Abstract
Purpose
In this study, we present the treatment of the psoriatic nail beds of patients refractory to standard therapies using high-dose-rate (HDR) brachytherapy. The custom-made micro applicators (CMMA) were designed and constructed for radiation dose delivery to small curvy targets with complicated topology. The role of the HDR brachytherapy treatment was to stimulate the T cells for an increased immune response.
Material and methods
The patient diagnosed with psoriatic nail beds refractory to standard therapies received monthly subunguinal injections that caused significant pain and discomfort in both hands. The clinical target was defined as the length from the fingertip to the distal interphalangeal joint. For the accurate and reproducible setup in the multi-fractional treatment delivery, the CMMAs were designed. Five needles were embedded into the dense plastic mesh and covered with 5 mm bolus material for each micro applicator. Five CMMAs were designed, resulting in the usage of 25 catheters in total.
Results
The prescription dose was planned to the depth of the anterior surface of the distal phalanx, allowing for the sparing of the surrounding tissue. The total number of the active dwell positions was 145 with step size of 5 mm. The total treatment time was 115 seconds with a 7.36 Ci activity of the 192Ir source. The treatment resulted in good pain control. The patient did not require further injections to the nail bed. After this initial treatment, additional two patients with similar symptoms received HDR brachytherapy. The treatment outcome was favorable in all cases.
Conclusions
The first HDR brachytherapy treatment of psoriasis of the nail bed is presented. The initial experience revealed that brachytherapy treatment was well-tolerated and resulted in adequate control of the disease. A larger cohort of patients will be required for additional conclusions related to the long-term clinical benefits.
Keywords: chronic psoriasis, HDR brachytherapy, micro applicators
Purpose
Psoriasis vulgaris is a genetic, systemic, inflammatory, chronic disorder that can be altered by environmental factors [1]. Psoriasis is predominantly manifested as a skin disorder, affecting about 2% of the population [1]. Many non-dermatological inflammatory disorders such as cardiovascular diseases, depression, inflammatory bowel disorders, and some cancers (lung, colon, and kidney) may be correlated with psoriasis. Guidelines of care and treatment recommendations for the management of psoriasis are well documented [1,2,3,4,5,6]. The standard of care for the treatment of psoriasis includes topical therapies, traditional systemic agents, phototherapy, and photochemotherapy. Topical agents provide a satisfactory efficacy-to-safety ratio, and they can be used adjunctively with either ultraviolet light, systemic or biologic medications [3]. The commonly used traditional systemic agents are: methotrexate, cyclosporine, and acitretin [4]. Phototherapy includes narrowband and broadband ultraviolet B-light, whereas photochemotherapy utilizes psoralen plus ultraviolet A light, alone or in combination with topical and systemic agents [5]. It was noticed that the patients could experience psoriasis exacerbation after hormonal therapy followed by curative radiation therapy of the prostate [7]. It was reported in [8,9,10,11,12,13] that the UV radiation, Grenz rays, and 50 kVp sources were utilized in treatments of psoriasis. In addition, the therapeutic regimen consisted of 6 to 8 treatments of radiographic therapy with a total dose ranging from 4 to 6 Gy for treatment of psoriasis was reported in [14]. Another superficial radiotherapy of psoriasis was performed in a randomized, double-blind study [15].
The purpose of the study is to report the treatment of the psoriatic lesions with high-dose-rate (HDR) brachytherapy using 192Ir source in a patient cohort refractory to standard therapies. The patients who received HDR brachytherapy were diagnosed with psoriatic nail beds and psoriasis of the hands. Custom-made micro applicators were used to deliver a radiation dose to such targets with complicated topologies. The design, manufacturing, dosimetric characteristics, clinical implementation, and immobilization technique of these applicators are described in detail. The specifics related to the treatment planning, such as plan normalization and optimization with non-standard applicators were described. In addition, the treatment delivery and the patient treatment outcomes were elaborated.
Material and methods
Patients
A total of three patients were enrolled in this study. The first patient diagnosed with psoriatic nail beds refractory to standard therapies received monthly subunguinal injections that caused significant pain and discomfort in both hands. The other two patients were diagnosed with psoriasis of the hands and were also refractory to standard therapies. The feasibility of the HDR brachytherapy treatment and the utility of technology were elaborated for the case of the first patient. The same treatment approach was used for the other two patients. The follow-up for all patients was reported as well. The timeframe to evaluate adequate control for all three patients ranges from 6 to 36 months. The role of HDR brachytherapy treatment was to stimulate T cells, a significant component of the inflammatory infiltrate of psoriatic lesions, for an increased immune response. This biological mechanism has been previously investigated [16,17,18]. The decision to offer radiation therapy was derived from the multidisciplinary clinic setting (radiation oncology, medical oncology, and dermatology) as a compassionate response to the significant pain caused by nail bed injections. This clinical decision was within the scope of practice based on a small amount of evidence and was not subjected to the IRB prospective approval. Informed consent outlining the potential benefits, risk, and alternatives was obtained within the normal scope of clinical radiation oncology practice.
Pre-treatment preparation
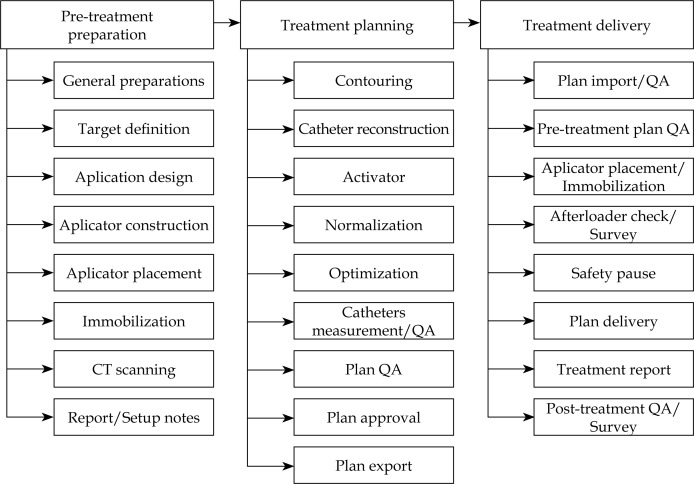
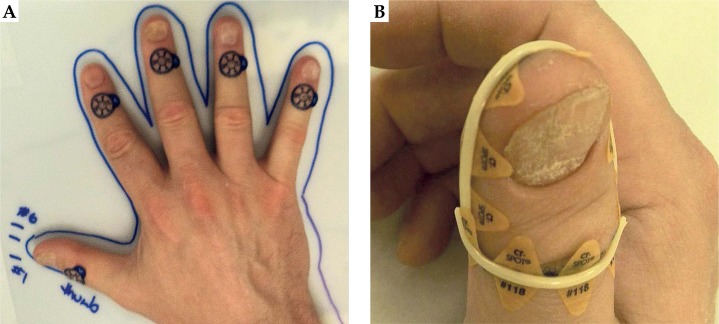
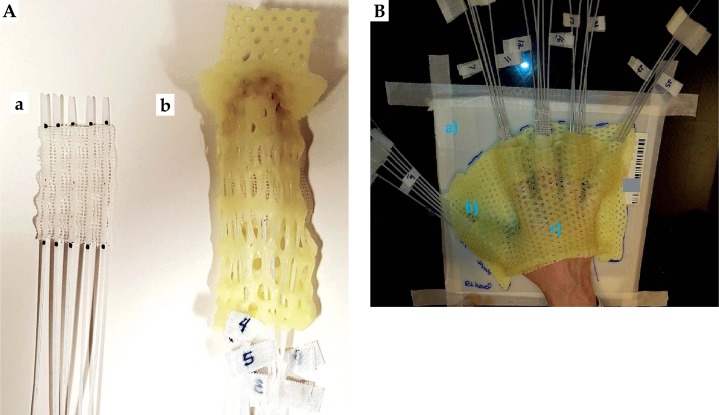
The complete treatment process consisted of three principal steps: pre-treatment preparation, treatment planning, and treatment delivery (Figure 1). The clinical target on each finger was defined as the length from the finger tip to the distal interphalangeal joint (first knuckle). The first knuckle on each finger was marked with a 1.5 mm lead-free metallic pellet X-SPOT® (Beekley Medical, Bristol, CT, USA), as presented in Figure 2A. The borders of the clinical target volume (CTV) were delineated on each finger using the radio-opaque non-metallic line CT-SPOT® (Beekley Medical, Bristol, CT, USA) (Figure 2B) to allow for good visibility of the CTV on the computed tomography (CT) images. The topology of the CTV was complicated and curvy; therefore, none of the commercially available applicators was suitable for such treatment. The anatomy of the fingers required the applicator to be adaptable, but fixed to the CTV once designed. For the accurate and reproducible setup in multi-fractional treatment delivery, it was decided to design and construct five custom-made micro applicators (CMMA) (one for each finger) capable of bringing the radiation dose to the CTV. The term ‘custom-made micro applicator’ was used to represent the applicator capable of conforming to the very small surface with curvy and complicated topology. The plastic mesh allows for catheter placement at small distances (shorter than 5 mm). The technical term ‘micro’ does not describe physical length. Five ProGuide (Elekta Brachytherapy, Stockholm, Sweden) needles with a length of 200 mm were embedded into the dense plastic mesh, and covered with 5 mm bolus material for each CMMA (Figure 3A) for homogeneous dose distribution. These brachytherapy needles were specially designed for interstitial procedures; however, the needles were utilized in the CMMAs due to its flexible yet strong CT-compatible material. Five CMMAs applicators were designed for each finger, resulting in the usage of 25 catheters in total. Each of the applicators was molded to the patient’s fingers and placed between two aquaplast sheets – one at the top and one at the bottom of the mesh, as presented in Figure 3B. As a result, each of the five CMMAs fitted to the patient’s fingers firmly resulted in the establishment of a highly reproducible setup. Due to the design of the applicator (explained in the sequel), it was possible to achieve the sub-millimeter setup accuracy. This was confirmed using the CT images obtained prior to the first fraction. The stability of the setup was achieved with a plastic base on which the patient positioned the hand, as presented in Figures 3A and 4. The aquaplast cover was molded to the dorsal hand to immobilize both the hand and the CMMAs during the simulation and treatment (Figure 4). The cover was taped to the plastic base for the treatment. Therefore, the cover stayed in place in only one possible position during the treatment; however, it was easily removed after the delivery of each fraction. This allowed for a comfortable setup and immobilization during the multi-fractional treatment course. For adequate visibility of the catheters in the CT images, twenty-five line marking wires were placed inside the catheters before the imaging. All the line markers were numbered in consecutive order for proper connection to the afterloader before the treatment. The patient was scanned using a GE Discovery (GE Healthcare, Fairfield, CT, USA) CT scanner. The image slice thickness was 1.25 mm to allow for accurate catheter reconstruction and planning. The quality of images was evaluated and approved by a physicist and a physician due to the increased complexity of the case. Upon completion of the CT scanning, the simulation report was generated. The simulation report contained clinical photos of the setup, the description of the setup, the immobilization procedure, the number, and the orientations of the catheters.
Fig. 1.
The detailed process map of the treatment was developed for improved treatment plan generation and quality assurance of the process
Fig. 2.
A) Metallic pellets were used to mark the distal position of the source (first knuckle) for the computed tomography-based treatment planning. B) Clinical target volume was delineated using a radio-opaque marker line
Fig. 3.
Left: a) A plastic mesh was used as a base of the micro applicators. The needles were embedded into the mesh; b) The aquaplast material (AM) was placed at the top of the custom-made micro applicators (CMMA), whereas the AM was molded at the bottom of the CMMA to uniquely fit the patient’s finger. Right: Simulation and treatment setup. The applicator consists of a) the plastic base, b) individual CMMAs, and c) the molded aquaplast cover that immobilizes the CMMAs to the patient
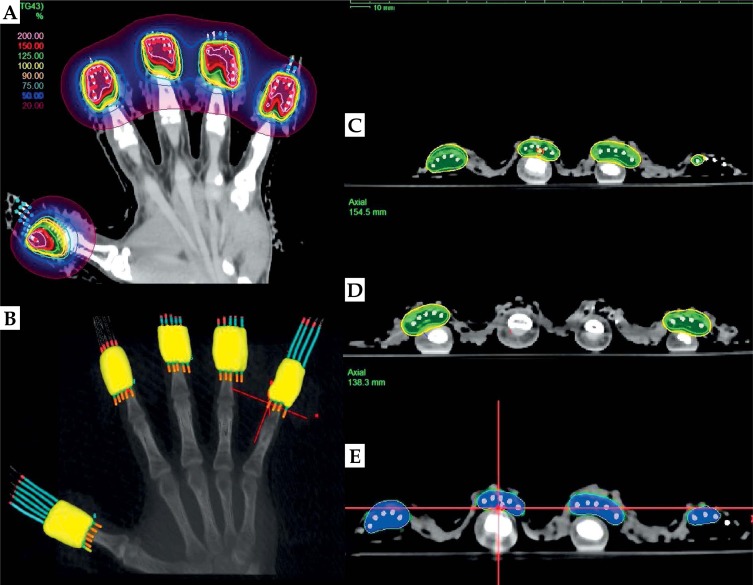
Fig. 4.
A) Isodose distribution of the optimized treatment plan of the psoriatic nail beds. B) Three-dimensional prescription 100% dose distribution with the custom-made micro applicators (CMMAs) (yellow clouds); C) and D) Prescription dose (yellow) planned at a depth of 3 mm or at the level of the anterior surface of the distal phalanx (OARs); E) The blue cloud that stays on the skin represents 125% of the prescription dose
Treatment planning
A treatment plan was generated using the Oncentra Brachy TPS (Version 4.5.2, Elekta Brachytherapy, Stockholm, Sweden). The prescription dose for the HDR treatment was 21.6 Gy in 12 fractions (1.8 Gy per fraction) to the CTV. The treatment was delivered five times per week. The minimal difference between two adjacent positions of the radioactive source (step size) was 5 mm.
For the generation of the treatment plan, the CTV was not contoured. The CTV was defined as the volume delineated with radio-opaque line (Figure 2B) with a depth of 3 mm, except when this depth interfered with the distal phalanx. In those cases, the position of the bone defined the depth of the CTV. With the CTV defined as described, it was possible to spare the bone yet to treat the dermis. However, the radio-opaque lines (markers) were contoured on each finger, so that the active position of the source could be defined during the treatment. All 25 catheters were reconstructed (digitized) starting from the distal end (catheter tip end) toward the proximal end (connector end). The reconstruction of the catheters resulted in the formation of uniform digital lines in the TPS due to the presence of the marking wires inside each catheter (Figure 4A). Image manipulation in the para-axial, -sagittal, and -coronal slices was used for the fast reconstruction of the catheters. Only the source positions that fell into the CTV were activated. This was achieved by following the positions of the markers in the CT images. The treatment plan was initially normalized to the multiple dose points at a depth of 3 mm under the skin. These points were chosen to be geometrically distant to force the dose calculation engine to generate as homogeneous dose distribution as possible in the first planning iteration. Due to the small target size, the plan optimization was localized to the individual segment of the CMMA, resulting in the adjustment of the isodose lines to the depth of 3 mm or to the bone level. Therefore, the healthy tissue unaffected by the disease was spared. One of the optimization goals was to keep the 125% isodose line off the skin, in order to spare the stem cells and vasculature, as presented in Figure 4C and 4D.
Results
Treatment planning
The final optimization resulted in the generation of a highly homogeneous and conformal treatment plan with a favorable dose distribution (Figure 4E). The total number of active dwell positions was 145. The treatment time for this plan was 115.3 s, when the associated activity of the source was 30.0435 mGy × m2 × h−1 (corresponded to 7.36 Ci). The treatment plan was optimized; thus, the dwell times were nearly the same. The smallest dwell time in the plan was 0.45 s, when the source activity was the highest – 11.8 Ci (air kerma strength equals to 48.168 mGy × m2 × h−1) eliminating the major influence of the transit dose to the prescribed dose. The undertaken treatment planning method was to achieve adequate coverage of the CTV and sparing of the healthy tissue with minimal deviation of the dwell times within each of the CMMA. The treatment time for each of the CMMAs was ranging from 20.9 s to 25.1 s, indicating that the treatment plan was optimal without high deviations of the individual dwell times. The contribution of the radiation dose from the adjacent nail beds to the middle finger was not more than 10%. Therefore, the dose received by each nail bed was delivered without being significantly affected by the adjacent targets due to design of the CMMAs. For the purpose of quality assurance, all catheters were measured in two independent ways: a) using the source position simulator, and b) by measuring the marking wires inside each catheter using a ruler. These two measurements for each catheter were within a tolerance level of 1 mm. The distal position of the source 192Ir (source extension) was 1,200 mm, which was to be expected since the CMMAs were constructed with needles with a length of 200 mm. These numbers were entered into the TPS to transfer the source extension of each channel to the driving mechanism of the afterloader. The treatment plan was checked against the written directive, and the dose was recalculated using an independent dose calculation program [19]. Additional details of the brachytherapy treatment of the skin and treatment planning can be found in [20,21].
Treatment delivery
The treatment plan was imported to the treatment console, and the pre-treatment quality assurance was performed. The patient placed their hand on the plastic base, as shown in Figure 2A, and the cover containing the CMMAs was placed on the top of the hand (Figure 3). The catheters were connected to the indexer of the afterloader, and the treatment plan was delivered. Due to the robust design, the applicator stayed firmly in place during the radiation dose delivery. The patient was treated in the sitting position with a hand placed on a table. The different scanning position of the (supine) patient did not influence the treatment setup and treatment in the sitting position due to the fixed design of the CMMA and its firm positioning – the CMMA fit the patient’s hand in only one possible way. Each arm was laterally extended during the treatment, so that the dose to the patient’s body should be minimized. This was done for both hands for all patients. The active dwell positions were located in the vicinity of the patient’s nail beds, so the patient’s head was at a distance of almost a meter from the active sources. The dose in air at 1 m from the active sources was calculated to be 0.05 cGy per fraction or 0.6 cGy for the entire treatment. Since the calculated dose was not significant, placement of any protective shields on the patient’s body (including lead apron, thyroid shield, and leaded glasses) was deemed unnecessary. The treatment time for the last (12th) fraction was 131.7 s, which was 16.4 s longer relative to the first received treatment. Therefore, the extended number of fractions did not result in a significant increase of the treatment time.
The setup on the day of the CT scan was identically reproduced during each treatment fraction. This daily reproducibility of the treatment setup was achieved by the rigid design of the applicator, which was in the form of a glove, as in Figure 3. The applicator rested on the CT couch. Since the attenuation caused by the couch is less than 2%, backscatter from this material did not contribute to the total dose. The parameters used in the TG-43 dose calculation method are derived by immersing the source in a sphere of water with radius 15 cm, which accounts for all the backscatter. The backscatter caused by material surrounding the source during treatment with surface applicators was much lower. A comparison of the in-air dose calculation to the TG-43 dose calculation at representative points within the target showed the difference to be less than 3%. The distance between the 100% and 125% isodose lines as a function of depth into the distal phalanges ranges between 1.7 and 3 mm. If any standard applicator was used in this case, it would not be easy to spare the stem cells and vasculature. Therefore, we utilized the CMMA with fixed geometry and sub-millimeter setup reproducibility. The position of the applicator was verified and confirmed before the treatments, using the planning CT images and the CT images obtained prior to the first fraction.
Overall, the patient tolerated the whole treatment course well. The treatment resulted in good pain control, and the patient did not require further injections to the nail bed. Following the treatment of the right hand, this patient received 20 Gy to their left hand with the described technique. The patient returned for their 6 months periodic check-up with a disease-free appearance of the nail beds. After this initial treatment, additional two patients with similar symptoms received HDR brachytherapy.
Discussion
Psoriasis is a skin condition that modifies the life cycle of the skin cells. The affected cells grow rapidly on the skin surface, causing dehydration, red patches, discomfort, and sometimes pain. The primary goal of the treatment is to slow or prevent the skin cells from growing. This principal approach is identical to that of skin cancer treatments. It is not uncommon that the patients diagnosed with psoriasis do not respond to conventional therapies. Therefore, it was decided to investigate the possibility for using the HDR brachytherapy treatment in patients refractory to standard therapies of psoriasis of the skin. The initial hypothesis was to use HDR brachytherapy to stimulate the T cells and to possibly cause a desirable immune response. No literature describing this treatment has been available.
The treatment outcome was favorable in all three cases. The correct amount of the follow-up time to make definitive statements on this disease varies [14,15] from 3 to 24 months. Stabilization of the nail growth and the disappearance of symptoms were noted in all 3 patients within the first 6 months (Figure 5). No further interventions were required in Patient 1 after a follow-up period of 36 months, and in Patients 2 and 3 after a follow-up period of 30 months. Consequently, the Patient 1 did not require subunguinal injections after the HDR brachytherapy treatment. In order to properly apply the presented technique in the clinical setup, the accurate design of the custom applicator is required.
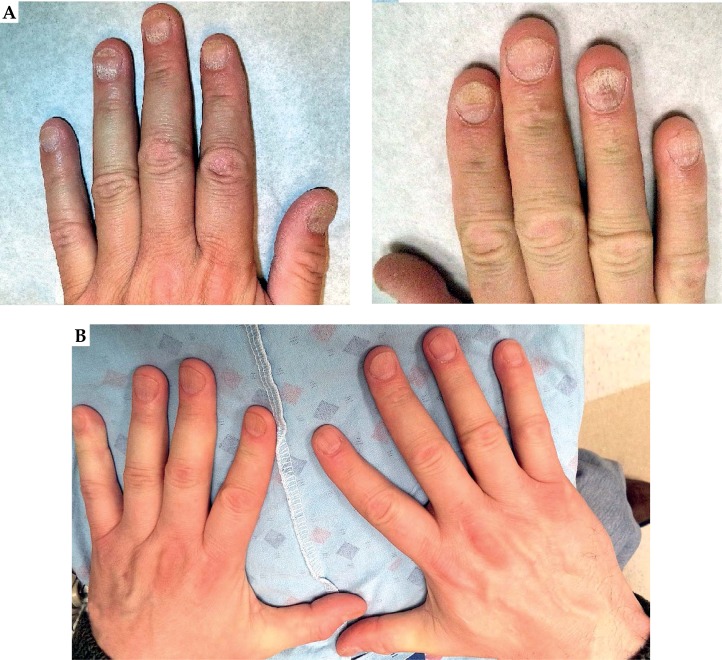
Fig. 5.
A) Patient 1 diagnosed with psoriasis of the nail before high-dose-rate brachytherapy treatment. B) Patient 1 at the 6-month follow-up
Any relative displacement of the target within small applicators would have caused uncertainty of the deliver dose to the adjacent CTVs and OARs. The proper design of the applicator should be able to eliminate this possibility, and to allow for an easy setup, immobilization, and reproducibility.
Conclusions
In this study, we presented the HDR brachytherapy treatment of the psoriasis of the nail bad. The main focus of the manuscript was to evolve the development of feasibility and utility of technology. The initial experience revealed that such technique was well-tolerated and resulted in adequate control of the disease. A larger cohort of patients will be required for additional conclusions related to the potential long-term clinical benefits. The intention of this group is to separately report its clinical series with a sufficient number and follow-ups. This experience can potentially initiate both the fundamental and clinical studies related to the usage of HDR brachytherapy for such diseases and sites in patients refractory to standard medical therapies.
Disclosure
Authors report no conflict of interest.
References
- 1.Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58:851–864. doi: 10.1016/j.jaad.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Menter A, Korman NJ, Elmets CA, et al. American Academy of Dermatology guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114–135. doi: 10.1016/j.jaad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Dermatology Work Group. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65:137–174. doi: 10.1016/j.jaad.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 7.Ziołkowska E, Biedka M, Zyromska A, et al. Psoriasis exacerbation after hormonotherapy in prostate cancer patient – case report. Rep Pract Oncol Radiother. 2010;15:103–106. doi: 10.1016/j.rpor.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harber LC. Clinical evaluation of radiation therapy in psoriasis. AMA Arch Derm. 1958;77:554–558. doi: 10.1001/archderm.1958.01560050062011. [DOI] [PubMed] [Google Scholar]
- 9.Johannesson A, Lindelöf B. Additional effect of Grenz rays on psoriasis lesions of the scalp treated with topical corticosteroids. Dermatology. 1987;175:290–292. doi: 10.1159/000248836. [DOI] [PubMed] [Google Scholar]
- 10.Lindelöf B, Eklund G. Incidence of malignant skin tumors in 14,140 patients after Grenz-ray treatment for benign skin disorders. Arch Dermatol. 1986;122:1391–1395. doi: 10.1001/archderm.1986.01660240055015. [DOI] [PubMed] [Google Scholar]
- 11.Edwards EK, Jr, Edwards EK., Sr Grenz ray therapy. Int J Dermatol. 1990;29:17–18. doi: 10.1111/j.1365-4362.1990.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Yosef R, Soyfer V, Vexler A. Radiation therapy in cancer patients with psoriasis. The fractionated daily dose and the Koebner phenomenon. Radiother Oncol. 2005;74:21–23. doi: 10.1016/j.radonc.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Stern R, Zierler S, Parrish J. Skin carcinoma in patients with psoriasis treated with topical tar and artificial ultraviolet radiation. Lancet. 1980;315:732–735. doi: 10.1016/s0140-6736(80)91231-3. [DOI] [PubMed] [Google Scholar]
- 14.Jiaravuthisan MM, Sasseville D, Vender RB, et al. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57:1–27. doi: 10.1016/j.jaad.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 15.Yu RC, King CM. A double-blind study of superficial radiotherapy in psoriatic nail dystrophy. Acta Derm Venereol. 1992;72:134. [PubMed] [Google Scholar]
- 16.Anderson RE, Warner NL. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- 17.Manda K, Glasow A, Paape D, et al. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front Oncol. 2012;2:102–110. doi: 10.3389/fonc.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hekim N, Cetin Z, Nikitaki Z, et al. Radiation triggering immune response and inflammation. Cancer Lett. 2015;368:156–163. doi: 10.1016/j.canlet.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Damato AL, Devlin PM, Buzurovic IM, et al. Independent brachytherapy plan verification software: Improving efficacy and efficiency. Radiother Oncol. 2014;113:420–424. doi: 10.1016/j.radonc.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Devlin PM, editor. Brachytherapy: applications and techniques. New York: Demos Medical Publishing; 2015. [Google Scholar]
- 21.Likhacheva AO, Devlin PM, Buzurovic IM. Brachytherapy: Applications and Techniques. New York: Demos Medical Publishing; 2015. Skin brachytherapy; pp. 211–234. [Google Scholar]