Abstract
Background
Epidermolysis bullosa simplex is a skin-blistering disorder caused by mutations in keratin (K)14 or K5. Treatment with nuclear factor (erythroid-derived 2)-like 2 inducer sulforaphane ameliorated skin blistering in Krt14-null mice, correlating with induction of K17. To be therapeutically useful for epidermolysis bullosa simplex, topical broccoli sprout extract (BSE), enriched for sulforaphane, would ideally induce the expression of homologous keratins (eg, K6, K17, K16) in the basal layer of human epidermis without impacting expression of defective keratins (K5/K14).
Objective
The purpose of this 1-week, randomized, split-body, single-blinded, placebo-controlled trial was to assess the impact of BSE on keratin expression.
Methods
Five subjects (34–71 years old) applied BSE (500 nmol of sulforaphane/mL) or vehicle alone to the inner aspect of the arm daily. Expression of keratin, nuclear factor (erythroid-derived 2)-like 2, and other markers was assessed using reverse transcription-polymerase chain reaction and indirect immunofluorescence.
Results
One subject (age 71 years) was excluded a posteriori because of poor tissue quality. Topical BSE activated nuclear factor (erythroid-derived 2)-like 2 and up-regulated K17 in the epidermis of all subjects, had variable effects on K16 and K6 expression, and did not alter expression of K14 or K5.
Limitations
Small sample size is a limitation.
Conclusion
BSE represents an attractive therapeutic candidate for K14-associated epidermolysis bullosa simplex.
Keywords: broccoli sprout extract, epidermolysis bullosa simplex, keratin, nuclear factor (erythroid-derived 2)-like 2, sulforaphane
Epidermolysis bullosa simplex (EBS) is a skin-blistering disorder caused by mutations in keratin (K)14 or K5.1 Studies involving Krt14-null mice showed that treatment with sulforaphane, an isothiocyanate enriched in cruciferous vegetables, markedly decreases blistering correlating with the induction of wound repair-associated K17 and K16.2 Sulforaphane is an inducer of nuclear factor (erythroid-derived 2)-like 2 (NRF2), and its ability to induce K16 and K17 occurs via NRF2-dependent and NRF2-independent mechanisms, respectively.3 Sulforaphane has been delivered orally and topically to human beings in the form of broccoli sprout extract (BSE).4 To be therapeutically useful for EBS, BSE would induce the expression of homologous keratins (eg, K6, K17, K16) in the basal layer of human epidermis without enhancing the expression of defective keratins (K5 or K14).1,2
METHODS
We conducted a 1-week, randomized, split-body, single-blinded, placebo-controlled trial to assess keratin expression after topical application of BSE containing 500 nmol of sulforaphane/mL (Appendix; available at http://www.jaad.org). Five healthy adult subjects (age 37, 37, 43, 44, and 71 years) applied BSE in vehicle or vehicle alone to the inner aspect of the arm daily. Expression of keratin and other markers was assessed using reverse transcription (RT)-polymerase chain reaction and indirect immunofluorescence (Fig 1, A). One subject (age 71 years) was excluded a posteriori because of poor tissue quality. Of the remaining subjects, 1 man and 1 woman were phototype V, and 1 man and 1 woman were phototype II.
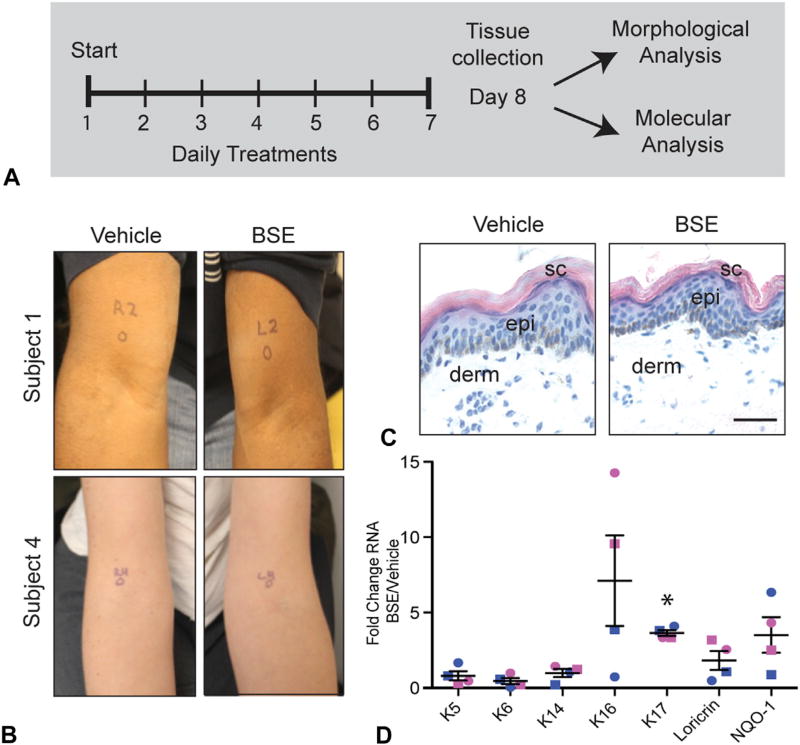
Fig 1.
Topical treatment of human skin with broccoli sprout extract (BSE). A, Treatment regimen. B, Vehicle- and BSE-treated sites of subjects 1 and 4. C, Hematoxylin-eosin staining of subject 1. Scale bar = 50 µm. D, Fold change RNA of keratin (K)5, K6, K14, K16, K17, loricrin, and NQO-1 of BSE-treated skin over vehicle. Each data point is the mean of 3 technical replicates for 1 patient. Blue, male; pink, female; circle, phototype V (subjects 1 and 3); square, phototype II (subjects 2 and 4). Error bars: mean + SEM of the biological replicates. Unpaired 2-tailed Student t test, *P value < .01. derm, Dermis; epi, epidermis; sc, stratum corneum.
RESULTS
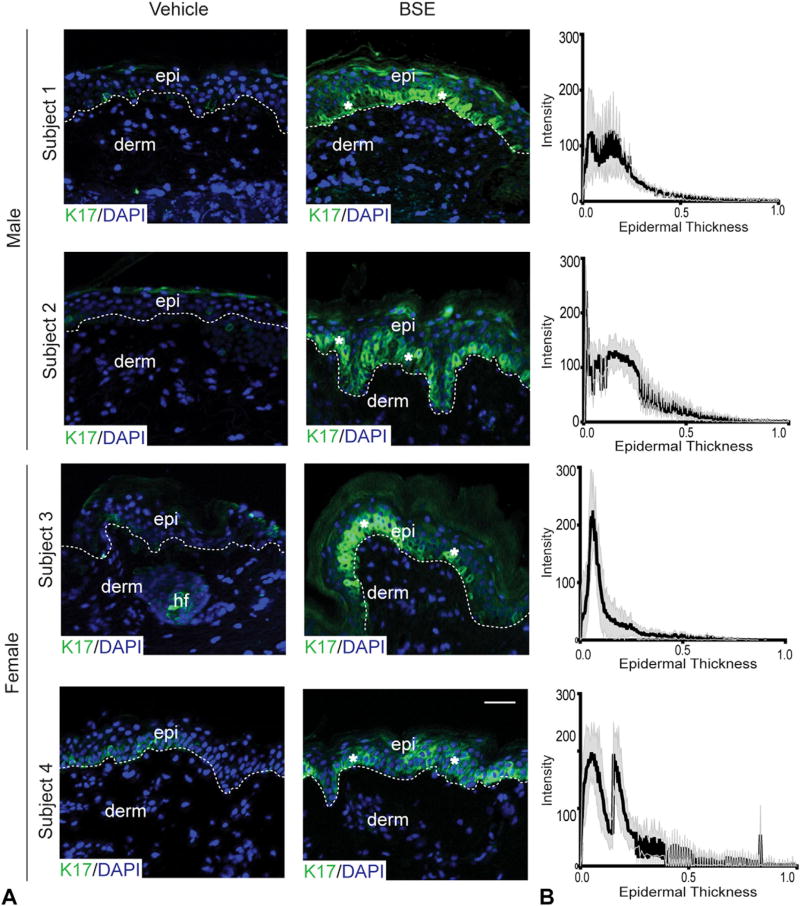
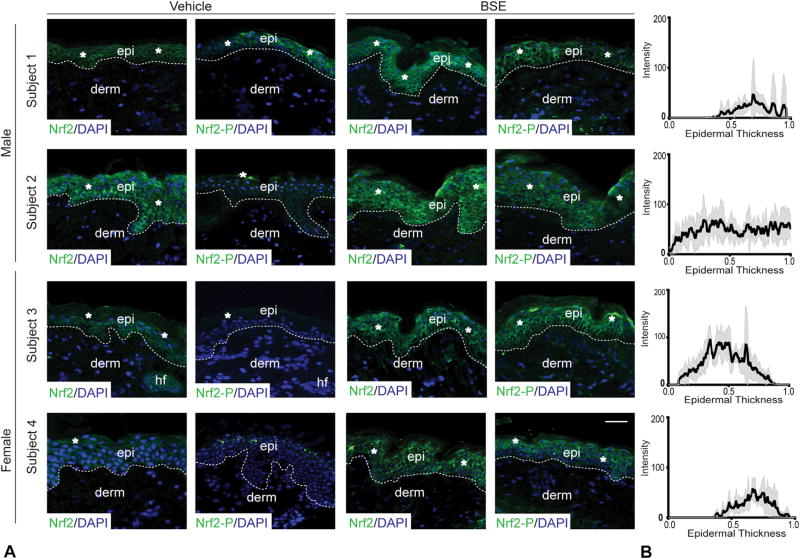
Both BSE- and vehicle-treated skin sites appeared normal on clinical examination and histologic analysis (Fig 1, B and C). All subjects denied any adverse effects. RT-polymerase chain reaction assays (Fig 1, D) evidenced increased levels of KRT17 messenger RNA in all subjects (average 3.6- ± 0.2-fold increase [mean ± SEM]; P value = .0022). KRT16 messenger RNA levels were increased after BSE in women by ~10- and ~14-fold. One man had a ~4-fold increase in KRT16 RNA after BSE, whereas levels were unchanged in the other man. There was no statistical difference in the average fold change of RNA levels of KRT6, KRT5, or KRT14, thus emulating past findings in mouse skin.2 In addition, there were ~3- and ~3.5-fold increases of loricrin RNA in the women, selectively. The RNA levels for a classic NRF2 target gene, NQO-1, were elevated in 3 of the 4 subjects, but the average fold change was not statistically different from control (Fig 1, D). Given that sample collection was ~24 hours after the final treatment, these findings suggest a finite impact of BSE on NRF2 activation at the transcript level, and/or reflect the diverse mechanisms through which sulforaphane induces keratin expression.3 Indirect immunofluorescence revealed a dramatic induction of K17 in BSE-treated skin, relative to control, in all subjects (Fig 2, A). Quantitation of signal intensity along an axis perpendicular to epidermis confirmed that K17 induction occurs primarily in the basal layer and exhibits a patchy character (Fig 2, B). K16 protein was elevated in the suprabasal layers of BSE-treated skin of subjects 2, 3, and 4 (data not shown). A modest induction of K6 protein also occurred in subjects 1, 3, and 4 in the basal and suprabasal layers (data not shown). In all subjects, BSE had no discernable impact on the distribution of K14, K1, or K5 antigens (data not shown). The vehicle-treated skin of the men had more total NRF2 present in the epidermis than the control samples from women (Fig 3, A). Despite the sex-based difference in baseline NRF2 (which deserves future examination), BSE treatment resulted in a strong induction of total and phosphorylated (active) NRF2 in the epidermis of all subjects (Fig 3, A) with a primarily suprabasal distribution (Fig 3, B).
Fig 2.
Status of keratin (K)17 after topical broccoli sprout extract (BSE) treatment. A, Indirect immunofluorescence for K17 in adult skin after vehicle or BSE treatment. Scale bar = 50 µm. *Areas of increased immunofluorescence. Dermoepidermal junction (dotted line). Occasionally, individual cells located in the upper layers of the epidermis (epi) show a strong signal for K17. The significance of this occurrence is unclear. B, Quantitation of fluorescence intensity of K17 staining in BSE-treated skin. Horizontal axis represents epidermal thickness from basal lamina extending to edge of stratum corneum (normalized to 1). Mean (black line) (n = 6) and SEM (gray area). DAPI, 4′,6-Diamidino-2-phenylindole nuclear stain; derm, dermis; hf, hair follicle.
Fig 3.
Status of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and phosphorylated Nrf2 (Nrf2-P) after topical broccoli sprout extract (BSE) treatment. A, Indirect immunofluorescence for Nrf2 in healthy adult skin with vehicle or BSE treatment. Scale bar = 50 µm. *Areas of increased immunofluorescence. Dermoepidermal junction (dotted line). B, Quantitation of fluorescent intensity of NRF2 staining in BSE-treated skin. Horizontal axis represents epidermal thickness from basal lamina extending to edge of stratum corneum. Mean (black line) (n = 6) and SEM (gray area). DAPI, 4′,6-Diamidino-2-phenylindole nuclear stain; derm, dermis; epi, epidermis; hf, hair follicle.
DISCUSSION
These findings suggest that the pharmacologic induction of K17 targeted to the basal layer of human epidermis is possible and worthy of additional consideration. Using an inducible transgenic mouse model, Cao et al5 showed that mosaic expression of mutant K14 did not compromise skin integrity and EBS-like blistering may be prevented by increasing the ratio of cells expressing wild-type K14. Even if mosaic, the expression of the K14-like K17 after topical BSE thus may suffice to afford a therapeutic benefit in individuals with EBS. Further studies with longer timelines, additional subjects, and the inclusion of patients with EBS are needed.
The authors are very grateful to the volunteers who participated in this study.
CAPSULE SUMMARY.
Sulforaphane ameliorates blistering in a Krt14-null mouse model of epidermolysis bullosa simplex, correlating with induction of homologous keratin 17.
We report keratin 17 up-regulation in human skin after topical broccoli sprout extract containing sulforaphane.
Broccoli sprout extract is a therapeutic candidate for keratin 14–related epidermolysis bullosa simplex.
Acknowledgments
Supported by internal funds at Johns Hopkins University.
Abbreviations used
- BSE
broccoli sprout extract
- EBS
epidermolysis bullosa simplex
- K
keratin
- NRF2
nuclear factor (erythroid-derived 2)-like 2
APPENDIX
Study design and approval
Five healthy adults were recruited from the parents of patients with epidermolysis bullosa simplex at the Johns Hopkins University pediatric dermatology clinic. Each subject was given broccoli sprout extract (BSE) in jojoba oil (containing 500 nmol of sulforaphane/mL) and another vial of jojoba oil alone. Subjects were blinded and applied 1 mL of the BSE-jojoba oil compound to a 3-cm circle on the flexural upper aspect of the arm every night and 1 mL of oil alone to the contralateral arm. Both arms were occluded with clear plastic cling wrap overnight. Subjects were advised to avoid topical medications, lotions, and a diet high in cruciferous plants. On the eighth day, photographs and a 6-mm punch biopsy specimen were taken. The study was approved by the Johns Hopkins Medicine Institutional Review Board after obtaining investigative new drug approval 126785 and registered on clinicaltrials.gov (NCT02592954).
BSE preparation
BSE was prepared5 from 3-day-old broccoli sprouts, grown from seeds that were tested by high-performance liquid chromatography for glucoraphanin content, and shown to contain levels sufficient to yield 3-day-old sprouts containing at least 6 µmol of glucoraphanin per gram (±10%). Regular cyclocondensation (chemical-) and enzyme induction (cellular-, or bio-) assays were conducted to augment the high-performance liquid chromatography analyses. Broccoli sprouts were added to boiling deionized water. The mixture was cooled to 37°C, filtered, and a small quantity of 7-day-old daikon sprouts (Japanese white radish; source of myrosinase) was added. The daikon sprouts were homogenized in BSE, and the mixture was incubated for about 4 hours at 37°C, poured into trays, rapidly frozen, and freeze-dried. A fixed amount of dry powder (250 mg) was placed in gel capsules, providing a titer of approximately 50 µmol of sulforaphane per capsule. The powder was dissolved in a minimal volume of room-temperature water, and mixed with 3 to 5 volumes of ethyl acetate. The ethyl acetate supernatant was removed and concentrated, and the resulting sulforaphane-rich solution was added to jojoba oil to achieve a final concentration of 5 mmol/L. Residual ethyl acetate was removed by vacuum evaporation and diluted with jojoba oil to a sulforaphane concentration of 500 µmol/L.
Antibodies
Commercial sources of the antibodies used are: nuclear factor (erythroid-derived 2)-like 2 (Santa Cruz sc-13032, Santa Cruz Biotechnology, Santa Cruz, CA); phosphorylated nuclear factor (erythroid-derived 2)-like 2 (NeoScientific PAR2020, NeoScientific, Cambridge, MA); keratin 1 (Abcam, Ab81623, Abcam, Cambridge, MA); keratin 5 (Covance AF138); and keratin 14 (Santa Cruz Sc-53253). Rabbit polyclonal antibodies to keratin 6, keratin 16, and keratin 17 were produced in the laboratory.2,3
Oligonuleotide primers
Oligonucleotide primers were obtained from Integrated DNA Technologies, Inc, Coralville, IA (Table I).
Table I.
Oligonucleotide primers for target-specific polymerase chain reaction assays
| Target | Forward primer | Reverse primer |
|---|---|---|
| HO-1 | 5′-GCAGAGAATGCTGAGTTCATG-3′ | 5′-CACATCTATGTGGCCCTGGAGGAGG-3′ |
| NQO-1 | 5′-GGGCAAGTCCATCCCAACTG-3′ | 5′-GCAAGTCAGGGAAGCCTGGA-3′ |
| KRT6 (all paralogs) | 5′-CCGGAATTCCAAGGGGCAGTGGTGGCCTG-3′ | 5′-CCGGAATTCGTCCTCCACCAGGTCCTGCAT-3′ |
| Loricrin | 5′-CATGATGCTACCCGAGGTTT-3′ | 5′-ACTGGGGTTGGGAGGTAGTT-3′ |
| KRT5 | 5′-CCTGTGGAGTGGGTGGCTAT-3′ | 5′-CCACTTGGTGTCCAGAACCT-3′ |
| KRT14 | 5′-TGAGCCGCATTCTGACCGAAG-3′ | 5′-GATGACTGCGATCCAGAGGA-3′ |
| KRT16 | 5′-GACCGGCGGAGATGTGAAC-3′ | 5′-CTGCTCGTACTGGTCACGC-3′ |
| KRT17 | 5′-GCTGGGTGGTGAGATCAATGT-3′ | 5′-CGCGGTTCAGTTCTTCTGTTC-3′ |
HO-1, Heme oxygenase-1; KRT, keratin; NQO-1, NAD(P)H, quinone oxidoreductase-1.
Footnotes
Conflicts of interest: None declared.
References
- 1.Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784–1793. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerns ML, DePianto D, Dinkova-Kostova AT, Talalay P, Coulombe PA. Reprogramming of keratin biosynthesis by sulforaphane restores skin integrity in epidermolysis bullosa simplex. Proc Natl Acad Sci U S A. 2007;104:14460–14465. doi: 10.1073/pnas.0706486104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerns M, DePianto D, Yamamoto M, Coulombe PA. Differential modulation of keratin expression by sulforaphane occurs via Nrf2-dependent and -independent pathways in skin epithelia. Mol Biol Cell. 2010;21:4068–4075. doi: 10.1091/mbc.E10-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahey JW, Holtzclaw WD, Wehage SL, Wade KL, Stephenson KK, Talalay P. Sulforaphane bioavailability from glucoraphanin-rich broccoli: control by active endogenous myrosinase. PLoS One. 2015;10:e0140963. doi: 10.1371/journal.pone.0140963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao T, Longley MA, Wang XJ, Roop DR. An inducible mouse model for epidermolysis bullosa simplex: implications for gene therapy. J Cell Biol. 2001;152:651–656. doi: 10.1083/jcb.152.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]