Abstract
During pregnancy, up‐regulation of heparin‐binding (HB‐) EGF and cyclooxygenase‐2 (COX‐2) in the uterine epithelium contributes to decidualization, a series of uterine morphological changes required for placental formation and fetal development. Here, we report a key role for the lipid mediator lysophosphatidic acid (LPA) in decidualization, acting through its G‐protein‐coupled receptor LPA 3 in the uterine epithelium. Knockout of Lpar3 or inhibition of the LPA‐producing enzyme autotaxin (ATX) in pregnant mice leads to HB‐EGF and COX‐2 down‐regulation near embryos and attenuates decidual reactions. Conversely, selective pharmacological activation of LPA 3 induces decidualization via up‐regulation of HB‐EGF and COX‐2. ATX and its substrate lysophosphatidylcholine can be detected in the uterine epithelium and in pre‐implantation‐stage embryos, respectively. Our results indicate that ATX–LPA–LPA 3 signaling at the embryo‐epithelial boundary induces decidualization via the canonical HB‐EGF and COX‐2 pathways.
Keywords: autotaxin, decidualization, embryo implantation, LPA3, lysophosphatidic acid
Subject Categories: Development & Differentiation, Signal Transduction
Introduction
Infertility is a global problem experienced by about 10–15% of couples during their reproductive years. In addition, in spite of recent advances in the artificial reproductive technology (ART), the success rate of pregnancy through ART is still low (~30%; Lim & Wang, 2010; Ramathal et al, 2010; Cha et al, 2012). These pregnancy failures are believed to be mainly due to defects in early pregnancy events including implantation and decidualization.
Decidualization is a series of uterine morphological changes during early pregnancy which is essential for the following placental formation and fetal development (Lim & Wang, 2010; Ramathal et al, 2010; Cha et al, 2012). In mice, decidualization occurs only in the vicinity of the embryos. In humans, this process occurs cyclically, whether or not an embryo is present (pre‐decidualization), although it is reinforced by embryo implantation (Cha et al, 2012). In the decidual process, the uterine epithelium breaks down, and extensive proliferation and angiogenesis occur in the subepithelial stroma (decidual reactions). The signaling required for proper decidualization is generated through two types of cell–cell interactions, i.e., embryo‐epithelial and epithelial–stromal interactions. Maternal factors such as heparin‐binding epidermal growth factor (HB‐EGF) and cyclooxygenase‐2 (COX‐2) are induced in the epithelium surrounding the embryos (embryo‐epithelial interaction) and then act on the stroma to induce the expression of bone morphogenic protein 2 (Bmp2) and wingless‐related MMTV integration site 4 (Wnt4) in stroma (epithelial–stromal interaction; Lim et al, 1997; Paria et al, 2001; Song et al, 2002; Wang et al, 2004; Xie et al, 2007; Large et al, 2014). Mice in which these maternal factors (HB‐EGF, COX‐2, Bmp2, and Wnt4) are knocked out showed obvious defects in decidual reactions (Lim et al, 1997; Wang et al, 2004; Lee et al, 2007; Xie et al, 2007; Franco et al, 2011; Li et al, 2013; Large et al, 2014). Based on these observations, it has been proposed that some factor(s) are present in the embryo‐epithelial boundary and induce the expression of HB‐EGF and COX‐2 in the epithelium, which in turn facilitate the following decidual reactions through up‐regulation of Bmp2 and Wnt4 (Paria et al, 2001; Cha et al, 2012).
Lysophosphatidic acid (LPA) has various roles as a lipid mediator through G‐protein‐coupled receptors (GPCR). So far, six LPA‐specific GPCRs named LPA1‐LPA6 have been identified (Aikawa et al, 2015; Sheng et al, 2015). LPA is mainly synthesized from lysophospholipids such as lysophosphatidylcholine (LPC) by a secretory enzyme autotaxin (ATX; Umezu‐Goto et al, 2002). Studies of knock‐out (KO) mice and human genetic diseases of these LPA‐related genes have shown that LPA has various pathophysiological roles including angiogenesis (Yukiura et al, 2011), hair follicle formation (Inoue et al, 2011; Hayashi et al, 2015), bone development (Nishioka et al, 2016), and neural development (Yung et al, 2015). We previously showed that LPA3, which is highly expressed in the uterine epithelium during the peri‐implantation period (Ye et al, 2005), has a critical role in the early pregnancy. Lpar3 KO mice show many reproductive defects, including significantly reduced COX‐2 (a key enzyme for synthesis of prostaglandins), delayed implantation, aberrant embryo spacing, defects in placental formation and fetal development, and reduced litter size (Ye et al, 2005; Hama et al, 2007). However, the defects in Lpar3 KO were only partially recovered by administration of prostaglandins (Ye et al, 2005), suggesting that other unidentified factors should operate downstream of LPA3. In addition, it is almost unclear what kind of cellular events are affected in Lpar3 KO uteri.
In this study, to gain insights into the signaling and cellular events downstream of LPA3, we administered a potent agonist for LPA3 into the mouse uterine cavity during the peri‐implantation period. Unexpectedly, mere activation of the epithelial LPA3 by the agonist induced prominent endometrial morphological changes, which were associated with up‐regulation of the above‐mentioned decidual factors (HB‐EGF, COX‐2, Bmp2, and Wnt4). Furthermore, we obtained evidences that endogenously LPA3 signaling was evoked by ATX, an LPA‐producing enzyme. These results lead us to propose a novel mechanism for decidualization elicited by embryos; that is, the ATX–LPA3 axis in the embryo‐epithelial boundary regulates decidualization by inducing maternal factors such as HB‐EGF and COX‐2.
Results
An LPA3 agonist, T13, induces decidualization
To clarify the molecular mechanisms and cellular events induced downstream of LPA3 signaling, we injected T13, a potent LPA3 agonist (EC50 ~0.2 nM; Fig EV1A–C; Tamaruya et al, 2004; Kano et al, 2008; Hama & Aoki, 2010), into the uterine cavities of pseudopregnant mice at 3.5 days post‐coitus (dpc). Interestingly, T13 induced dramatic uterine hypertrophy throughout the uterine horns at 5.5 dpc (Figs 1A and B, and 2A). The T13‐induced hypertrophy was completely absent in the uteri of Lpar3 KO uteri mice (Figs 1 and 2A), indicating T13 evokes uterine hypertrophy through the activation of LPA3. T13 induced several cellular changes, which resembled the changes that occur during decidual reactions in normal pregnancy. At 4.5 dpc, stromal proliferation as judged by bromodeoxyuridine (BrdU) labeling was evident in the stromal cells surrounding the embryo (primary decidual zone; PDZ; Fig 2B, upper row). At 5.5 dpc, the proliferative area expanded outside the PDZ (Fig 2B, lower row). In addition, angiogenesis as judged by anti‐CD31 staining was prominent in the stromal layer (Fig 2A). At this time, the luminal epithelium collapsed (LE‐breakdown) at the antimesometrial (AM) pole, as shown by E‐cadherin staining in T13‐treated uteri (Fig 2C). We also confirmed that T13‐injected uteri showed high alkaline phosphatase activity which is an indicator of decidualized stromal cells (Appendix Fig S1). LPA3 activation seems to induce some factor(s) in the epithelial layer, which then evoke the decidual reactions in the stromal layer. It should be noted that oil‐induced decidualization was similarly observed both in wild‐type and Lpar3 KO uteri (Fig EV2), confirming that the intrinsic mechanism for decidualization was not affected in Lpar3 KO uteri. This suggests that LPA does not induce decidualization directly but contributes to the induction of decidualization by up‐regulating some decidual factors via LPA3. Accordingly, we concluded that all the decidual reactions (LE‐breakdown, stromal proliferation, and angiogenesis) could be induced in the absence of embryos solely by activating LPA3.
Figure EV1. T13 is a potent and selective agonist of LPA 3 .
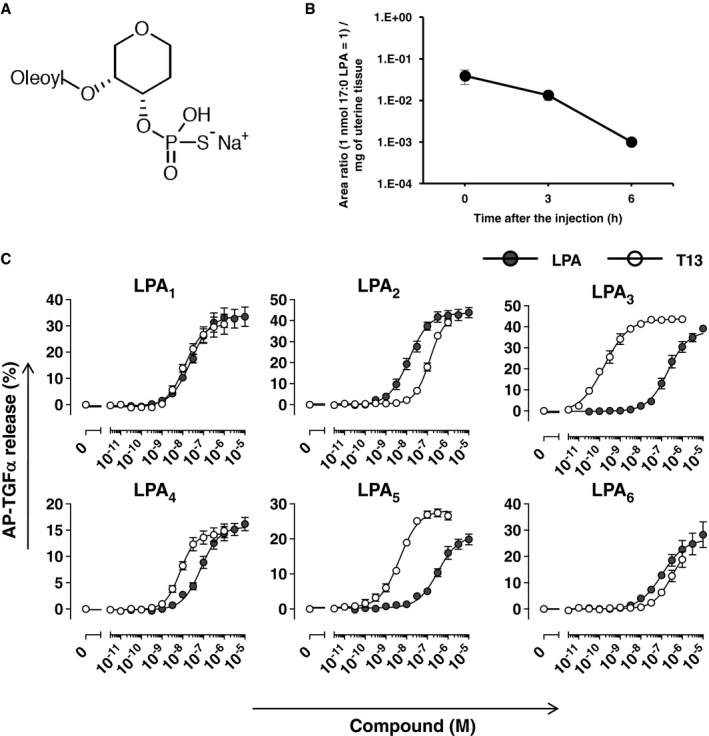
- The structure of T13. T13 was synthesized based on the structure of 2‐oleoyl LPA and thiophosphate group and ring structure were introduced to make it more stable and resistant for phosphatase.
- The pharmacokinetics of T13 in uteri after the intrauterine injection. A single data point was measured in three biological replicates. Data are means ± SEM.
- Each LPA receptors and AP‐TGFα plasmids were co‐transfected to HEK293 cells which endogeneously express a protease, TACE, responsible for ectodomain‐shedding of TGFα. Activation of each LPA receptor by T13 (open circles) and LPA (closed circles) was evaluated by TGFα shedding assay. T13 has a potent agonistic effect on LPA3. For each experiment, a single data point was measured in three biological replicates. Receptor‐specific responses were calculated by subtracting AP‐TGFα release signals in mock‐transfected cells from those in LPA receptor plasmid‐transfected cells. Data are means ± SEM, respectively, of three (for LPA1, LPA2, LPA3, and LPA6) or four (for LPA4 and LPA5) independent experiments.
Figure 1. Intrauterine injection of a potent LPA 3 agonist, T13, causes uterine hypertrophy.
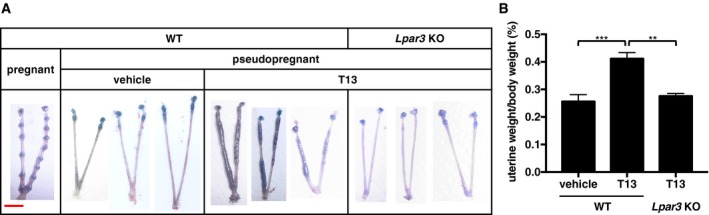
-
A, BRepresentative photographs of pregnant or T13‐injected pseudopregnant uteri on 5.5 dpc (A) and the average mass of uteri (B, n = 5 for WT with vehicle and Lpar3 KO with T13, n = 7 for WT with T13). At 3.5 dpc, vehicle or an LPA3 agonist (T13) was injected into pseudopregnant uteri and mice were dissected at 5.5 dpc. In the normal pregnant uterus, only implantation sites were enlarged because of decidualization. On the other hand, pseudopregnant uteri showed no enlargement since they had no blastocysts in the luminal cavity. The injection of T13 into pseudopregnant uteri caused dramatic hypertrophy throughout the uterine horns in an LPA3‐dependent manner. Each image in (A) is a representative from at least three independent experiments. Scale bar = 1 cm. Data are means + SEM. **P < 0.01, ***P < 0.001 by ANOVA.
Figure 2. Activation of LPA 3 evokes decidual reactions.
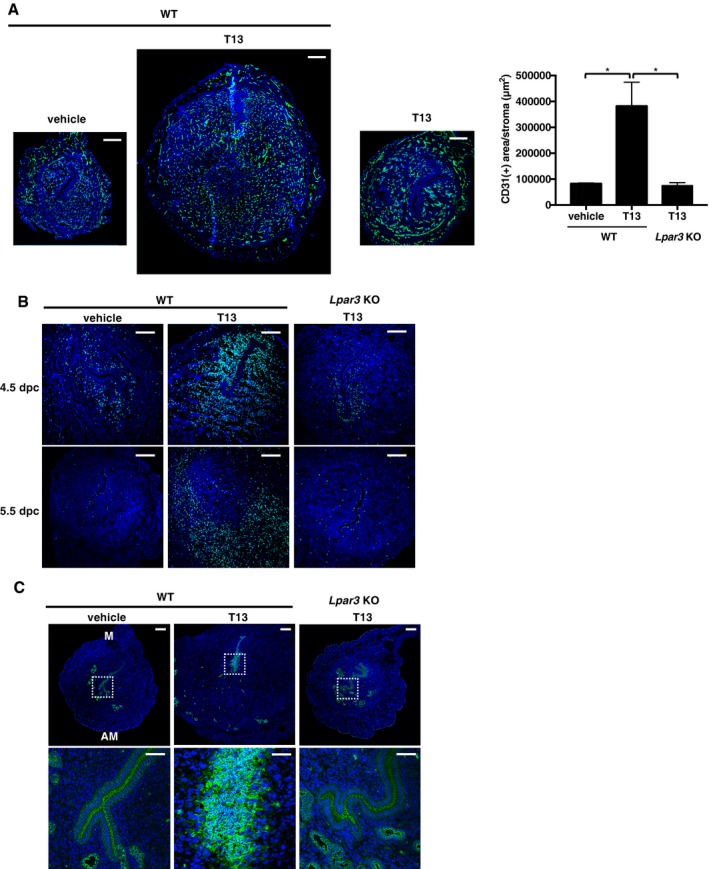
-
A–CImmunostaining of CD31 (angiogenesis, A), BrdU (cell proliferation, B), and E‐cadherin (LE‐breakdown, C) in T13‐treated pseudopregnant uteri showed that T13 induced prominent decidual reactions in an LPA3‐dependent manner. (A) In T13‐injected uteri on 5.5 dpc, fine vascular formation was observed in the AM pole. The CD31 (+) area was calculated by ImageJ (right, n = 3 for WT with vehicle and Lpar3 KO with T13, n = 4 for WT with T13). Data are means + SEM. *P < 0.01 by ANOVA. (B) T13‐treated mice were injected with BrdU on the morning of 4.5 or 5.5 dpc. After 2‐h chasing, uteri were dissected. Cell proliferation occurred in the PDZ on 4.5 dpc and then SDZ on 5.5 dpc. Higher magnification images of boxed regions are shown in the lower panels. (C) T13 caused LE‐breakdown in the AM pole on 5.5 dpc. In each image, nuclei were counterstained with DAPI (blue). M, mesometrial pole; AM, anti‐mesometrial pole. Scale bar: 200 μm (A, B and upper row in C) and 50 μm (lower low in C). Each image is a representative from at least three independent experiments.
Source data are available online for this figure.
Figure EV2. Oil‐induced decidualization is not affected in Lpar3 KO mice.

Representative photographs (left) and the average mass of oil‐infused uteri 2 days after the oil infusion (n = 10 for WT mice and n = 12 for Lpar3 KO mice). Each image is a representative from at least three independent experiments. Scale bar: 1 cm. Data are means + SEM, n.s.: not significant by Student's t‐test.
LPA3 evokes decidual reactions through up‐regulation of HB‐EGF, COX‐2, and Bmp2/Wnt4 signalings
To understand the molecular mechanism underlying T13‐induced decidualization, we then performed cDNA microarray analyses. Several tens of genes were up‐regulated 2 h after the T13 injection (Table 1). Among the genes, we focused on Hbegf and Ptgs2 (encoding COX‐2), because they are responsible for decidualization and knockout of these genes interfered with implantation (Lim et al, 1997; Song et al, 2002; Wang et al, 2004; Xie et al, 2007; Large et al, 2014), as was observed in Lpar3 KO mice (Ye et al, 2005). Both Hbegf and Ptgs2 were transiently induced, peaking at 2–9 h after the T13 injection (Fig 3A). Both genes were predominantly up‐regulated in the epithelial layer (Fig 3B). Among the EGF family members, only Hbegf was up‐regulated by T13 (Fig EV3). In agreement with the up‐regulation of Ptgs2, a lipidomics analysis confirmed the increase of PGE2 and PGF2α in T13‐injected uteri 9 h after the injection. The increase of PGE2 and PGF2α were markedly suppressed by the COX‐2 inhibitor (Fig EV4), suggesting that these PGs are involved in T13‐induced decidualization.
Table 1.
Numbers of genes up‐ and down‐regulated by at least a factor of two in uteri treated with T13
| Type of regulation | No. of genes regulated at time after T13 injection (h) | ||
|---|---|---|---|
| 0.5 | 1 | 2 | |
| Up‐regulateda | 10 | 10 | 29 |
| Down‐regulatedb | 7 | 8 | 12 |
The data have been deposited in the NCBI GEO database (accession no. GSE87116). The following genes are up‐ or down‐regulated in 2 h after the T13 injection. Hbegf and Ptgs2 (in bold), responsible genes for uterine functions, were up‐regulated.
Mup1, Cftr, Vmn1r12/Vmn1r14, Gjb2, A2m, Ptgs2, Vmn2r88, P2yr14, Edn1, Vnn1, Ly6a, Cyp4a11, Gpcr5a, Hsd3b1, Csn2, Ifi16, Cyp3a25, Rcan1, Ramp3, Lcn2, Crtac1, mir‐15, Hbegf, Scl40s1, Ighm, Gadd45 g, Sirpb1, Dip2, Egr3.
Lpin2, Tp53i11, Rnasel, Mcpt4, Ccl5, Sult5a1, Tac3, Ceacam1, Cxcl10, Cxcl9, Igk‐v28, H2‐t22.
Figure 3. HB‐EGF and COX‐2 were highly induced on the epithelial layer in the T13‐injected uteri.
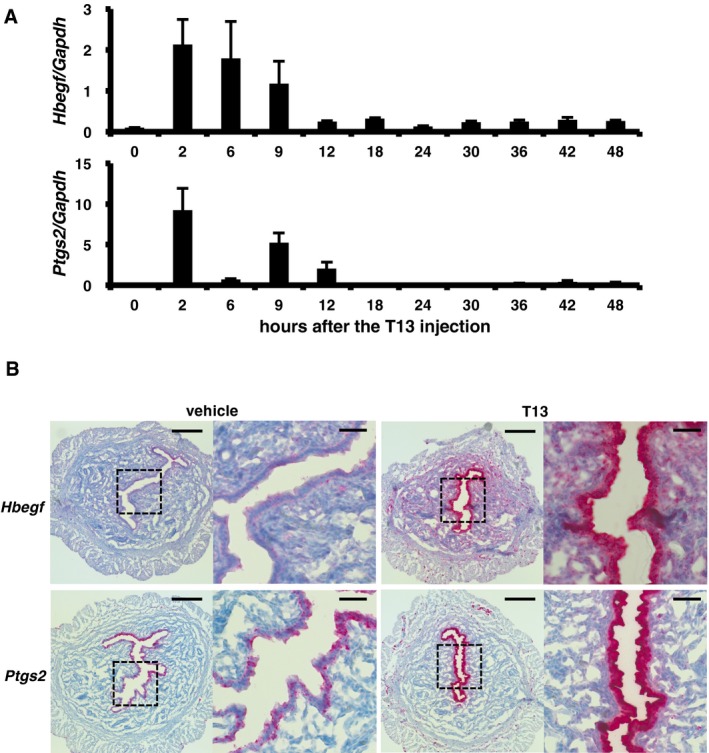
- Time course of qRT–PCR quantification of Hbegf and Ptgs2 mRNAs in T13‐injected pseudopregnant uteri (n = 8 for 2 h, n = 4 for 6 and 9 h, n = 5 for 0 and 12–48 h of Hbegf; n = 12 for 6 h, n = 4 for 9 h, n = 5 for 0, 2 and 12–48 h of Ptgs2). Both Hbegf and Ptgs2 were transiently up‐regulated after the treatment. Data are means + SEM.
- Representative ISH images of Hbegf and Ptgs2 2 h after the injection. T13 strongly induced both transcripts in the epithelial layer. Higher magnification images of boxed regions are shown in each right panel. Each image is a representative from at least three independent experiments. Scale bar: 200 μm (each left panel) and 50 μm (each right panel).
Figure EV3. T13 induces Hbegf specifically among the EGF family members.
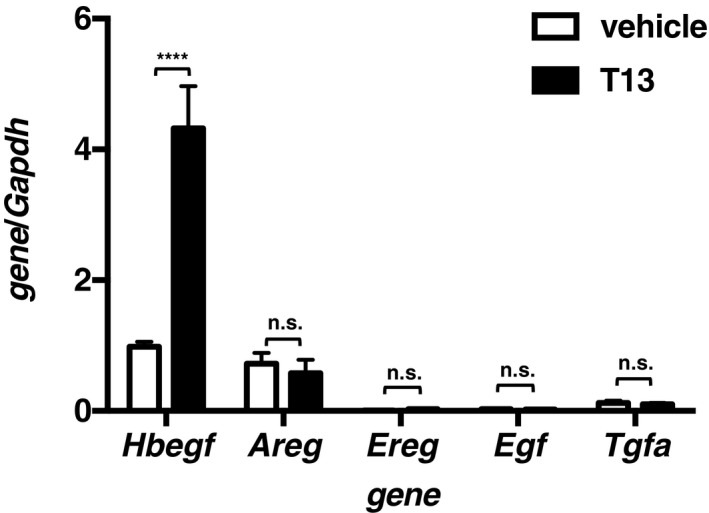
Two hours after the T13 injection, expression of each EGF family member was evaluated by qRT–PCR (n = 5 for each bar). Data are means + SEM, ****P < 0.0001, n.s.: not significant by ANOVA.
Figure EV4. Levels of prostaglandins and related lipids in T13‐injected uteri.
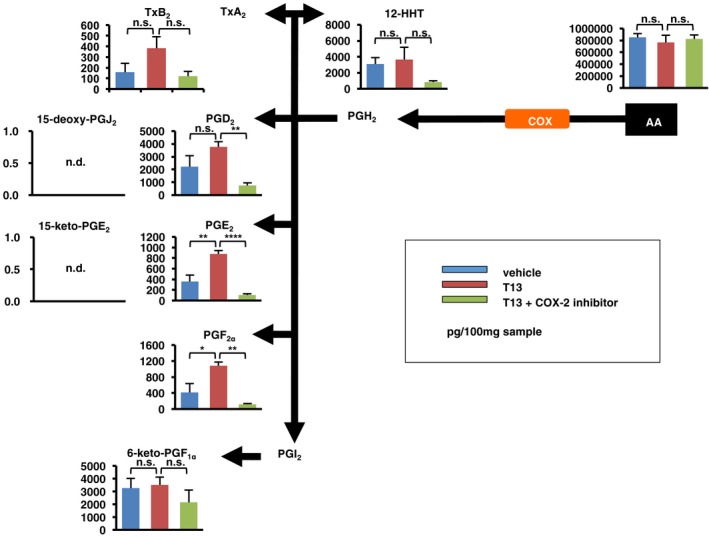
Whole uteri were isolated from T13‐injected mice 9 h after the T13 injection and were subjected to LC‐MS/MS‐based lipidomics analysis, mainly focused on fatty acids and their derivatives. Arachidonic acid (AA)‐derived products as well as AA itself were quantified by LC‐MS/MS. Effect of COX‐2 inhibitor, Celecoxib, was also examined. In T13‐injected uteri, PGE2 and PGF2α were significantly up‐regulated in COX‐2‐dependent manner (n = 5 for each bar). Data are means + SEM. *P < 0.05, **P < 0.01, ****P < 0.0001, n.s.: not significant, n.d.: not detected. TxB2: thromboxane B2, TxA2: thromboxane A2, 12‐HHT: 12‐hydroxyheptadecatrienoic acid, 15‐keto‐PGE2: 15‐keto‐prostaglandin E2, 15‐deoxy‐PGJ2: 15‐deoxy‐prostaglandin J2, 6‐keto‐PGF1α: 6‐keto‐prostaglandin F1α.
The microarray analysis also revealed that the expressions of several thousand genes (3,220 genes at 24 h, 3,365 genes at 30 h, and 3,949 genes at 36 h) differed by a factor of at least two between T13‐injected uteri and the control (Table 2). Ingenuity pathway analysis (IPA) revealed that genes involved in the cell cycle and DNA replication were highly affected by T13 injection (Table 3), which is in agreement with the observation that T13 induced the uterine hypertrophy. We also found that downstream of LPA3, Bmp2/Wnt4 signaling (Table 2 and Fig 4A) was activated. Bmp2 and Wnt4 are well‐known decidual factors acting downstream of HB‐EGF signaling (Paria et al, 2001; Lee et al, 2007; Franco et al, 2011; Li et al, 2013; Large et al, 2014). Interestingly, a significant negative correlation of gene expression profiles was observed between T13‐injected uteri and uteri null for either Egfr (a target of HB‐EGF), Bmp2, and Wnt4 (Large et al, 2014; Fig 4B). By upstream analysis using the IPA, we also found a significant positive correlation between genes affected by LPA3 and E2 signaling, the latter of which is important for the endometrial proliferation and the establishment of early pregnancy (Lim & Wang, 2010; Ramathal et al, 2010; Cha et al, 2012; Pawar et al, 2015; Table 4). Conversely, there was a negative correlation between genes affected by LPA3 and an ERα antagonist (fulvestrant; Table 4). These results are consistent with the recent observation that E2 signaling is down‐regulated in pregnant Lpar3 KO uteri (Diao et al, 2015).
Table 2.
Gene expression profiling of uteri treated with T13
| Parameter | Time after T13 injection (h) | ||
|---|---|---|---|
| 24 | 30 | 36 | |
| No. genes up‐regulated > 2 fold | 776 | 903 | 1,341 |
| No. genes down‐regulated > 2 fold | 2,444 | 2,462 | 2,608 |
| Bmp2 fold increase | 2.6↑ | 6.3↑ | 11.9↑ |
| Wnt4 fold increase | 1.3↑ | 2.8↑ | 12.2↑ |
The data have been deposited in the NCBI GEO database (accession no. GSE87161).
Table 3.
Top five Molecular and Cellular Functional annotations for differently expressed genes between T13 vs. vehicle‐treated uteri 24 h after the injection
| Annotation | No. genes |
|---|---|
| Cell cycle | 282 |
| Cellular assembly and organization | 98 |
| DNA replication, recombination, and repair | 215 |
| Molecular transport | 200 |
| Cell‐to‐cell signaling and interaction | 247 |
Functional annotations were analyzed with Ingenuity Pathway Analysis (IPA) software (Qiagen, CA, USA).
Figure 4. Bmp2 and Wnt4 were up‐regulated in the T13‐injected uteri, downstream of EGFR, COX‐2, and ERα signals.
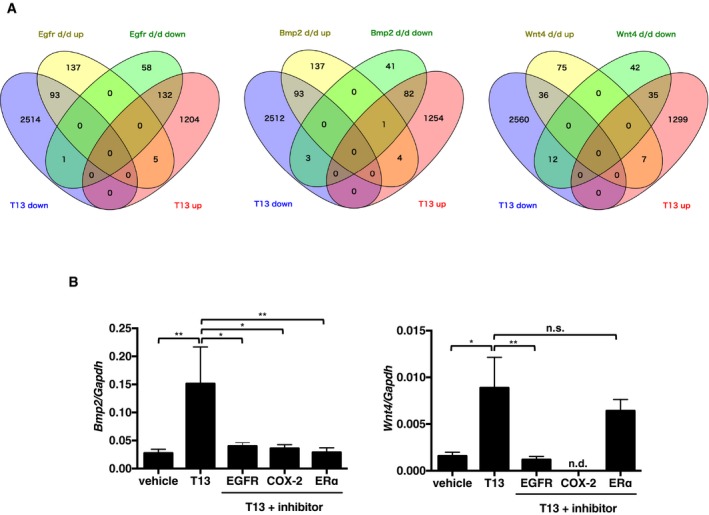
- Venn diagrams comparing gene expression signatures between T13‐injected uteri (36 h after the injection) and uteri from either KO of Egfr, Bmp2, or Wnt4 (24 h after the artificial decidual stimuli). A significant negative correlation in the gene expression patterns was observed between T13‐injected and each KO uteri.
- qRT–PCR quantification of Bmp2 and Wnt4 mRNAs in T13‐injected uteri 36 h after the treatment (n = 8 for vehicle, n = 7 for T13, n = 8 for T13 + EGFR inhibitor, n = 10 for T13 + COX‐2 inhibitor, and n = 9 for T13 + ERα antagonist). Pharmacological inhibition of EGFR, COX‐2 or ERα prominently reduced T13‐induced Bmp2 and Wnt4 expressions. Data are means + SEM. *P < 0.05, **P < 0.01, n.s.: not significant by ANOVA. n.d.: not detected. See also Tables 1, 2, 3, 4.
Table 4.
Upstream analysis 24 h after the T13 injection
| Upstream regulator | Molecule type | Predicted activation state | P‐value of overlap |
|---|---|---|---|
| CSF2 | Cytokine | ↑ | 4.43E‐21 |
| EP400 | Other | ↑ | 1.95E‐14 |
| E2F1 | Transcription regulator | ↑ | 5.49E‐13 |
| E2f | Group | ↑ | 4.18E‐10 |
| Vegf | Group | ↑ | 2.50E‐09 |
| Estrogen | Chemical drug | ↑ | 4.82E‐08 |
| Cephaloridine | Chemical drug | ↑ | 2.29E‐07 |
| FOXM1 | Transcription regulator | ↑ | 8.46E‐07 |
| CDKN1A | Kinase | ↓ | 1.91E‐23 |
| 1‐alpha, 25‐dihydroxy vitamin D3 | Chemical drug | ↓ | 3.96E‐20 |
| Rb | Group | ↓ | 2.07E‐13 |
| IRGM | Other | ↓ | 1.17E‐12 |
| Fulvestrant | Chemical drug | ↓ | 2.60E‐10 |
| SMARCB1 | Transcription regulator | ↓ | 1.13E‐08 |
| PTF1A | Transcription regulator | ↓ | 4.94E‐08 |
| BNIP3L | Other | ↓ | 1.08E‐07 |
Biological networks and pathways were analyzed with Ingenuity Pathway Analysis (IPA) software (Qiagen, CA, USA). Estrogen signaling was significantly affected by T13 injection (in bold).
We next performed in vivo experiments to evaluate each signal for the development of T13‐induced decidualization. Administration of an EGFR inhibitor (AST1306) reduced the levels of T13‐induced Bmp2 and Wnt4 (Fig 4B). A COX‐2 selective inhibitor (Celecoxib) also down‐regulated both Bmp2 and Wnt4 (Fig 4B), suggesting that, in addition to HB‐EGF signaling, epithelial COX‐2 signaling is required for the induction of Bmp2 and Wnt4 in the stromal layer. By contrast, fulvestrant decreased the Bmp2 level, but not Wnt4 level (Fig 4B). As expected, neither AST1306 nor Celecoxib decreased the expression of Hbegf and Ptgs2 themselves (Appendix Fig S2). Likewise, fulvestrant failed to affect the expression of Hbegf and Ptgs2 (Appendix Fig S2), indicating that LPA3 signaling in the epithelial layer was not affected by E2. The signaling pathways identified above can be inhibited by several factors, including an EGFR inhibitor (AST1306), a COX‐2 inhibitor (Celecoxib), an ERα antagonist (fulvestrant), a BMPR inhibitor (LDN193189), or a β‐catenin inhibitor (XAV939). Each of these factors strongly suppressed T13‐induced decidual events including uterine hypertrophy (Fig 5A), stromal cell proliferation (Fig 5B), angiogenesis (Fig EV5), and LE‐breakdown (Fig EV6). These results demonstrate that all these signals were essential for T13‐induced decidualization.
Figure 5. LPA 3 facilitates decidualization through HB‐EGF, COX‐2, Bmp2/Wnt4, and ERα signaling pathways.
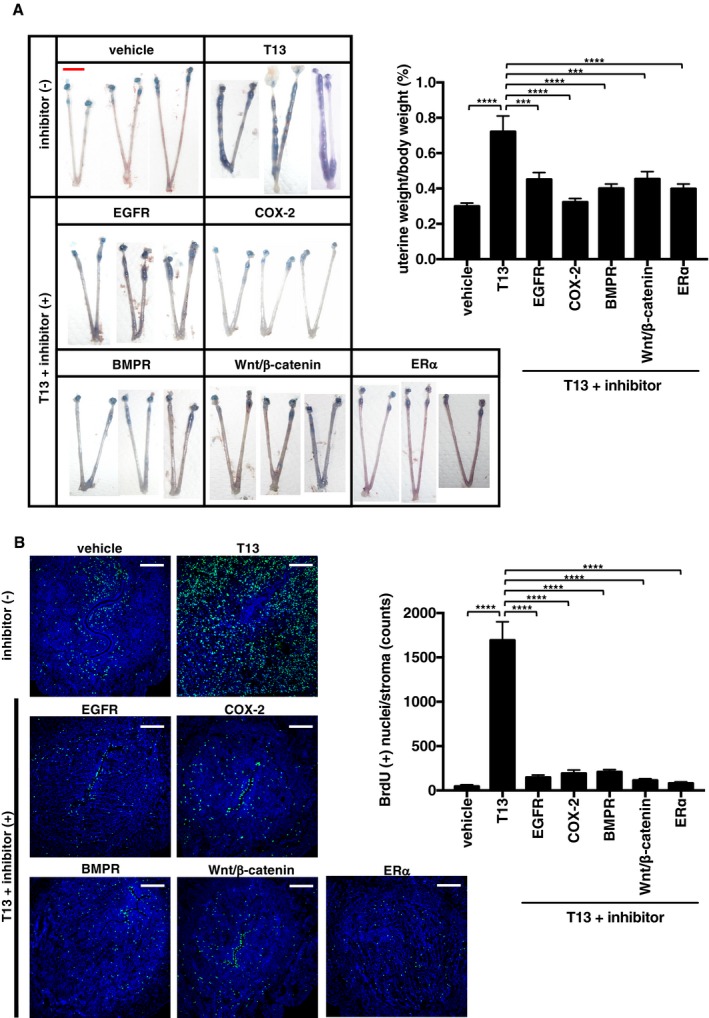
- Representative photographs of pseudopregnant uteri treated with T13 and either one of inhibitors (left) and the average uterine weight (right) on 5.5 dpc were shown. Pharmacological inhibition of each signaling molecule suppressed T13‐induced hypertrophy (n = 7 for vehicle, n = 8 for T13, T13 + Wnt/β‐catenin inhibitor and T13 + ERα antagonist, n = 9 for T13 + EGFR inhibitor and T13 + BMPR inhibitor, n = 10 for T13 + COX‐2 inhibitor).
- Representative images of BrdU immunostaining in pseudopregnant uteri treated with T13 and either one of inhibitors (2 h BrdU chasing on 5.5 dpc) (left) and the average BrdU (+) counts (right, n = 3 for each bar). T13‐induced stromal cell proliferation was canceled by treatment of each inhibitor. In each image, nuclei were counterstained with DAPI (blue). The counts of BrdU were calculated using ImageJ.
Figure EV5. Effects of each signal inhibitor on T13‐induced uterine angiogenesis.
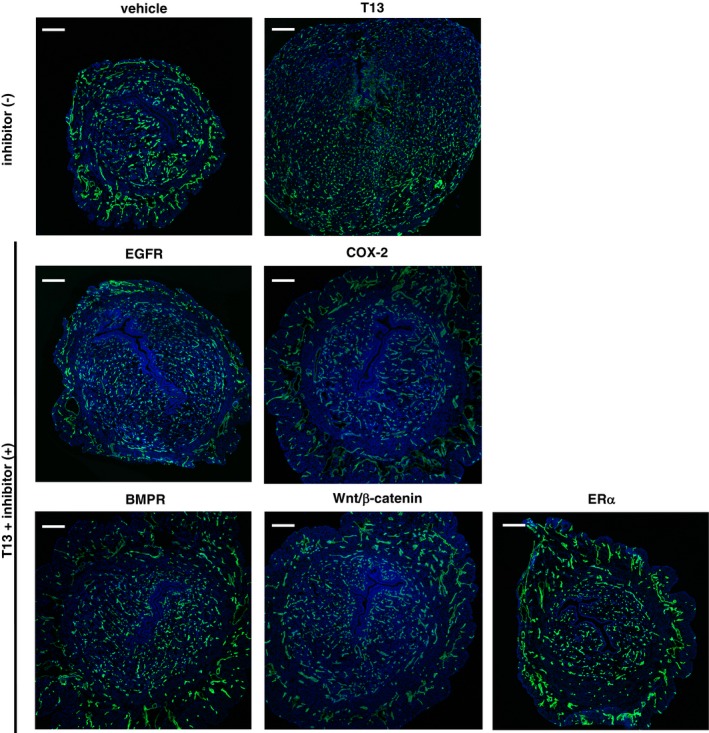
Immunohistochemical images of uterine cross‐sections from pseudopregnant mice using anti‐CD31 antibodies 2 days after the injection of both T13 and either one of the inhibitors. Each inhibitor prominently blocked T13‐induced angiogenesis characterized by fine vascular formation in the AM pole. Scale bar: 200 μm.
Figure EV6. Effects of each signal inhibitor on T13‐induced LE‐breakdown.

Immunohistochemical images of uterine cross‐sections from pseudopregnant mice using anti‐E‐cadherin antibodies 2 days after the injection of both T13 and either of inhibitors, showing that T13‐induced LE‐breakdown was prominently blocked by each inhibitor. Scale bar = 50 μm.
ATX–LPA3 axis endogenously contributes to decidualization
To know the role of endogenous LPA3 signaling in decidualization, we looked into the decidual reactions in Lpar3 KO uteri. Consistent with the data using the LPA3 agonist, stromal cell proliferation in pregnant Lpar3 KO uteri was markedly reduced as judged by incorporation of BrdU (Fig 6A and B) and the number of nuclei in the stromal layer (Fig 6C). In addition, Lpar3 KO uteri showed weakened angiogenesis (Fig 6D and E). LE‐breakdown was rarely observed in Lpar3 uteri (Fig 6F). All these data suggested that endogenous LPA3 signaling is important for the development of decidualization. The expressions of Hbegf, Ptgs2, Bmp2, and Wnt4 were also concomitantly reduced (Fig 6G and H), which further explains the reduced decidual reactions in Lpar3 KO uteri.
Figure 6. Endogenous LPA 3 signaling regulates decidual reactions in normal pregnancy.
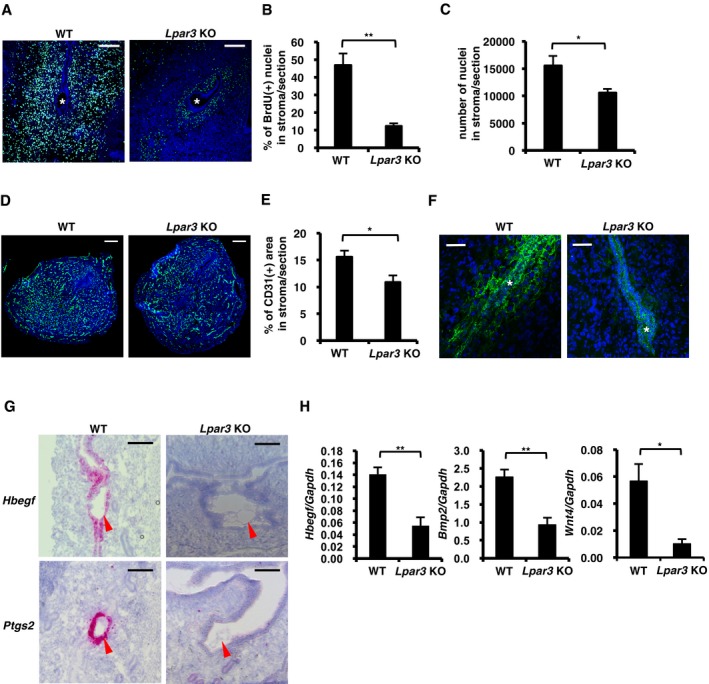
-
A–F(A, D and F) Immunostaining of BrdU (cell proliferation on 4.5 dpc, A), CD31 (angiogenesis on 5.5 dpc, D), and E‐cadherin (LE‐breakdown on 5.5 dpc, F) in pregnant uteri showed that decidual reactions were poorly occurred in Lpar3 KO mice. For BrdU IHC, pregnant mice were treated with BrdU 2 h before the dissection. In each image, nuclei were counterstained with DAPI (blue). Asterisks indicate the position of embryos. (B) Number of BrdU (+) nuclei in the stromal layer per section in (A) was counted by ImageJ software (n = 5 for each bar). (C) Number of nuclei in the stromal layer per section on 5.5 dpc was counted by ImageJ software (n = 5 for WT and n = 7 for Lpar3 KO uteri). (E) CD31(+) area in the stromal layer per section in (D) was calculated by ImageJ software (n = 5), showing reduced angiogenesis in Lpar3 KO pregnant uteri.
-
G, HThe expression of Hbegf, Ptgs2, Bmp2, and Wnt4 were significantly lower in Lpar3 KO pregnant uteri. (G) Representative ISH images of Hbegf and Ptgs2 in Lpar3 KO pregnant uteri on 4.0 dpc. Arrowheads indicate the position of embryos. (H) qRT–PCR quantification of Hbegf, Bmp2 and Wnt4 mRNAs in Lpar3 KO pregnant uteri on 4.0 dpc (Hbegf, n = 7 for WT and n = 12 for Lpar3 KO uteri) and 5.5 dpc (Bmp2 and Wnt4, n = 7 for WT and n = 9 for Lpar3 KO uteri).
A remaining question is how LPA is produced in the vicinity of the embryos. It is possible that LPA is derived from embryos and/or uteri. Blastocysts null for either Enpp2 [encoding ATX) or Liph (encoding phosphatidic acid‐preferential phospholipase A1α (PA‐PLA1α, another LPA‐producing enzyme)] implanted normally (Tanaka et al, 2006; Inoue et al, 2011), suggesting that embryonic ATX and PA‐PLA1α are not involved in implantation. In addition, Liph KO female mice showed normal reproductive activity (Inoue et al, 2011). By contrast, the role of maternal ATX remains to be determined, since Enpp2 null mice were embryonic lethal (Tanaka et al, 2006). Anti‐ATX staining showed that ATX was predominantly expressed in the uteri and localized throughout the epithelial layer (Fig 7A). Administration of an ATX inhibitor (S15‐00826, Fig EV7A–E) into the uterine cavity of pregnant mice resulted in abnormal embryo spacing and implantation failure at 5.5 dpc (Fig 7B–D), as was observed in Lpar3 KO uteri (Ye et al, 2005; Hama et al, 2007). The expression levels of Hbegf, Ptgs2, Bmp2 and Wnt4 were also reduced by the ATX inhibitor (Fig 7E and F). These results indicate that epithelially expressed ATX is responsible for the activation of LPA3 during the peri‐implantation period.
Figure 7. Autotaxin is responsible for LPA 3 in uteri during early pregnancy.
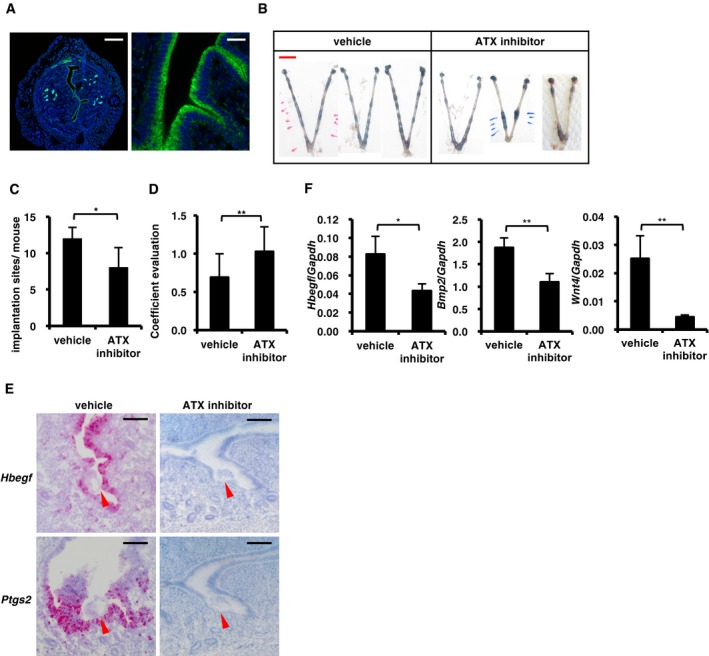
-
AImmunostaining of ATX in WT pregnant uteri at 3.5 dpc. The signal was highly detected in the luminal epithelium.
-
B–DEmbryo implantation was impaired by the inhibition of ATX like Lpar3 KO uteri. (B) Representative photographs of pregnant uteri on 5.5 dpc from mice injected with ATX inhibitor at 3.5 dpc. (C and D) The number of implantation sites (C) and the degree of equidistance (D) in (B) (n = 7 for vehicle and n = 9 for ATX inhibitor).
-
E, FThe expression of Hbegf, Ptgs2, Bmp2, and Wnt4 were significantly lower in pregnant uteri treated with the ATX inhibitor. (E) Representative ISH images of Hbegf and Ptgs2 in pregnant uteri on 4.0 dpc injected with the ATX inhibitor at 3.5 dpc. Arrowheads indicate the position of embryos. (F) qRT–PCR quantification of Hbegf, Bmp2, and Wnt4 mRNAs in pregnant uteri on 4.0 dpc (Hbegf, n = 8 for vehicle and n = 10 for ATX inhibitor) and 5.5 dpc (Bmp2 and Wnt4, n = 15 for vehicle and n = 16 for ATX inhibitor) after treated with the ATX inhibitor at 3.5 dpc.
Figure EV7. The character of the ATX inhibitor.
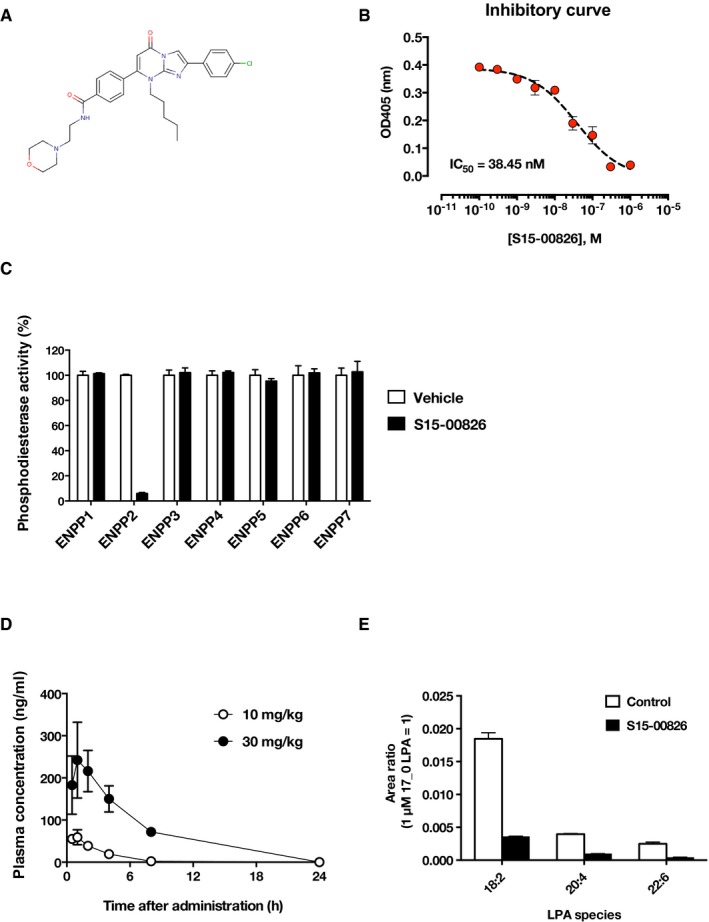
- The chemical structure of S15‐00826.
- Inhibitory curve of recombinant ATX by S15‐00826. Data are means ± SD (n = 3). The IC50 value for S15‐00826 is ˜38 nM in ATX assay using p‐nitrophenyl TMP as a substrate.
- The selectivity of S15‐00826 was evaluated using phosphodiesterase activity assay. Among the six ENPP family members, S15‐00826 inhibited the activity of ENPP2 (= ATX) selectively. A single data point was measured by two biological replicates. Data are means + SD.
- Pharmacokinetics in mice. The compound was p.o. administered to mice and collected bloods were analyzed in LC‐MS/MS (n = 3 for each bar). Data are means ± SD.
- The effect of S15‐00826 on circulating LPA in mice. The compound was administered to mice (20 mg/kg, i.p.), and plasma LPA level was determined by LC‐MS/MS analysis. Data are means + SD (n = 3).
Discussion
Our results indicate that LPA is produced in a maternal ATX‐dependent manner and present in the vicinity of the embryo, then activates LPA3 in the epithelial layer (Fig 8). We tried to detect LPA in the eggs as well as in the luminal fluid using LC‐MS/MS. While we could not detect LPA in the eggs, small amount of LPA (0.1–0.2 nM) was found in the uterine flushing fluids from the pregnant mice (Appendix Fig S3). Interestingly, LPA with an unsaturated fatty acid (oleic or linoleic acid), a potent ligand for LPA3 (Bandoh et al, 2000), was detected when the uteri were flushed with the saline containing albumin which is capable of extracting lysophospholipids from outer leaflet of the cells (Okudaira et al, 2014; Appendix Fig S3). LPA was hardly recovered in the albumin‐free flushing fluids (Appendix Fig S3), indicating clearly that LPA is present in the extracellular milieu. The estimated egg volume is ~6 × 10−14 m3 (provided that the diameter of the egg is 50 μm), while the volume of uterine cavity is ~5 × 10−9 m3: i.e., the approximate ratio of them = 1:105. Assuming that LPA is produced only in the embryo‐epithelial boundary, we can estimate that a high concentration of LPA enough to activate LPA3 (normally μM order) is present there. Since ATX is present almost exclusively in the epithelial layer (Fig 7A), ATX substrates should be either derived from embryos or produced as a result of embryo‐epithelial interactions. Interestingly, lysophosphatidylcholine (LPC) species with an unsaturated fatty acid (oleic or linoleic acid), one of the preferable ATX substrates (Nishimasu et al, 2011), were detected in blastocysts on 3.5 dpc by LC‐MS/MS (Appendix Fig S4). This is consistent with a report that the luminal epithelium surrounding embryos at 3.5 dpc was rich in unsaturated fatty acid‐containing phosphatidylcholine (Burnum et al, 2009), the most likely precursor of unsaturated LPC. These data raise the possibility that embryo‐derived LPC is converted to LPA by the epithelial ATX, leading to the activation of LPA3.
Figure 8. Proposed model for LPA‐induced decidualization through ATX–LPA 3 axis.
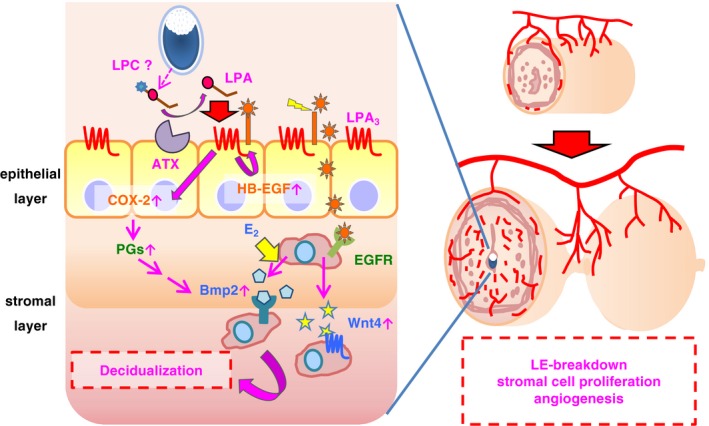
LPA is produced at the embryo‐epithelial boundary in ATX‐dependent manner and then activates epithelial LPA3. Embryos are the possible source of LPC. LPA3 activation induces heparin‐binding EGF (HB‐EGF) and cyclooxygenase‐2 (COX‐2) in the epithelial layer, which up‐regulates Bmp2 and Wnt4 cooperatively with estrogen (E2) signaling. Totally, LPA3 signaling contributes LE‐breakdown, angiogenesis, and cell proliferation in the stromal layer.
The present results also demonstrate a novel mechanism for GPCR‐induced cell growth in which LPA3 is the key player. In our results, activation of LPA3 in the epithelial layer leads to enhanced production of HB‐EGF and COX‐2, which then induces stromal cell growth in an EGFR‐Bmp2/Wnt4‐dependent manner. GPCRs such as β1, AT1, and LPA6 were previously found to be co‐expressed with EGFR in epithelial cells and to activate (actually transactivate) EGFR by stimulating the ectodomain‐shedding of membrane bound EGF precursors (Zhai et al, 2006; Noma et al, 2007; Inoue et al, 2011). Interestingly, activation of LPA3 also induced ectodomain‐shedding of HB‐EGF in vitro (Appendix Fig S5), which indicates that the LPA3 signaling leads to the activation of EGF signaling not only by up‐regulating the expression of HB‐EGF but also by ectodomain‐shedding of HB‐EGF and the following transactivation of EGFR.
The expression of LPA3 in female reproductive tissues is conserved in mammals. Indeed, in mouse, sheep, pig and human, LPA3 was expressed in the uterine epithelial layer in a female sex hormone‐dependent manner (Hama et al, 2006; Kamińska et al, 2008; Liszewska et al, 2012; Guo et al, 2013). In addition, ATX and LPA were detected in the reproductive biological fluids such as follicular fluids and uterine luminal fluids (Liszewska et al, 2009; Seo et al, 2012; Yamamoto et al, 2016). Thus, LPA3 appears to regulate the female reproductive systems in wide range of mammalian species including human, although there are some slight differences in the process of decidualization between species (Cha et al, 2012).
Our findings may lead to new treatments for infertility. In in vitro fertilization (IVF), endometrial thickness is highly correlated with the success rate of implantation and pregnancy (Noyes et al, 1995; Lim & Wang, 2010). Interestingly, patients with recurrent implantation failure in IVF treatment have reduced LPA3 levels (Achache et al, 2010). Thus, treatment of human females with an LPA3 agonist may increase the pregnancy rate by promoting the endometrial cell growth and increasing the endometrial thickness in IVF. This idea is supported by the facts that expression of Lpar3 in mice is up‐ and down‐regulated by P4 and E2, respectively (Hama et al, 2006) and that the balance of female sex hormones is also critical for establishment of pregnancy in both species (Lim & Wang, 2010; Ramathal et al, 2010; Cha et al, 2012).
HB‐EGF, COX‐2, and Wnt4 have been implicated in the progression of endometriosis, the abnormal growth of endometrial tissues outside the uterus (Ota et al, 2001; Uno et al, 2010; Arosh et al, 2015; Miller et al, 2015). Interestingly, in our mouse endometriosis model, endometrial tissues from Lpar3 KO mice were significantly less developed (Appendix Fig S6). Thus, LPA–LPA3 signaling is critical in the development of endometriosis. Furthermore, HB‐EGF, COX‐2, and Wnt4 as well as Bmp2 have been shown to be risk factors for sex hormone‐dependent diseases, such as prostatic hyperplasia, breast cancer, and ovarian cancer (Bentley et al, 1992; Ferrandina et al, 2002; Levin, 2003; Zong et al, 2012). Since LPA3 is highly expressed in the prostate, mammary gland, and ovary (Bandoh et al, 1999), LPA3 signaling might contribute to the progression of such diseases.
In summary, we propose a novel mechanism for LPA3‐mediated decidualization during the peri‐implantation period as illustrated in Fig 8. (i) LPA is produced in the vicinity of the embryo in an ATX‐dependent manner; (ii) LPA thus generated activates LPA3 in the epithelial layer; (iii) activation of LPA3 up‐regulates HB‐EGF and COX‐2 in the epithelial layer; (iv) Both Bmp2 and Wnt4 are induced in the stromal layer via activation of EGFR, COX‐2, and ERα, leading to the decidual reactions including LE‐breakdown, stromal proliferation, and angiogenesis. Because expression of LPA3 and LPA3 signaling is highly dependent on female sex hormones (P4 and E2, respectively; Hama et al, 2006; Diao et al, 2015), this mechanism may operate not only in female reproduction but also in pathological diseases such as endometriosis and female reproductive cancers.
Materials and Methods
Reagents
LPA (1‐oleoyl (18:1)) was purchased from Avanti Polar Lipids. A potent LPA3 agonist T13 was synthesized as described previously (Tamaruya et al, 2004; Kano et al, 2008; Hama & Aoki, 2010). LPA and T13 were dried under nitrogen gas and dissolved in 0.01% fatty acid‐free bovine serum albumin (Sigma‐Aldrich)‐PBS using water bath sonication and stocked in −20°C. COX‐2 selective inhibitor (Celecoxib) was from TCI. EGFR inhibitor (AST1306), BMPR inhibitor (LDN193189), and Tankyrase inhibitor (XAV939) were obtained from Adooq Bioscience. Estrogen receptor (ERα) antagonist (fulvestrant) was obtained from Sigma‐Aldrich. All inhibitors and the ERα antagonist were dissolved in DMSO and stored at −20°C. ATX inhibitor (S15‐00826) was kindly donated by Shionogi pharmaceutical company. The selectivity toward ENPP family members and pharmacokinetics of the ATX inhibitor was shown in Fig EV7. Other chemicals were purchased from Wako Pure Chemical Industries unless otherwise indicated.
Antibodies
Anti‐autotaxin (ATX) polyclonal antibody (pAb) made in guinea pig was a kind gift from Drs Masahiko Watanabe and Masanori Tachikawa (Hokkaido University, Japan). The following antibodies were purchased from distributors: anti‐mouse CD31 (PECAM) monoclonal antibody (mAb) made in rat (BD Pharmingen); anti‐BrdU mAb conjugated with fluorescein made in mouse (Roche); anti‐mouse E‐cadherin mAb made in Rabbit (CST); anti‐guinea pig, anti‐rat, anti‐fluorescein secondary antibodies (biotinylated; Vector); anti‐rabbit secondary antibody‐Alexa488 (Invitrogen). ATX, CD31, and BrdU were detected using biotinylated secondary antibody followed by TSA Alexa Fluor 488 kit (Invitrogen).
Mice
ICR mice were purchased from CLEA Japan. Lpar3 −/− mice (ICR background) were generated by crossing C57BL/6J Lpar3 +/− males with ICR wild‐type females over eight times.
TGFα shedding assay
TGFα shedding assay was performed as described previously (Inoue et al, 2012). Briefly, HEK293 cells were seeded in 12‐well dishes at a density of 2 × 105 cells/dish and cultured at 37°C for 24 h. Then, the cells were transfected with cDNAs encoding alkaline phosphatase (AP)‐tagged TGFα (AP‐TGFα, 0.25 μg), LPA receptors (each 0.1 μg) using Lipofectamine 2000 as transfection reagent. The cells were seeded in 96‐well plate at a density of 4 × 105 cells/ml (90 μl) in HBSS containing 5 mM HEPES (pH 7.4). After incubation at 37°C for 30 min, the cells were stimulated with LPA or T13 and incubated at 37°C for 1 h. After 1 h, the cells centrifuged at 190 g for 3 min and supernatant (80 μl) was moved into a new 96‐well plate. An amount of 80 μl of 10 mM p‐nitrophenyl phosphate (pNPP) in 2× pNPP buffer [40 mM Tris–HCl (pH 9.5)], 40 mM NaCl and 10 mM MgCl2)/well was added to the supernatant and the cells, and measured at optical density at 405 nm (OD405). After incubation at 37°C for 1 h, both the supernatant and the cells were measured at OD405. TGFα shedding activity was calculated by OD405 of 0 and 1 h.
Intrauterine injection of T13 and inhibitors
Adult females (8–12 weeks) were mated with vasectomized males. The day a plug was found after mating was designed as 0.5 dpc of pseudopregnancy. At 3.5 dpc, females were anesthetized with three types of reagent cocktail (M/M/B; 0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol) i.p. and then subjected intrauterine injection, as previously described (Wu & Gu, 1981). Briefly, 5 μl/horn of T13 solution (1 mM) or 0.01% BSA‐PBS was injected into the uterine cavity adjacent to the ovary. Inhibitors for EGFR, BMPR, or Tankyrase (100 μM of final concentration) were mixed with T13 solution before injection, respectively. ERα antagonist (100 μg/mouse in 100 μl DMSO) or COX‐2 inhibitor (10 mg/mouse in 100 μl DMSO) was subcutaneously injected into mice 3 h before or just before T13 injection, respectively. The mice were intravenously injected with 200 μl of 1% Evans Blue solution to visualize decidualized area at 5.5 dpc.
Oil infusion
Corn oil was infused intraluminally into the uterine horns as described previously (Chen et al, 2011) with some modifications. Briefly, 50 μl corn oil was infused at 3.5 dpc of pseudopregnancy. On 5.5 dpc, the mice were intravenously injected with 200 μl of 1% Evans Blue solution to detect deciduoma, and uterine weights were recorded to assess the extent of decidualization.
Evaluation of implantation in mice treated with autotaxin inhibitor
Adult female mice were mated with fertile WT males. At 3.5 dpc, females were injected with 5 μl/horn of autotaxin inhibitor (1 mM) or vehicle (3.3% DMSO in PBS) in a similar manner as T13 injection. On 5.5 dpc, mice were injected with 1% Evans Blue solution to visualize embryo implantation sites. The distance between each blastocyst was calculated by National Institutes of Health Image software. The coefficient of evaluation (= the degree of equidistance) was calculated by dividing the standard deviation by the mean distance between blastocysts in a horn.
Immunohistochemistry
Uteri were fixed in 4% formaldehyde in phosphate‐buffered saline (PBS) at 4°C overnight and embedded in O.C.T. compound (Sakura Finetek). Tissues were cryosectioned at 8 μm. The cryosections were plated on MAS‐coated glass slides (Matsunami Glass), blocked in IgG from the same animal species of the secondary antibody for 30 min at room temperature, incubated at 4°C overnight with primary antibodies, incubated with biotinylated secondary antibody and TSA Alexa Fluor 488 kit (Invitrogen) for 5 min, counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI, Sigma‐Aldrich) to reveal the nuclei and photographed with a Zeiss LSM700 confocal fluorescence microscope. Number of nuclei and CD31‐positive area were calculated by ImageJ (National Institutes of Health). For Figs 2A–C (upper panel), 5B, 6A and D, the brightness and contrast was changed using ImageJ (National Institutes of Health).
Bromo‐deoxyuridine (BrdU) in vivo incorporation assay
Females were injected with BrdU (100 mg per kg body weight) on the morning of 3.5 dpc (10:00). The mice were killed 2 h after injection, and the uteri were freshly embedded in O.C.T. compound (Sakura Finetek). The embedded uteri were cryosectioned, and the sections were fixed in methanol for 10 min at room temperature and immersed in 2N HCl at 37°C to denature DNA for immunohistochemical detection.
Quantitative RT–PCR analysis
Total RNA from whole uterine tissues was isolated with a GenElute Mammalian Total RNA Miniprep kit (Sigma‐Aldrich) and then reverse‐transcribed with a High‐Capacity cDNA RT Kits (Applied Biosystems) according to the manufacturer's instructions. PCRs were performed with SYBR Premix Ex Taq II (Takara Bio) and were monitored by ABI Prism 7300 (Applied Biosystems). Standard plasmids ranging from 103 to 108 copies per well were used to quantify the absolute number of transcripts of cDNA samples. The numbers of transcripts were normalized to the number of a house‐keeping gene, Gapdh, in the same sample. PCR was performed using the following primers: 5′‐TGACCCCAGCTCAGGGAAAG‐3′ and 5′‐GGGCTTAATCACCTGTTCAACTCTG‐3′ for Areg; 5′‐GATCTGTACCGCAGGCACTC‐3′ and 5′‐CCACGGCTTCTTCGTGATGG‐3′ for Bmp2; 5′‐ACTACAGGACTCGGAAGCAG‐3′ and 5′‐AAGGTTGGGGACAAGAAGCC‐3′ for Egf; 5′‐GACGCTGCTTTGTCTAGGTTC‐3′ and 5′‐CACACGGGGATCGTCTTCC‐3′ for Ereg; 5′‐AGGAGCGAGACCCCACTAAC‐3′ and 5′‐CGGAGATGATGACCCTTTTG‐3′ for Gapdh; 5′‐TGCTGCCGTCGGTGATG‐3′ and 5′‐CAGACTCTCACCGGTCACCAA‐3′ for Hbegf; 5′‐AAGCGAGGACCTGGGTTCA‐3′ and 5′‐AAGGCGCAGTTTATGTTGTCTGT‐3′ for Ptgs2; 5′‐GCGAGCAATTGGCTGTAC‐3′ and 5′‐TTGTGTCACCACCTTCCCA‐3′ for Wnt4.
RNA in situ hybridization
Uteri were fixed overnight in 4% (w/v) paraformaldehyde at 4°C and then embedded in O.C.T. compound (Sakura Finetek). The tissues were cryosectioned at 8 μm and plated on MAS‐coated glass slides (Matsunami Glass) and processed for RNA in situ detection using the RNAscope 2.0 High Definition RED kit according to manufacturer's instructions (ACDBio).
LC‐MS/MS analysis of prostaglandins and related lipids
T13‐injected uteri were collected 9 h after the injection and processed for LC‐MS‐/MS‐based lipidomics analyses as previously described (Arita, 2012). MS/MS analyses were performed in negative ion mode. Arachidonic acid‐derived metabolites were identified and quantified by multiple reaction monitoring.
LC‐MS/MS analysis of LPA
To obtain a plasma sample, a blood sample was collected 1 h after the administration of ATX inhibitor (20 mg/kg, i.p.) and then centrifuged at 1,500 g for 5 min. Quantification of LPA was performed as previously described (Kato et al, 2016) using the highly sensitive LC‐MS/MS system.
Microarray analysis
The quantity and quality of total RNA were determined with an Agilent 2100 Bioanalyzer (RNA Nano 6000) and Nanodrop, respectively. For each of six time points (0.5, 1, 2, 24, 30, and 36 h after the T13 injection) and two conditions (vehicle control and T13‐injected), total RNA was prepared from three individuals and pooled. DNA microarray analyses were carried out using Whole mouse genome oligo DNA microarray kit ver2.0 (4×44 K; Agilent Technology, for time points 0.5, 1, and 2 h) and GeneChip Mouse Gene 2.0 ST Array (Affymetrix, for time points 24, 30, and 36 h) according to the manufacturer's protocol. Up‐regulated and down‐regulated genes were defined as those whose expression levels were increased and decreased, respectively, by a factor of at least 2. Biological networks and pathways were analyzed with Ingenuity Pathway Analysis (IPA) software (Qiagen, CA, USA). The data have been deposited to the NCBI GEO database, with accession no. GSE87116 (0.5–2 h after the T13 injection) and GSE87161 (24–36 h after the T13 injection). The raw data of uterine‐specific Egfr or Wnt4 KO were kindly disclosed by the corresponding author of the previous article (Large et al, 2014). The data of Bmp2 (Lee et al, 2007; accession no. GSE10193), Egfr, or Wnt4 KO were analyzed using R (https://www.R-project.org/). Venn diagrams were created by Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html).
Pharmacokinetics of T13 and ATX inhibitor
T13 (10 nmol) was injected into uteri, and then, uteri were collected 0, 3, and 6 h after the injection. 20–30 mg of uterine tissues was homogenized in methanol. For the analysis of the ATX inhibitor, the inhibitor was injected to mice p.o. (10 or 30 mg/kg), and then, bloods were collected. Collected samples were processed for LC‐MS/MS as previously performed (Okudaira et al, 2014).
Phosphodiesterase activity assay
Phosphodiesterase activity was measured colorimetrically as previously described (Sakagami et al, 2005) with some modifications. Each mouse ENPP protein was transiently expressed in HEK293A cells using Lipofectamine 2000 as transfection reagent. For the analysis of the specificity of ATX inhibitor to ENPP proteins, culture supernatants were incubated with substrates, p‐nitrophenyl thymidine monophosphate (4 mM, pNP‐TMP, for ENPP1‐5) or p‐nitrophenylphosphorylcholine (4 mM, pNP‐PC, for ENPP6, 7), in the presence or absence of the ATX inhibitor (1 μM) in a 96‐well microplate at 37°C for 30 min. The amount of p‐nitrophenolate (p‐NP) was determined by the absorbance at 405 nm with a SpectraMax190 microplate reader (Molecular Devices). The phosphodiesterase activity was calculated assuming the inhibitor‐free absorbance as 100% activity. For the determination of IC50 value, ENPP2 protein was incubated with 1 mM pNP‐TMP in the presence of ATX inhibitor for 40 min. A four‐parameter sigmoid curve for IC50 value was fitted to concentration−response plots using GraphPad Prism 6 (GraphPad, USA).
Statistical analysis
The significance of differences between groups was determined by Student's t‐test or a multiway analysis of variance (ANOVA) followed by a Bonferroni post hoc analysis using GraphPad Prism 6 (GraphPad).
Study approval
Mice were maintained according to the Guidelines for Animal Experimentation of Tohoku University, and the protocol was approved by the Institutional Animal Care and Use Committee at Tohoku University (Approval number: 2014PhLMO‐018).
Author contributions
SA and KK designed the study and carried out most of the experiments. SA wrote the draft of the manuscript. JW, KK, and DS performed the LC‐MS/MS experiments. TN, TF, MK, HI, and YY designed the endometriosis experiment and gave many useful comments. YH designed the experiments on decidual reactions. ST, YT, YS, and MM designed and carried out the experiment on DNA microarray. MA designed and performed mediator lipidomics experiment. JC provided the LPA3 KO mice and gave many useful comments. JA supervised all aspects of the study, including experimental design, discussion, data interpretation, and modified the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 2
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
We thank Shionogi pharmaceutical Co., Ltd for the gift of the ATX inhibitor. We also thank Dr Yasumasa Nishito (Core Technology and Research Center, Tokyo Metropolitan Institute of Medical Science) for DNA microarray analysis. The present work was supported partly by AMED‐CREST (Japan Agency for Medical Research and Development, Core Research for Evolutional Science and Technology) for J.A. and M.M., PRESTO (Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology) for A.I., Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant‐in‐Aid for Scientific Research for J.A. and M.M.
The EMBO Journal (2017) 36: 2146–2160
References
- Achache H, Tsafrir A, Prus D, Reich R, Revel A (2010) Defective endometrial prostaglandin synthesis identified in patients with repeated implantation failure undergoing in vitro fertilization. Fertil Steril 94: 1271–1278 [DOI] [PubMed] [Google Scholar]
- Aikawa S, Hashimoto T, Kano K, Aoki J (2015) Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem 157: 81–89 [DOI] [PubMed] [Google Scholar]
- Arita M (2012) Mediator lipidomics in acute inflammation and resolution. J Biochem 152: 313–319 [DOI] [PubMed] [Google Scholar]
- Arosh JA, Lee J, Balasubbramanian D, Stanley JA, Long CR, Meagher MW, Osteen KG, Bruner‐Tran KL, Burghardt RC, Starzinski‐Powitz A, Banu SK (2015) Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc Natl Acad Sci USA 112: 9716–9721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami‐Murofushi K, Tsujimoto M, Arai H, Inoue K (1999) Molecular cloning and characterization of a novel human G‐protein‐coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem 274: 27776–27785 [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K (2000) Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure‐activity relationship of cloned LPA receptors. FEBS Lett 478: 159–165 [DOI] [PubMed] [Google Scholar]
- Bentley H, Hamdy FC, Hart KA, Seid JM, Williams JL, Johnstone D, Russell RG (1992) Expression of bone morphogenetic proteins in human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer 66: 1159–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnum KE, Cornett DS, Puolitaival SM, Milne SB, Myers DS, Tranguch S, Brown HA, Dey SK, Caprioli RM (2009) Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J Lipid Res 50: 2290–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18: 1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Peng H, Lei L, Kuang H, Zhang L, Ning L, Cao Y, Duan E (2011) Transient {beta}2‐adrenoceptor activation confers pregnancy loss by disrupting embryo spacing at implantation. J Biol Chem 286: 4349–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H, Li R, El Zowalaty AE, Xiao S, Zhao F, Dudley EA, Ye X (2015) Deletion of lysophosphatidic acid receptor 3 (Lpar3) disrupts fine local balance of progesterone and estrogen signaling in mouse uterus during implantation. Biol Reprod 93: 123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandina G, Lauriola L, Zannoni GF, Fagotti A, Fanfani F, Legge F, Maggiano N, Gessi M, Mancuso S, Ranelletti FO, Scambia G (2002) Increased cyclooxygenase‐2 (COX‐2) expression is associated with chemotherapy resistance and outcome in ovarian cancer patients. Ann Oncol 13: 1205–1211 [DOI] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ (2011) WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25: 1176–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Gong F, Luo KL, Lu GX (2013) Cyclic regulation of LPA3 in human endometrium. Arch Gynecol Obstet 287: 131–138 [DOI] [PubMed] [Google Scholar]
- Hama K, Aoki J (2010) LPA(3), a unique G protein‐coupled receptor for lysophosphatidic acid. Prog Lipid Res 49: 335–342 [DOI] [PubMed] [Google Scholar]
- Hama K, Aoki J, Bandoh K, Inoue A, Endo T, Amano T, Suzuki H, Arai H (2006) Lysophosphatidic receptor, LPA3, is positively and negatively regulated by progesterone and estrogen in the mouse uterus. Life Sci 79: 1736–1740 [DOI] [PubMed] [Google Scholar]
- Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, Kanai M, Ye X, Chun J, Matsuki N, Suzuki H, Shibasaki M, Arai H (2007) Embryo spacing and implantation timing are differentially regulated by LPA3‐mediated lysophosphatidic acid signaling in mice. Biol Reprod 77: 954–959 [DOI] [PubMed] [Google Scholar]
- Hayashi R, Inoue A, Suga Y, Aoki J, Shimomura Y (2015) Analysis of unique mutations in the LPAR6 gene identified in a Japanese family with autosomal recessive woolly hair/hypotrichosis: establishment of a useful assay system for LPA6. J Dermatol Sci 78: 197–205 [DOI] [PubMed] [Google Scholar]
- Inoue A, Arima N, Ishiguro J, Prestwich GD, Arai H, Aoki J (2011) LPA‐producing enzyme PA‐PLA1α regulates hair follicle development by modulating EGFR signalling. EMBO J 30: 4248–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Ishiguro J, Kitamura H, Arima N, Okutani M, Shuto A, Higashiyama S, Ohwada T, Arai H, Makide K, Aoki J (2012) TGFα shedding assay: an accurate and versatile method for detecting GPCR activation. Nat Methods 9: 1021–1029 [DOI] [PubMed] [Google Scholar]
- Kamińska K, Wasielak M, Bogacka I, Blitek M, Bogacki M (2008) Quantitative expression of lysophosphatidic acid receptor 3 gene in porcine endometrium during the periimplantation period and estrous cycle. Prostaglandins Other Lipid Mediat 85: 26–32 [DOI] [PubMed] [Google Scholar]
- Kano K, Arima N, Ohgami M, Aoki J (2008) LPA and its analogs‐attractive tools for elucidation of LPA biology and drug development. Curr Med Chem 15: 2122–2131 [DOI] [PubMed] [Google Scholar]
- Kato K, Ikeda H, Miyakawa S, Futakawa S, Nonaka Y, Fujiwara M, Okudaira S, Kano K, Aoki J, Morita J, Ishitani R, Nishimasu H, Nakamura Y, Nureki O (2016) Structural basis for specific inhibition of Autotaxin by a DNA aptamer. Nat Struct Mol Biol 23: 395–401 [DOI] [PubMed] [Google Scholar]
- Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadgill DW, Lydon JP, Jeong JW, DeMayo FJ (2014) The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet 10: e1004451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ (2007) Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27: 5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (2003) Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 17: 309–317 [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC (2013) WNT4 acts downstream of BMP2 and functions via β‐catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology 154: 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK (1997) Multiple female reproductive failures in cyclooxygenase 2‐deficient mice. Cell 91: 197–208 [DOI] [PubMed] [Google Scholar]
- Lim HJ, Wang H (2010) Uterine disorders and pregnancy complications: insights from mouse models. J Clin Invest 120: 1004–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewska E, Reinaud P, Billon‐Denis E, Dubois O, Robin P, Charpigny G (2009) Lysophosphatidic acid signaling during embryo development in sheep: involvement in prostaglandin synthesis. Endocrinology 150: 422–434 [DOI] [PubMed] [Google Scholar]
- Liszewska E, Reinaud P, Dubois O, Charpigny G (2012) Lysophosphatidic acid receptors in ovine uterus during estrous cycle and early pregnancy and their regulation by progesterone. Domest Anim Endocrinol 42: 31–42 [DOI] [PubMed] [Google Scholar]
- Miller MA, Moss ML, Powell G, Petrovich R, Edwards L, Meyer AS, Griffith LG, Lauffenburger DA (2015) Targeting autocrine HB‐EGF signaling with specific ADAM12 inhibition using recombinant ADAM12 prodomain. Sci Rep 5: 15150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimasu H, Okudaira S, Hama K, Mihara E, Dohmae N, Inoue A, Ishitani R, Takagi J, Aoki J, Nureki O (2011) Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat Struct Mol Biol 18: 205–212 [DOI] [PubMed] [Google Scholar]
- Nishioka T, Arima N, Kano K, Hama K, Itai E, Yukiura H, Kise R, Inoue A, Kim SH, Solnica‐Krezel L, Moolenaar WH, Chun J, Aoki J (2016) ATX‐LPA1 axis contributes to proliferation of chondrocytes by regulating fibronectin assembly leading to proper cartilage formation. Sci Rep 6: 23433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki‐Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA (2007) Beta‐arrestin‐mediated beta1‐adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 117: 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes N, Liu HC, Sultan K, Schattman G, Rosenwaks Z (1995) Endometrial thickness appears to be a significant factor in embryo implantation in in‐vitro fertilization. Hum Reprod 10: 919–922 [DOI] [PubMed] [Google Scholar]
- Okudaira M, Inoue A, Shuto A, Nakanaga K, Kano K, Makide K, Saigusa D, Tomioka Y, Aoki J (2014) Separation and quantification of 2‐acyl‐1‐lysophospholipids and 1‐acyl‐2‐lysophospholipids in biological samples by LC‐MS/MS. J Lipid Res 55: 2178–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Igarashi S, Sasaki M, Tanaka T (2001) Distribution of cyclooxygenase‐2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod 16: 561–566 [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL (2001) Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA 98: 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S, Laws MJ, Bagchi IC, Bagchi MK (2015) Uterine epithelial estrogen receptor‐α controls decidualization via a paracrine mechanism. Mol Endocrinol 29: 1362–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK (2010) Endometrial decidualization: of mice and men. Semin Reprod Med 28: 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami H, Aoki J, Natori Y, Nishikawa K, Kakehi Y, Arai H (2005) Biochemical and molecular characterization of a novel choline‐specific glycerophosphodiester phosphodiesterase belonging to the nucleotide pyrophosphatase/phosphodiesterase family. J Biol Chem 280: 23084–23093 [DOI] [PubMed] [Google Scholar]
- Seo H, Choi Y, Shim J, Kim M, Ka H (2012) Analysis of the lysophosphatidic acid‐generating enzyme ENPP2 in the uterus during pregnancy in pigs. Biol Reprod 87: 77 [DOI] [PubMed] [Google Scholar]
- Sheng X, Yung YC, Chen A, Chun J (2015) Lysophosphatidic acid signalling in development. Development 142: 1390–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK (2002) Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on‐time’ embryo implantation that directs subsequent development. Development 129: 2879–2889 [DOI] [PubMed] [Google Scholar]
- Tamaruya Y, Suzuki M, Kamura G, Kanai M, Hama K, Shimizu K, Aoki J, Arai H, Shibasaki M (2004) Identifying specific conformations by using a carbohydrate scaffold: discovery of subtype‐selective LPA‐receptor agonists and an antagonist. Angew Chem Int Ed Engl 43: 2834–2837 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H (2006) Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 281: 25822–25830 [DOI] [PubMed] [Google Scholar]
- Umezu‐Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 158: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y (2010) A genome‐wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet 42: 707–710 [DOI] [PubMed] [Google Scholar]
- Wang H, Ma WG, Tejada L, Zhang H, Morrow JD, Das SK, Dey SK (2004) Rescue of female infertility from the loss of cyclooxygenase‐2 by compensatory up‐regulation of cyclooxygenase‐1 is a function of genetic makeup. J Biol Chem 279: 10649–10658 [DOI] [PubMed] [Google Scholar]
- Wu JT, Gu Z (1981) The effect of intrauterine injection of concanavalin A on implantation in mice and rats. Contraception 23: 667–675 [DOI] [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK (2007) Maternal heparin‐binding‐EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA 104: 18315–18320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Omura M, Tuchiya K, Hidaka M, Kuwahara A, Irahara M, Tanaka T, Tokumura A (2016) Preferable existence of polyunsaturated lysophosphatidic acids in human follicular fluid from patients programmed with in vitro fertilization. Prostaglandins Other Lipid Mediat 126: 16–23 [DOI] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J (2005) LPA3‐mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukiura H, Hama K, Nakanaga K, Tanaka M, Asaoka Y, Okudaira S, Arima N, Inoue A, Hashimoto T, Arai H, Kawahara A, Nishina H, Aoki J (2011) Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J Biol Chem 286: 43972–43983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Mirendil H, Chun J (2015) Lysophosphatidic Acid signaling in the nervous system. Neuron 85: 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J (2006) An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II‐mediated cardiac hypertrophy. Circ Res 99: 528–536 [DOI] [PubMed] [Google Scholar]
- Zong Y, Huang J, Sankarasharma D, Morikawa T, Fukayama M, Epstein JI, Chada KK, Witte ON (2012) Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling. Proc Natl Acad Sci USA 109: E3395–E3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 2
Source Data for Figure 5
Source Data for Figure 6


