During pregnancy, uterine vasculature undergoes dramatic adaptation to meet the nutrient demand of fetal development and growth, leading to increased uterine blood flow to perfuse the placenta. Oestrogen is a master regulator of the adaptive changes during pregnancy including angiogenesis. Angiogenesis is the formation of new blood vessels from existing vasculature and endothelial cells play a critical role in this process. Angiogenesis is tightly regulated, involving migration, growth, and differentiation of endothelial cells (https://www.ncbi.nlm.nih.gov/books/NBK53242/). Mitogen‐activated protein kinases (MAPKs), consisting of the extracellular signal‐regulated kinases (ERKs), the c‐Jun N‐terminal kinases (JNK)/stress‐activated protein kinases (SAPK), and the p38 isoforms, are essential elements in cell proliferation.
17‐β‐Oestradiol (E2β) undergoes hydroxylation at either the C2 or C4 position by cytochrome P450 enzymes to generate 2‐hydroxyoestradiol and 4‐hydroxyoestradiol. These two metabolites can be further converted to 2‐methoxyoestradiol and 4‐methoxyoestradiol, respectively, through methylation catalysed by catechol‐O‐methyltransferase (COMT). Importantly, aberrant metabolism of oestrogen is implicated in the pathophysiology of pre‐eclampsia (Kanasaki et al. 2008; Jobe et al. 2013), a pregnancy complication occurring in ∼5% of pregnancies with high fetal and maternal morbidity and mortality. In pre‐eclamptic pregnancy, levels of oestrogen and its metabolites are decreased (Jobe et al. 2013) and deletion of the COMT gene results in a pre‐eclampsia‐like phenotype in pregnant mice due to loss of 2‐methoxyoestradiol (Kanasaki et al. 2008). Vascular endothelial growth factor A (VEGF‐A), expression of which is regulated by oestrogen during pregnancy, is a major player in triggering angiogenesis. Recent studies from the Magness laboratory suggest that oestrogen could also regulate uterine artery endothelial cell proliferation directly or indirectly through its metabolites (Jobe et al. 2010, 2011). Interestingly, E2β induces proliferation via activation of the oestrogen receptor‐β, whereas 2‐hydroxyoestradiol and 4‐hydroxyoestradiol, owing to the catechol moiety in their structure, exert their proliferative action via interaction with β2/β3 adrenergic receptors but not the oestrogen receptor‐β (Jobe et al. 2011). Thus, oestrogen, in addition to activating ERβ, diversifies its proliferative effects through interaction of its metabolites with G protein‐coupled receptors during pregnancy. It is interesting to note that β2/β3 adrenergic receptors are mitogenic in uterine artery endothelial cells following the transition from non‐pregnant state to pregnancy, which may represent an adaptive mechanism. However, the signalling pathway(s) for the regulation of uterine artery endothelial cell proliferation by oestrogen and its metabolites remains elusive. The study by Landeros et al. in this issue of The Journal of Physiology demonstrates that E2β, 2‐hydroxyoestradiol, 4‐hydroxyoestradiol, norepinephrine, and epinephrine stimulate proliferation of uterine artery endothelial cells via activation of three convergent MAPK pathways (ERK1/2, p38 and JNK) (Landeros et al. 2017). The observations in the present and previous studies from the Magness laboratory are interesting. E2β, catecholoestradiols and catecholamines, though activating distinct sets of receptors, utilize the same MAPK/ERK pathway activated by VEGF as well as MAPK/JNK and MAPK/p38 pathways to promote endothelial cell proliferation during pregnancy (Fig. 1). It appears that evolutionary pressure has resulted in the development of multiple mechanisms that converge at MAPKs for oestrogen to promote endothelial cell proliferation and angiogenesis in the uteroplacental circulation to ensure successful pregnancy.
Figure 1. Overview of signalling pathways in uterine artery endothelial cell (EC) proliferation during pregnancy.
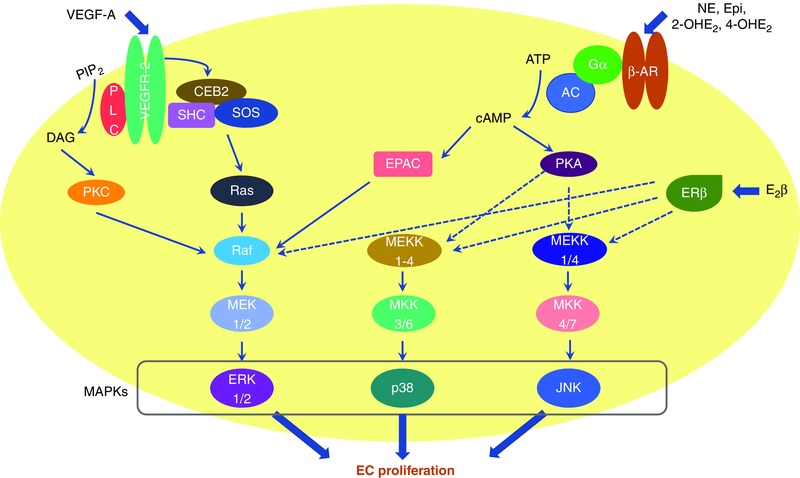
VEGF, oestrogen and its metabolites via interaction with distinct receptors activate the MAPK pathways, leading to endothelial cell proliferation during pregnancy. VEGF‐A interacts with VEGFR‐2 to activate the Ras–MEK–ERK pathway to promote endothelial cell proliferation (Gualandi L & Claesson‐Welsh L. (2011). Signal transduction by vascular endothelial growth factor receptors. Biochem J 437, 169–183). The findings from the Magness laboratory suggest that this pathway is also utilized by 17‐β‐oestradiol (E2β), norepinephrine (NE), epinephrine (Epi), 2‐hydroxyoestradiol (2‐OHE2) and 4‐hydroxyoestradiol (4‐OHE2) in uterine arterial endothelial cells. Exchange protein directly activated by cAMP (EPAC) probably links β2/β3 adrenergic receptors (ARs) to the Ras–MEK–Erk pathway (Borland G, Smith BO & Yarwood SJ. (2009). EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol 158, 70–86.). In addition, E2β, catecholestradiols and catecholamines also stimulate uterine arterial endothelial cell proliferation via activation of p38 mitogen‐activated protein kinase (p38) and c‐Jun N‐terminal kinases (JNK).
Angiogenesis in uteroplacental circulation begins from early gestation. The endothelial cells used for proliferation investigations by the Magness laboratory are from uterine arteries of late pregnancy sheep (120–130 days; term, 147 days). Therefore, knowledge of the regulation of angiogenesis by oestrogen and its metabolites in early gestation is lacking. Moreover, the same group reported that oestrogen and its metabolites were unable to stimulate proliferation of uterine arterial endothelial cells from non‐pregnant animals (Jobe et al. 2010, 2011). This implies that pregnancy has primed endothelial cells via factors yet to be determined, leading to increased susceptibility to the proliferative stimuli of oestrogen and its metabolites. Furthermore, it would be great of interest to uncover whether VEGF, E2β and catecholoestradiols contribute differently to angiogenesis in different stages of pregnancy and under pathophysiological conditions.
Additional information
Competing interests
None declared.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Institutes of Health Grants HD083132 (L. Zhang), HL128209 (L. Zhang), HL110125 (L. Zhang).
Linked articles This Perspective highlights an article by Landeros et al. To read this article, visit https://doi.org/10.1113/JP274119.
References
- Jobe SO, Fling SN, Ramadoss J & Magness RR (2011). A novel role for an endothelial adrenergic receptor system in mediating catecholestradiol‐induced proliferation of uterine artery endothelial cells. Hypertension 58, 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J & Magness RR (2010). Estradiol‐17β and its cytochrome P450‐ and catechol‐O‐methyltransferase‐derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor‐α versus estrogen receptor‐β. Hypertension 55, 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe SO, Tyler CT & Magness RR (2013). Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 61, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF & Kalluri R (2008). Deficiency in catechol‐O‐methyltransferase and 2‐methoxyoestradiol is associated with pre‐eclampsia. Nature 453, 1117–1121. [DOI] [PubMed] [Google Scholar]
- Landeros RV, Jobe SO, Aranda‐Pino G, Lopez GE, Zheng J & Magness RR (2017). Convergent ERK1/2, p38, and JNK mitogen activated protein kinases (MAPKs) signalling mediate catecholoestradiol‐induced proliferation of ovine uterine artery endothelial cells. J Physiol 595, 4663–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]


