, which had at least one FXYD gene. Modern Cyclostomata inherited at least one FXYD gene that shows sequence‐inferred evolutionary affinity to FXYD5. In the FXYD5‐like gene in lampreys (Petromyzontidae), the FXYD motif mutated to LTYD. The Gnathostomata ancestor (ca 525 mya) likely possessed at least six FXYD genes: FXYD5, FXYD6, FXYD7, FXYD3/4 (the common ancestor of FXYD3 and FXYD4 in modern tetrapods) and a recently duplicated pair FXYD2 and FXYD2f (Table 2). Modern Chondrichthyes preserved this gene complement. There is no evidence of any chromosome association between these genes existing at that point, but the Euteleostomi (bony vertebrates) ancestor (ca 455 mya) likely had the tandem arrangement of FXYD2 and FXYD6.
Several duplications and rearrangements occurred in the lineage leading to the Actinopterygii clade ca 300–455 mya. FXYD6 duplicated, producing a fish‐specific variant FXYD6f, aka fxyd8 (Tipsmark, 2008) (Table 2); the latter was established upstream of FXYD7. In different fish clades, FXYD2 and FXYD2f were probably involved in homologous recombination, exchanging their position downstream from FXYD6. FXYD3/4, aka FXYD10 or PLMS (Mahmmoud et al. 2000; Cornelius et al. 2005), duplicated in tandem, producing two fish‐specific genes FXYD3/4‐1, aka fxyd11 (Tipsmark, 2008), and FXYD3/4‐2, aka fxyd9 (Tipsmark, 2008) (Table 2). The emergence of additional fish FXYD homologues is consistent with the third, fish‐specific, whole‐genome duplication, which occurred in ray‐finned fishes prior to the emergence of Teleostei (Amores et al. 1998, 2004; Jaillon et al. 2004).
In the Tetrapoda ancestor clade (ca 360–455 mya), the FXYD2f gene was lost, whereas FXYD3/4 duplicated into FXYD3 and FXYD4 proper. The FXYD1–FXYD7–FXYD5 gene neighbourhood was established. The same configuration was apparently inherited by the Amniota ancestor (ca 325 mya). Evolution of Amphibia saw the emergence of a narrowly distributed FXYD2a, possibly by duplication of FXYD2, although the evidence is scant. In the clade leading to Eutheria (placental mammals) ancestor (ca 100–325 mya), FXYD3 joined the upstream region of the FXYD1–FXYD7–FXYD5 gene neighbourhood. Late in the primate lineage (Simiiformes ancestor ca 30 mya), two more gene duplications occurred, with FXYD6 producing FXYD6p and FXYD8 variants (Table 2). Both new genes either emerged as pseudogenes from the start (possibly, by retroposition of mRNA‐derived cDNA), or deteriorated in the Homo–Pan clade ca 6–9 mya, turning into pseudogenes and reverting the human genome to the standard mammalian FXYD gene complement (FXYD1, FXYD2, FXYD3, FXYD4, FXYD5, FXYD6 and FXYD7) (Fig. 9).
A case for revision of the current FXYD classification
A consistent phylogeny‐based classification simplifies the investigation of any physiological function of orthologous proteins in different species. However, establishing orthology between the fish and tetrapod FXYDs has been challenging. Whenever a newly discovered fish FXYD was not an obvious orthologue of a tetrapod FXYD, it was simply assigned the next consecutive number (e.g. FXYD10) (Mahmmoud et al. 2003; Cornelius et al. 2005) with or without additional prefixes (e.g. OdFXYD9, OlFXYD9) (Yang et al. 2013) and suffixes denoting the species (e.g. FXYD9dr) (Sweadner & Rael, 2000). Although such classification was deemed provisional in the original descriptions of the FXYD gene family (Sweadner & Rael, 2000) and FXYD10 (Mahmmoud et al. 2000; Mahmmoud et al. 2003), it has persisted for the description of fish FXYDs. According to this current classification, fishes lack orthologues of tetrapod FXYD1, FXYD3, and FXYD4, but have additional FXYDs that are lacking in mammals (FXYD8–12). Based on this classification, fish and tetrapods share only four FXYDs (FXYD2, FXYD5, FXYD6 and FXYD7), while all the other FXYDs are either fish‐specific or tetrapod‐specific.
By supplementing sequence analyses with analyses of chromosomal neighbourhoods, we show that the fish FXYD9 and FXYD11, as well as the tetrapod FXYD3 and FXYD4, originated from the same ancestor (FXYD10). Furthermore, we show that fish FXYD12 corresponds to the tetrapod FXYD2. Thus, the current FXYD classification is not entirely congruent; that is FXYDs with common phylogenetic origins are classified under different names in fish and tetrapods. Obtaining relevant and accurate data that can be extrapolated from fish to humans is contingent upon having a unified FXYD classification that closely reflects the evolutionary history of the FXYD family. We therefore suggest a revised FXYD classification (Table 2), which reflects the likely phylogeny of FXYDs more closely than the current one.
Our revision is indirectly supported by previous analyses indicating FXYD10 (aka PLMS) might be homologous with the mammalian FXYD3, as well as the teleost FXYD9 and FXYD11 (Mahmmoud et al. 2003; Tipsmark, 2008; Wang et al. 2008). FXYD12 was also identified as a possible homologue of the tetrapod FXYD2 (Tipsmark, 2008). However, without additional information, sequence homology was deemed too low to designate these fish FXYDs as orthologues of tetrapod FXYDs (Mahmmoud et al. 2003). Our analysis of conserved chromosomal neighbourhoods now provides this additional information and allows for a reliable reclassification of fish FXYDs (Table 2).
Evolutionary history of SERCA regulators
Compared with the FXYD family, the history of other small transmembrane regulators of ion transport was relatively uneventful (Fig. 8). Phospholamban, as well as sarcolipin, to which phospholamban is structurally related (Toyoshima et al. 2013), is not found beyond Cyclostomata. Thus, they likely originated at the same time frame in the common ancestor of the Craniata lineage. Unlike FXYDs, phospholamban is strongly conserved across the whole range of the organisms harbouring this gene. Most vertebrate species have only a single copy of phospholamban, which suggests that this family evolves under evolutionary constraints preventing its duplication and functional diversification. The sarcolamban gene in Drosophila has similar functional properties as phospholamban (Magny et al. 2013) and possibly shares a common pre‐vertebrate ancestor with phospholamban. The history of the myoregulin peptide proper cannot be traced with confidence beyond the Eutheria (the placental mammal) clade. Indirect evidence, however, such as the conservation of the CCDC6–[MRLN]–SLC16A9 gene context across Euteleostomi (Fig. 4), suggests the possibility that it was already present in the common ancestor of Euteleostomi, that is the ancestor to all Actinopterygii and Sarcopterygii. There is no evidence for the presence of myoregulin in Chondrichthyes or Cyclostomata. Two copies of the CCDC6–[MRLN]–SLC16A9 gene neighbourhood were retained after the whole‐genome duplication event(s) in Actinopterygii, but there is no indication of subfunctionalization of the two putative myoregulin genes.
Phosphorylation sites in small transmembrane regulators of ion transport
FXYDs from all clades except the FXYD2 clade contain one or more putative phosphorylation sites in their cytoplasmic segments. Earlier analyses of mammalian (Sweadner & Rael, 2000; Yamaguchi et al. 2001; Crambert et al. 2004) and salmon FXYDs (Tipsmark, 2008) produced a similar result. Our analysis allows for the following conclusions. Firstly, predicted phosphorylation motifs are not conserved across the FXYD clades, consistent with low sequence similarity of FXYDs outside the signature motif (Sweadner & Rael, 2000). Secondly, orthologous or paralogous FXYDs frequently have similar phosphorylation motifs. For instance, the phosphorylation motif [–RSS63IRR(L/M)S68(T/S)69R–] is well conserved in FXYD1 across the Tetrapoda. Similarly, the phosphorylation motif [–KRTRS75NS77–] of shark FXYD3/4 resembles the predicted phosphorylation motif in its paralogue FXYD3/4‐2 in Actinopterygii. Thirdly, although similar phosphorylation motifs suggest common ancestry of FXYDs, different phosphorylation motifs do not preclude it. Indeed, paralogous FXYDs can have different phosphorylation motifs. For instance, predicted phosphorylation motifs in FXYD3/4‐1 differ from those in FXYD3/4 and FXYD3/4‐2 (Fig. 6).
Excepting FXYD1 and FXYD3/4 (FXYD10) (Mahmmoud et al. 2000; Mahmmoud et al. 2003; Cornelius et al. 2005; Despa et al. 2005; Bibert et al. 2008), phosphorylation sites in FXYDs are only predictions and would require thorough functional characterization. Nevertheless, by extrapolating from the role of phosphorylation in regulation of FXYD1 (Despa et al. 2005; Bibert et al. 2008) and phospholamban (Simmerman et al. 1986; James et al. 1989; Simmerman & Jones, 1998), we may infer that FXYDs that contain phosphorylation motifs are likely to be dynamically regulated by kinases. Conversely, FXYDs that lack phosphorylation sites or that are less likely to possess functionally important phosphorylation sites might be preferentially regulated by other mechanisms, including regulation of gene expression by hormones (Attali et al. 1995; Wald et al. 1996; Capurro et al. 1997; Brennan & Fuller, 1999; Shi et al. 2001), alternative splicing and mRNA editing (Sweadner et al. 2011; Arystarkhova, 2016). Taken together, differences in phosphorylation motifs of paralogous FXYDs are likely to reflect diversification of their physiological function and modes of regulation.
Functional perspectives of early diversification of small transmembrane regulators of ion transport
Small transmembrane regulators of ion transport diversified early in the vertebrate lineage, which was likely to have been one of the drivers behind acquisition of new physiological properties in vertebrates. The origin of multiple inhibitors of SERCA, including the muscle‐specific myoregulin (Anderson et al. 2015), as well as DWORF, which activates SERCA (Nelson et al. 2016), maps to the same lineage where the excitation–contraction coupling in skeletal muscle and heart acquired new functional characteristics. This co‐emergence probably indicates that regulators of SERCA, which play a major role in Ca2+ homeostasis in skeletal muscle and heart, might have been important for the evolution of this mechanism in the vertebrate lineage. Notably, one unique feature of vertebrates, including Cyclostomata, is that skeletal muscle contraction depends on the release of Ca2+ from the sarcoplasmic reticulum and does not require extracellular Ca2+ (Armstrong et al. 1972; Inoue et al. 2002). Conversely, entry of extracellular Ca2+ is required for contraction of invertebrate skeletal muscle (Hagiwara et al. 1971). Interestingly, the vertebrate heart evolved from an organ that is almost entirely dependent on extracellular Ca2+ to generate contraction, such as in fish and amphibians, to an organ that heavily relies on the release of Ca2+ from the sarcoplasmic reticulum, such as in birds and mammals (Shiels & Galli, 2014), which suggests a role for regulators of SERCA. Also, the emergence of FXYD1, which regulates Ca2+ extrusion from the cardiomyocytes by inhibiting the Na+/Ca2+ exchanger (Zhang et al. 2003), might have been important for the evolution of cardiac Ca2+ homeostasis in the tetrapod lineage.
FXYDs in fishes and mammals are regulated by hormones (Despa et al. 2005; Tipsmark et al. 2010, 2011; Tang et al. 2012; Pirkmajer & Chibalin, 2016). Expression of tetrapod FXYD4 is regulated by aldosterone (Capurro et al. 1997; Brennan & Fuller, 1999; Shi et al. 2001), a major mineralocorticoid in terrestrial vertebrates (Rossier et al. 2015). Similarly, expression of FXYD3/4‐1 (fxyd11) and FXYD3/4‐2 (fxyd9) responds to salinity changes and/or cortisol (Tipsmark et al. 2010, 2011; Tang et al. 2012), which is a major mineralocorticoid in Actinopterygii (Rossier et al. 2015). Tetrapod FXYD4 is co‐orthologous to FXYD3/4 in cartilaginous fishes, which were the first vertebrates with a separate mineralocorticoid and glucocorticoid receptor (Baker et al. 2013; Rossier et al. 2015). Conversely, lampreys, which have only one corticosteroid receptor, lack FXYD3/4 (Baker et al. 2013; Rossier et al. 2015). Aldosterone first appeared in lobe‐finned fishes (Baker et al. 2013), which was probably an important step in the evolution of terrestrial vertebrates (Rossier et al. 2015). The duplication of FXYD3/4 into FXYD3 and the aldosterone‐responsive FXYD4 occurred in the Tetrapoda ancestor (Figs 8 and 9), thus apparently coinciding with the appearance of aldosterone and the transition from the aquatic to the terrestrial life (Rossier et al. 2015). Taken together, diversification of the FXYD3/4 clade seems to have closely paralleled development of the mineralocorticoid system.
Conclusions
Collectively, our study provides evidence of early vertebrate origins and diversification of small transmembrane regulators of ion transport. In addition, new insights into evolutionary history of FXYDs are provided. We propose a revised FXYD classification, which reflects the likely evolutionary history of the family and is consistent between the fish and tetrapod genes. Our findings open new avenues for investigating the evolution of small transmembrane regulators of ion transport and their roles in health and disease.
Additional information
Competing interests
The authors declare they have no relevant conflicts of interest.
Author contributions
A.V.C. conceived the study. S.P., K.S.M. and Y.I.W. conducted database searches for FXYDs and SERCA regulators. H.K., P.V.Z. and L.L. performed identification and sequencing of FXYD homologue in Petromyzon marinus and analysed these data. S.P., K.S.M., Y.I.W. and A.V.C identified and evaluated phosphorylation sites in FXYDs. K.S.M and Y.I.W. performed computational analysis of small transmembrane regulators of ion transport. S.P., K.S.M., Y.I.W., J.R.Z. and A.V.C designed the study, interpreted the data, and wrote the paper. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported with funding from the Swedish Research Council, Novo Nordisk Research Foundation, and the Strategic Research Programme in Diabetes at Karolinska Institutet. M.K.S. and Y.I.W. are supported by intramural funds of the US Department of Health and Human Services (to the National Library of Medicine). S.P. is supported by the Slovenian Research Agency (grants P3‐0043, J7‐7138, and J3‐6794).
Acknowledgements
We thank Drs Marc Gilbert, Dan Larhammar, Alexey Shipunov, Kathleen Sweadner and Lev Yampolsky for productive discussions and critical reading of the manuscript.
References
- Abascal F, Zardoya R & Posada D (2005). ProtTest: selection of best‐fit models of protein evolution. Bioinformatics 21, 2104–2105. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W & Lipman DJ (1997). Gapped BLAST and PSI‐BLAST: a new generation of protein database search programs. Nucleic Acids Res 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M & Postlethwait JH (1998). Zebrafish hox clusters and vertebrate genome evolution. Science 282, 1711–1714. [DOI] [PubMed] [Google Scholar]
- Amores A, Suzuki T, Yan YL, Pomeroy J, Singer A, Amemiya C & Postlethwait JH (2004). Developmental roles of pufferfish Hox clusters and genome evolution in ray‐fin fish. Genome Res 14, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Anderson KM, Chang CL, Makarewich CA, Nelson BR, McAnally JR, Kasaragod P, Shelton JM, Liou J, Bassel‐Duby R & Olson EN (2015). A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla FM & Horowicz P (1972). Twitches in the presence of ethylene glycol bis(β‐aminoethyl ether)‐N,N′‐tetracetic acid. Biochim Biophys Acta 267, 605–608. [DOI] [PubMed] [Google Scholar]
- Arystarkhova E (2016). Beneficial renal and pancreatic phenotypes in a mouse deficient in FXYD2 regulatory subunit of Na,K‐ATPase. Front Physiol 7, 88 https://doi.org/10.3389/fphys.2016.00088. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnani A & Peterson RT (2014). The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Dis Model Mech 7, 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B, Latter H, Rachamim N & Garty H (1995). A corticosteroid‐induced gene expressing an "IsK‐like" K+ channel activity in Xenopus oocytes. Proc Natl Acad Sci USA 92, 6092–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ME, Funder JW & Kattoula SR (2013). Evolution of hormone selectivity in glucocorticoid and mineralocorticoid receptors. J Steroid Biochem Mol Biol 137, 57–70. [DOI] [PubMed] [Google Scholar]
- Bhupathy P, Babu GJ, Ito M & Periasamy M (2009). Threonine‐5 at the N‐terminus can modulate sarcolipin function in cardiac myocytes. J Mol Cell Cardiol 47, 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibert S, Roy S, Schaer D, Horisberger JD & Geering K (2008). Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K‐ATPase isozymes. J Biol Chem 283, 476–486. [DOI] [PubMed] [Google Scholar]
- Brennan FE & Fuller PJ (1999). Acute regulation by corticosteroids of channel‐inducing factor gene messenger ribonucleic acid in the distal colon. Endocrinology 140, 1213–1218. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J & Wittwer CT (2009). The MIQE guidelines: minimum information for publication of quantitative real‐time PCR experiments. Clin Chem 55, 611–622. [DOI] [PubMed] [Google Scholar]
- Cai SY, Lionarons DA, Hagey L, Soroka CJ, Mennone A & Boyer JL (2013). Adult sea lamprey tolerates biliary atresia by altering bile salt composition and renal excretion. Hepatology 57, 2418–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K & Madden TL (2009). BLAST+: architecture and applications. BMC Bioinformatics 10, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro C, Coutry N, Bonvalet JP, Escoubet B, Garty H & Farman N (1997). Specific expression and regulation of CHIF in kidney and colon. Ann N Y Acad Sci 834, 562–564. [DOI] [PubMed] [Google Scholar]
- Cole KS & Curtis HJ (1939). Electric impedance of the squid giant axon during activity. J Gen Physiol 22, 649–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius F, Mahmmoud YA, Meischke L & Cramb G (2005). Functional significance of the shark Na,K‐ATPase N‐terminal domain. Is the structurally variable N‐Terminus involved in tissue‐specific regulation by FXYD proteins? Biochemistry 44, 13051–13062. [DOI] [PubMed] [Google Scholar]
- Crambert G, Fuzesi M, Garty H, Karlish S & Geering K (2002). Phospholemman (FXYD1) associates with Na,K‐ATPase and regulates its transport properties. Proc Natl Acad Sci USA 99, 11476–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crambert G, Li C, Claeys D & Geering K (2005). FXYD3 (Mat‐8), a new regulator of Na,K‐ATPase. Mol Biol Cell 16, 2363–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crambert G, Li C, Swee LK & Geering K (2004). FXYD7, mapping of functional sites involved in endoplasmic reticulum export, association with and regulation of Na,K‐ATPase. J Biol Chem 279, 30888–30895. [DOI] [PubMed] [Google Scholar]
- Crane RK (1960). Intestinal absorption of sugars. Physiol Rev 40, 789–825. [DOI] [PubMed] [Google Scholar]
- Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL & Bers DM (2005). Phospholemman‐phosphorylation mediates the β‐adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97, 252–259. [DOI] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC & Zon LI (2000). Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781. [DOI] [PubMed] [Google Scholar]
- Edgar RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J (1996). Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol 266, 418–427. [DOI] [PubMed] [Google Scholar]
- Fujii J, Zarain‐Herzberg A, Willard HF, Tada M & MacLennan DH (1991). Structure of the rabbit phospholamban gene, cloning of the human cDNA, and assignment of the gene to human chromosome 6. J Biol Chem 266, 11669–11675. [PubMed] [Google Scholar]
- Geering K (2008). Functional roles of Na,K‐ATPase subunits. Curr Opin Nephrol Hypertens 17, 526–532. [DOI] [PubMed] [Google Scholar]
- Gorski PA, Glaves JP, Vangheluwe P & Young HS (2013). Sarco(endo)plasmic reticulum calcium ATPase (SERCA) inhibition by sarcolipin is encoded in its luminal tail. J Biol Chem 288, 8456–8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski PA, Trieber CA, Ashrafi G & Young HS (2015). Regulation of the sarcoplasmic reticulum calcium pump by divergent phospholamban isoforms in zebrafish. J Biol Chem 290, 6777–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramolini AO, Trivieri MG, Oudit GY, Kislinger T, Li W, Patel MM, Emili A, Kranias EG, Backx PH & Maclennan DH (2006). Cardiac‐specific overexpression of sarcolipin in phospholamban null mice impairs myocyte function that is restored by phosphorylation. Proc Natl Acad Sci USA 103, 2446–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Henkart MP & Kidokoro Y (1971). Excitation‐contraction coupling in amphioxus muscle cells. J Physiol 219, 233–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SB, Marin J, Suleski M, Paymer M & Kumar S (2015). Tree of life reveals clock‐like speciation and diversification. Mol Biol Evol 32, 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbrunn LV & Wiercinski FJ (1947). The action of various cations on muscle protoplasm. J Cell Physiol 29, 15–32. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF & Katz B (1952). Measurement of current‐voltage relations in the membrane of the giant axon of Loligo. J Physiol 116, 424–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Li S, Zhong Y, Mu X, Gui L & Zhang J (2014). Identification of fxyd genes from the spotted scat (Scatophagus argus): molecular cloning, tissue‐specific expression, and response to acute hyposaline stress. Comp Biochem Physiol B Biochem Mol Biol 174, 15–22. [DOI] [PubMed] [Google Scholar]
- Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K & Hirohashi S (2002). Dysadherin, a cancer‐associated cell membrane glycoprotein, down‐regulates E‐cadherin and promotes metastasis. Proc Natl Acad Sci USA 99, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I, Tsutsui I & Bone Q (2002). Excitation‐contraction coupling in skeletal and caudal heart muscle of the hagfish Eptatretus burgeri Girard. J Exp Biol 205, 3535–3541. [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Brunet F, Petit JL, Stange‐Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf‐Costaz C, Bernot A, Nicaud S, Jaffe D, Fisher S, Lutfalla G, Dossat C, Segurens B, Dasilva C, Salanoubat M, Levy M, Boudet N, Castellano S, Anthouard V, Jubin C, Castelli V, Katinka M, Vacherie B, Biemont C, Skalli Z, Cattolico L, Poulain J, De Berardinis V, Cruaud C, Duprat S, Brottier P, Coutanceau JP, Gouzy J, Parra G, Lardier G, Chapple C, McKernan KJ, McEwan P, Bosak S, Kellis M, Volff JN, Guigo R, Zody MC, Mesirov J, Lindblad‐Toh K, Birren B, Nusbaum C, Kahn D, Robinson‐Rechavi M, Laudet V, Schachter V, Quetier F, Saurin W, Scarpelli C, Wincker P, Lander ES, Weissenbach J & Roest Crollius H (2004). Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto‐karyotype. Nature 431, 946–957. [DOI] [PubMed] [Google Scholar]
- James P, Inui M, Tada M, Chiesi M & Carafoli E (1989). Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature 342, 90–92. [DOI] [PubMed] [Google Scholar]
- Kohno S, Nakai Y, Satoh S, Yoshida M & Kobayashi H (1986). Chromosome elimination in the Japanese hagfish, Eptatretus burgeri (Agnatha, Cyclostomata). Cytogenet Cell Genet 41, 209–214. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange‐Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette‐Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry‐Mieg D, Thierry‐Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S & Chen YJ (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. [DOI] [PubMed] [Google Scholar]
- Lubarski I, Asher C & Garty H (2011). FXYD5 (dysadherin) regulates the paracellular permeability in cultured kidney collecting duct cells. Am J Physiol Renal Physiol 301, F1270–F1280. [DOI] [PubMed] [Google Scholar]
- Lubarski I, Pihakaski‐Maunsbach K, Karlish SJ, Maunsbach AB & Garty H (2005). Interaction with the Na,K‐ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem 280, 37717–37724. [DOI] [PubMed] [Google Scholar]
- MacRae CA & Peterson RT (2015). Zebrafish as tools for drug discovery. Nat Rev Drug Discov 14, 721–731. [DOI] [PubMed] [Google Scholar]
- Magny EG, Pueyo JI, Pearl FM, Cespedes MA, Niven JE, Bishop SA & Couso JP (2013). Conserved regulation of cardiac calcium uptake by peptides encoded in small open reading frames. Science 341, 1116–1120. [DOI] [PubMed] [Google Scholar]
- Mahmmoud YA, Cramb G, Maunsbach AB, Cutler CP, Meischke L & Cornelius F (2003). Regulation of Na,K‐ATPase by PLMS, the phospholemman‐like protein from shark: molecular cloning, sequence, expression, cellular distribution, and functional effects of PLMS. J Biol Chem 278, 37427–37438. [DOI] [PubMed] [Google Scholar]
- Mahmmoud YA, Vorum H & Cornelius F (2000). Identification of a phospholemman‐like protein from shark rectal glands. Evidence for indirect regulation of Na,K‐ATPase by protein kinase C via a novel member of the FXYDY family. J Biol Chem 275, 35969–35977. [DOI] [PubMed] [Google Scholar]
- Manson MD, Tedesco P, Berg HC, Harold FM & Van der Drift C (1977). A protonmotive force drives bacterial flagella. Proc Natl Acad Sci USA 74, 3060–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsura S, Shioi J & Imae Y (1977). Motility in Bacillus subtilis driven by an artificial protonmotive force. FEBS Lett 82, 187–190. [DOI] [PubMed] [Google Scholar]
- Mitchell P (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemi‐osmotic type of mechanism. Nature 191, 144–148. [DOI] [PubMed] [Google Scholar]
- Navarre C, Ghislain M, Leterme S, Ferroud C, Dufour JP & Goffeau A (1992). Purification and complete sequence of a small proteolipid associated with the plasma membrane H+‐ATPase of Saccharomyces cerevisiae . J Biol Chem 267, 6425–6428. [PubMed] [Google Scholar]
- Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, Reese AL, McAnally JR, Chen X, Kavalali ET, Cannon SC, Houser SR, Bassel‐Duby R & Olson EN (2016). A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351, 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A, Becker S, Khanna VK, Kurzydlowski K, Leisner E, Pette D & MacLennan DH (1998). Sarcolipin regulates the activity of SERCA1, the fast‐twitch skeletal muscle sarcoplasmic reticulum Ca2+‐ATPase. J Biol Chem 273, 12360–12369. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Taschner PE, Scherer SW, Beatty B, Khanna VK, Cornblath DR, Chaudhry V, Yee WC, Schrank B, Karpati G, Breuning MH, Knoers N & MacLennan DH (1997). Characterization of the gene encoding human sarcolipin (SLN), a proteolipid associated with SERCA1: absence of structural mutations in five patients with Brody disease. Genomics 45, 541–553. [DOI] [PubMed] [Google Scholar]
- Okuda J, Hayashi N, Okamoto M, Sawada S, Minagawa S, Yano Y & Gotoh N (2010). Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na,K‐ATPase regulator, FXYD3. Infect Immun 78, 4511–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CJ, Scott BT & Jones LR (1991). Purification and complete sequence determination of the major plasma membrane substrate for cAMP‐dependent protein kinase and protein kinase C in myocardium. J Biol Chem 266, 11126–11130. [PubMed] [Google Scholar]
- Pirkmajer S & Chibalin AV (2016). Na,K‐ATPase regulation in skeletal muscle. Am J Physiol Endocrinol Metab 311, E1–E31. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS & Arkin AP (2009). FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26, 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt KD, Brown GR, Hiatt SM, Thibaud‐Nissen F, Astashyn A, Ermolaeva O, Farrell CM, Hart J, Landrum MJ, McGarvey KM, Murphy MR, O'Leary NA, Pujar S, Rajput B, Rangwala SH, Riddick LD, Shkeda A, Sun H, Tamez P, Tully RE, Wallin C, Webb D, Weber J, Wu W, DiCuccio M, Kitts P, Maglott DR, Murphy TD & Ostell JM (2014). RefSeq: an update on mammalian reference sequences. Nucleic Acids Res 42, D756–D763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier BC, Baker ME & Studer RA (2015). Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95, 297–340. [DOI] [PubMed] [Google Scholar]
- Saito K, Nakamura N, Ito Y, Hoshijima K, Esaki M, Zhao B & Hirose S (2010). Identification of zebrafish Fxyd11a protein that is highly expressed in ion‐transporting epithelium of the gill and skin and its possible role in ion homeostasis. Front Physiol 1, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Levy‐Holzman R, Cluzeaud F, Farman N & Garty H (2001). Membrane topology and immunolocalization of CHIF in kidney and intestine. Am J Physiol Renal Physiol 280, F505–F512. [DOI] [PubMed] [Google Scholar]
- Shiels HA & Galli GL (2014). The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology (Bethesda) 29, 456–469. [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Collins JH, Theibert JL, Wegener AD & Jones LR (1986). Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem 261, 13333–13341. [PubMed] [Google Scholar]
- Simmerman HK & Jones LR (1998). Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev 78, 921–947. [DOI] [PubMed] [Google Scholar]
- Skou JC (1957). The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta 23, 394–401. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Antonacci F, Eichler EE & Amemiya CT (2009). Programmed loss of millions of base pairs from a vertebrate genome. Proc Natl Acad Sci USA 106, 11212–11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soding J (2005). Protein homology detection by HMM‐HMM comparison. Bioinformatics 21, 951–960. [DOI] [PubMed] [Google Scholar]
- Studer RA, Person E, Robinson‐Rechavi M & Rossier BC (2011). Evolution of the epithelial sodium channel and the sodium pump as limiting factors of aldosterone action on sodium transport. Physiol Genomics 43, 844–854. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ, Pascoa JL, Salazar CA & Arystarkhova E (2011). Post‐transcriptional control of Na,K‐ATPase activity and cell growth by a splice variant of FXYD2 protein with modified mRNA. J Biol Chem 286, 18290–18300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner KJ & Rael E (2000). The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68, 41–56. [DOI] [PubMed] [Google Scholar]
- Tada M, Ohmori F, Yamada M & Abe H (1979). Mechanism of the stimulation of Ca2+‐dependent ATPase of cardiac sarcoplasmic reticulum by adenosine 3′:5′‐monophosphate‐dependent protein kinase. Role of the 22,000‐dalton protein. J Biol Chem 254, 319–326. [PubMed] [Google Scholar]
- Tang CH, Lai DY & Lee TH (2012). Effects of salinity acclimation on Na+/K+‐ATPase responses and FXYD11 expression in the gills and kidneys of the Japanese eel (Anguilla japonica). Comp Biochem Physiol A Mol Integr Physiol 163, 302–310. [DOI] [PubMed] [Google Scholar]
- Tipsmark CK (2008). Identification of FXYD protein genes in a teleost: tissue‐specific expression and response to salinity change. Am J Physiol Regul Integr Comp Physiol 294, R1367–R1378. [DOI] [PubMed] [Google Scholar]
- Tipsmark CK, Breves JP, Seale AP, Lerner DT, Hirano T & Grau EG (2011). Switching of Na+,K+‐ATPase isoforms by salinity and prolactin in the gill of a cichlid fish. J Endocrinol 209, 237–244. [DOI] [PubMed] [Google Scholar]
- Tipsmark CK, Mahmmoud YA, Borski RJ & Madsen SS (2010). FXYD‐11 associates with Na+‐K+‐ATPase in the gill of Atlantic salmon: regulation and localization in relation to changed ion‐regulatory status. Am J Physiol Regul Integr Comp Physiol 299, R1212–R1223. [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Iwasawa S, Ogawa H, Hirata A, Tsueda J & Inesi G (2013). Crystal structures of the calcium pump and sarcolipin in the Mg2+‐bound E1 state. Nature 495, 260–264. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu‐Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn‐Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes‐Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A & Zhu X (2001). The sequence of the human genome. Science 291, 1304–1351. [DOI] [PubMed] [Google Scholar]
- Wald H, Goldstein O, Asher C, Yagil Y & Garty H (1996). Aldosterone induction and epithelial distribution of CHIF. Am J Physiol Renal Physiol 271, F322–F329. [DOI] [PubMed] [Google Scholar]
- Wang PJ, Lin CH, Hwang HH & Lee TH (2008). Branchial FXYD protein expression in response to salinity change and its interaction with Na+/K+‐ATPase of the euryhaline teleost Tetraodon nigroviridis . J Exp Biol 211, 3750–3758. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Yamaguchi K, Tai Y, Sugimoto K & Tokuda M (2001). Molecular cloning and characterization of a novel phospholemman‐like protein from rat hippocampus. Brain Res Mol Brain Res 86, 189–192. [DOI] [PubMed] [Google Scholar]
- Yang WK, Kang CK, Chang CH, Hsu AD, Lee TH & Hwang PP (2013). Expression profiles of branchial FXYD proteins in the brackish medaka Oryzias dancena: a potential saltwater fish model for studies of osmoregulation. PLoS One 8, e55470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Qureshi A, Song J, Carl LL, Tian Q, Stahl RC, Carey DJ, Rothblum LI & Cheung JY (2003). Phospholemman modulates Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 284, H225–H233. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Wang J, Song J, Rabinowitz J, Chen X, Houser SR, Peterson BZ, Tucker AL, Feldman AM & Cheung JY (2015). Regulation of L‐type calcium channel by phospholemman in cardiac myocytes. J Mol Cell Cardiol 84, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


