Abstract
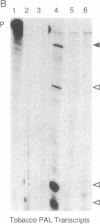
Biosynthesis of phenylpropanoid natural products in tobacco was perturbed by introduction of a heterologous (bean) phenylalanine ammonia-lyase (PAL; L-phenylalanine ammonia-lyase, EC 4.3.1.5) gene, modified by inclusion of cauliflower mosaic virus 35S enhancer sequences in its promoter. These transgenic plants can exhibit a series of unusual phenotypes including localized fluorescent lesions, altered leaf shape and texture, reduced signification in xylem, stunted growth, reduced pollen viability, and altered flower morphology and pigmentation. Genetic analysis of a transformant with severe symptoms showed that symptom development was inherited as a single, partially dominant trait and cosegregated with reduced levels of PAL activity and soluble phenylpropanoid products. Accumulation of transcripts encoded by the endogenous tobacco PAL genes was suppressed. We conclude that the transgene disrupts PAL regulation and that some of the phenotypes reflect interference with putative signals dependent on phenylpropanoid biosynthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey P. N., Chua N. H. Regulated genes in transgenic plants. Science. 1989 Apr 14;244(4901):174–181. doi: 10.1126/science.244.4901.174. [DOI] [PubMed] [Google Scholar]
- Bevan M., Shufflebottom D., Edwards K., Jefferson R., Schuch W. Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989 Jul;8(7):1899–1906. doi: 10.1002/j.1460-2075.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R. A semi-dominant allele, niv-525, acts in trans to inhibit expression of its wild-type homologue in Antirrhinum majus. EMBO J. 1988 Apr;7(4):877–883. doi: 10.1002/j.1460-2075.1988.tb02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkin K., Edwards R., Edington B., Dixon R. A. Stress Responses in Alfalfa (Medicago sativa L.): I. Induction of Phenylpropanoid Biosynthesis and Hydrolytic Enzymes in Elicitor-Treated Cell Suspension Cultures. Plant Physiol. 1990 Feb;92(2):440–446. doi: 10.1104/pp.92.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoisington D. A., Neuffer M. G., Walbot V. Disease lesion mimics in maize. I. Effect of genetic background, temperature, developmental age, and wounding on necrotic spot formation with Les1. Dev Biol. 1982 Oct;93(2):381–388. doi: 10.1016/0012-1606(82)90125-7. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Keller B., Templeton M. D., Lamb C. J. Specific localization of a plant cell wall glycine-rich protein in protoxylem cells of the vascular system. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1529–1533. doi: 10.1073/pnas.86.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini L. M., Bradford S., Rothstein S. Peroxidase-Induced Wilting in Transgenic Tobacco Plants. Plant Cell. 1990 Jan;2(1):7–18. doi: 10.1105/tpc.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Cramer C. L., Dixon R. A., Lamb C. J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem. 1989 Aug 25;264(24):14486–14492. [PubMed] [Google Scholar]
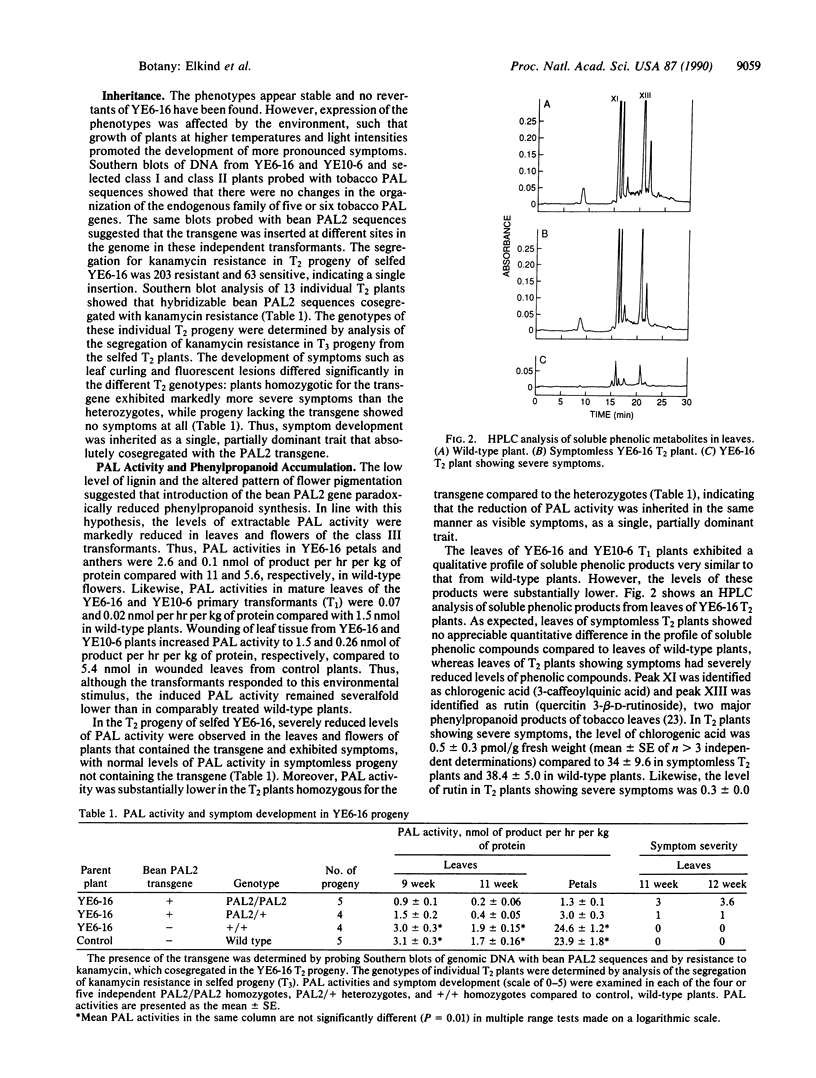
- Liang X. W., Dron M., Schmid J., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a phenylalanine ammonia-lyase-beta-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9284–9288. doi: 10.1073/pnas.86.23.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke M. A., Primig M., Trnovsky J., Matzke A. J. Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J. 1989 Mar;8(3):643–649. doi: 10.1002/j.1460-2075.1989.tb03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990 Apr;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schmid J., Doerner P. W., Clouse S. D., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a bean chalcone synthase promoter in transgenic tobacco. Plant Cell. 1990 Jul;2(7):619–631. doi: 10.1105/tpc.2.7.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmülling T., Schell J., Spena A. Single genes from Agrobacterium rhizogenes influence plant development. EMBO J. 1988 Sep;7(9):2621–2629. doi: 10.1002/j.1460-2075.1988.tb03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S. E., Wingate V. P., Lamb C. J. Dual control of phenylalanine ammonia-lyase production and removal by its product cinnamic acid. Eur J Biochem. 1982 Apr 1;123(2):389–395. doi: 10.1111/j.1432-1033.1982.tb19781.x. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Goldberg M. L. The Drosophila zeste gene and transvection. Trends Genet. 1989 Jun;5(6):189–194. doi: 10.1016/0168-9525(89)90074-7. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Mur L. A., Beld M., Mol J. N., Stuitje A. R. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990 Apr;2(4):291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]