Abstract
Hematopoietic Stem and Progenitor Cells (HSPCs) sustain life-long hematopoiesis and are first detected in the embryo by transplantation at embryonic day 10.5. (E10.5). HSPCs are mesodermal in origin and ultimately emerge from a subset of arterial endothelium (i.e. hemogenic endothelium), which is highly concentrated in the aorta-gonad-mesonephros region (AGM) of the mid-gestation embryo. Here, we employ clonal ex vivo assays in which endothelial cells isolated from the mid-gestation aorta and vitelline and umbilical arteries are co-cultured on supportive stroma to show that only about 0.1%, 1.3% and 0.29% of E9.5, E10.5 and E11.5 endothelium are functional hemogenic endothelium, respectively. We further show high phenotypic and functional variability in the hematopoietic potential of individual hemogenic endothelial precursors. Notably, by employing unique niche stroma capable of providing the signals necessary for definitive hematopoietic stem cell (dHSC) induction, we demonstrate this variability in hemogenic endothelium includes their potential to support phenotypic dHSCs. These data suggest the presence of either a continuum of maturing hemogenic endothelium with distinct hematopoietic potential or that hemogenic endothelium represents a heterogeneous pool of precursors that give rise to HSPCs with disparate hematopoietic potential.
Keywords: Hematopoietic progenitor cells, hemogenic endothelium, hematopoietic development
Introduction
Hematopoiesis arises in multiple waves during embryonic development, beginning with primitive hematopoietic progenitors at embryonic day 7.5 (E7.5), followed by definitive erythro-myeloid progenitors (EMPs) at E8.5. A third wave of hematopoiesis begins around E10 and yields dHSCs that originate from arterial endothelium throughout the embryo, although their emergence is concentrated in the AGM and the vitelline (VA) and umbilical arteries (UA) (reviewed in Medvinsky et al., 2011). dHSCs are defined by their ability to reconstitute hematopoiesis when transplanted into conditioned recipients. dHSCs that can reconstitute adult recipients are first detected at E10.5 in the embryo (Müller et al., 1994, Medvinsky and Dzierzak, 1996, Kumaravelu et al., 2002), while cells capable of reconstituting conditioned newborns can be detected earlier, between E9–E10 (Yoder et al., 1997a; Yoder et al., 1997b; Fraser et al., 2002).
dHSCs emerge during development from a subset of “blood-forming” endothelium known as “hemogenic endothelium” (HE), a concept that was first proposed in the 19th century when embryologists reported that the “blood islands” of the yolk sac appeared to bud from developing vascular tissue (Maximow, 1909; Sabin, 1920; Smith and Glomski, 1982; Tavian et al., 1996; reviewed in Medvinsky et al., 2011). The demonstration that endothelial cells purified from murine embryos and embryonic stem cells (ES) possessed lymphopoietic potential (Nishikawa et al., 1998a; Nishikawa et al., 1998b), that labeling of vascular endothelium before the onset of hematopoiesis resulted in labeled hematopoietic clusters (Jaffredo et al., 1998), and the identification of a progenitor with both hematopoietic and endothelial potential supported this model (Choi et al., 1998). Recent lineage trace studies using endothelial-specific promoters and live imaging of hematopoietic cells emerging from the endothelium of zebrafish and murine aortas have definitively cemented the HE hypothesis (Zovein et al., 2008; Chen et al., 2009; Bertrand et al., 2010; Boisset et al., 2010; Swiers et al., 2010).
Much work has gone into identifying HE in the developing embryo. As mentioned, functional dHSCs cannot be detected via transplantation into adult recipients prior to E10.5 (Müller et al., 1994; Medvinsky and Dzierzak, 1996; Yoder et al., 1997; Kumaravelu et al., 2002; Fraser ST et al., 2002), making identification of earlier developing HSPCs and their precursors technically difficult. To overcome this, several experimental platforms have been developed in which E11, E10 or E9 AGM-derived cells can be cultured ex vivo such that ongoing specification and expansion of dHSCs is preserved (Taoudi, et al., 2008; Rybtsov et al., 2011; Rybtsov et al., 2014; Rybtsov et al., 2016). As such, a series of precursors at E11, E10 and E9 have been identified using these assays. However, the exact frequency of the HE precursors within these populations or their precise hematopoietic potential has not yet been clearly defined. We recently developed a novel system that also supports the ongoing specification and expansion of HSPCs from HE ex vivo (Hadland et al., 2015). Here, E11 AGM-derived endothelial cells are engineered to express Myr-AKT (AGM AKT-endothelial cells or AA-ECs). These cells constitute an endothelial niche that is sufficient to support the specification and maturation of dHSCs ex vivo, including the maturation of E9 VE-Cadherin+c-Kit+ cells to transplantable dHSCs (Hadland et al., 2015). Here, we employ this system to demonstrate at the clonal level that the hematopoietic output of HE is highly heterogeneous with respect to both cell surface phenotype and function. To our knowledge, our study is the first to clonally interrogate the phenotype and function of HSPCs emerging from HE ex vivo. In parallel, we also employed OP9 stromal cells, a widely used hematopoietic supportive cell line, to assess the frequency and heterogeneity of HE in E9.5, E10.5 and E11.5 embryos (Nakano et al., 1994; Nishikawa et al., 1998a; Nishikawa et al., 1998b; Hadland et al., 2015). We find that only 0.1%, 1.3% and 0.29% of the E9.5, E10.5 and E11.5 VE-Cadherin+CD45− endothelium, respectively, generate hematopoietic colonies after OP9 co-culture. Further, only about 9% of these colonies contain phenotypic dHSCs ex vivo, detection of which is critically dependent upon exposure to relevant niche signals, as AA-ECs displayed superior support of phenotypic dHSC and multi-lineage hematopoietic progenitors compared with OP9 stroma.
Methods
Mice
C57BL/6J mice were acquired from The Jackson Laboratory (Bar Harbor, Maine) and housed in a pathogen-free facility. All animal experiments were carried out according to procedures approved by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee. Timed pregnancies were set up overnight, vaginal plugs were assessed before 8 am the next morning (designated as E0.5). Embryos were staged based on somite pair (sp) counts: E9.5 (19–23 sp), E10.5 (32–36 sp) and E11.5 (46–47 sp).
Co-culture of AGM cells with AA-ECs or OP9 cells to assess hemogenic potential ex vivo
E9.5 caudal halves and E10.5/E11.5 AGMs were dissociated with collagenase (0.0012 g/ml, Sigma-Aldrich, St. Louis, MO) in PBS (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% FCS (Omega Scientific, Tarzana, CA) and then stained with CD144-PE (11D4.1, BD Biosciences, San Diego, CA) and CD45-FITC (30-F11, BD Bioscience, San Diego, CA). VE-Cadherin+CD45− cells were then sorted by fluorescence activated cell sorting (FACS) via a FACSAria III (BD Biosciences, San Diego, CA) into 384-well or 96-well cell-culture treated plates (Sigma-Aldrich, St. Louis, MO) at 1, 2, 5, 10 or 20 cells/well. Each 384-well plate was pre-plated with 1000 AA-ECs overnight after collagen coating (StemCell Technologies, Vancouver, Canada) and each 96-well plate was pre-plated with 3000 OP9 cells or 3000 AA-ECs to ensure equivalent cell densities in 96- and 384-well plates. Each 96- and 384-well contained 150 or 75 uL of X-vivo 20 serum-free media (Lonza, Walkersville, MD, USA) supplemented with 100ng/ml of recombinant murine SCF, recombinant murine FLT3L, recombinant murine IL-3 and 20 ng/ml of recombinant murine TPO (Peprotech, Rocky Hill, NJ). Sorted cells were co-cultured with either AA-ECs or OP9 cells for seven days before each well was inspected for the presence of hematopoietic colonies. Individual colonies were then either re-plated in M3434 (Stem Cell Technologies, Vancouver, Canada) or stained with the following antibodies and analyzed by flow cytometry on a BD LSRFortessa (BD Biosciences, San Diego, CA): c-Kit-APC (2B8), CD4-e605 (RM4-5), CD8-e605 (53-6.7), Gr1-e605 (RB6-8C5), B220-e605 (RA3-6B2), Ter119-e605 (Ter119), CD4-Brilliant Violet 605™ (RM4-5), CD8- Brilliant Violet 605™ (53-6.7), Gr1- Brilliant Violet 605™ (RB6-8C5), B220- Brilliant Violet 605™ (RA3-6B2), Ter119- Brilliant Violet 605™ (Ter119), Sca-1-PerCPCy5.5 (E13-161.7), CD150-PE (TC15-12F12.2), CD48- FITC (HM48-1). All antibodies were obtained from Biolegend, (San Diego, CA) except c-Kit-APC (2B8), which was obtained from eBiosciences (San Diego, CA). Colony forming units on M3434 were scored 10 days after plating.
Maintenance of OP9 stromal cells
OP9 stromal cells (CRL2749, ATCC, Manassas VA) were maintained in MEM-20 media (MEM Alpha 1× media (12561, Gibco), FBS 20% (Omega), 1% Penicillin-streptomycin (Hyclone, Thermo Scientific)) and kept in culture in medium density to avoid cell differentiation.
AA-EC cells cultures
AA-ECs were derived and cultured as previously described (Hadland et al., 2015).
Statistics
The Shapiro-Wilk test was performed to test for normality of the data. (Exact) Chi-square tests and two-sample t-tests or (Exact) Wilcoxon Rank Sum tests, depending on the normality, were used to compare two groups for categorical and continuous variables, respectively. Box-plot, bar plot with error bars, and bar chart were used to present the data. All analyses were performed in SAS version 9.4. (SAS Institute Inc 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary, NC: SAS Institute Inc.). For limiting dilution analysis (LDA). parameters were estimated using a generalized linear model with a complementary log-log link. The Chi-square (Pearson and Deviance) was used to assess the goodness-of-fit to the LDA model. Differences in frequencies between groups was assessed by relying on the asymptotic normality of Maximum Likelihood Estimator. LDA were performed using L-Calc (Stem Cell Technologies, Vancouver, CA).
Results
Ex vivo hematopoietic potential of E9.5, E10.5 and E11.5 VE-Cadherin+CD45− endothelial cells
OP9 co-culture is widely utilized in the maintenance of HSPCs from adults, embryos and differentiating ESCs (Nakano et al., 1994; Nishikawa et al., 1998a; Nishikawa et al., 1998b; McKinney-Freeman et al., 2009; Hadland et al., 2015). Here, we employed OP9 co-culture to interrogate the hematopoietic output of mid-gestation endothelium from E9.5, E10.5 and E11.5 embryos (Fig. 1A). VE-Cadherin+CD45− (VE+CD45−) cells were isolated by FACS from E9.5 caudal halves and from E10.5 or E11.5 AGM, UA and VA and co-cultured at limiting dilution with OP9 cells in 96-wells plates (Fig. 1, Supplemental Fig. 1). This population represents a mixture of hemogenic and non-hemogenic endothelium (Rybtsov S. et al., 2011) but should capture all the embryonic endothelium (Ayalon et al., 1994; Matsuyoshi et al., 1997; Vittet et al., 1997; Nishikawa et al., 1998a; Nishikawa et al., 1998b). Individual wells were scrutinized seven days later for hematopoietic colonies (Fig. 1A, 1B). Only 0.1%, 1.3% and 0.29% of E9.5, E10.5 and E11.5 VE+CD45− cells generated hematopoietic colonies, respectively (Fig. 1B, Table 1, Supplemental Table 1). Thus, hemogenic activity in this cell population peaks at E10.5. In line with this finding, the frequency of HE-derived c-Kit+ hematopoietic clusters also peaks at E10.5 in the mouse (Yokomizo et al., 2010).
Figure 1. Ex vivo limiting dilution assay for hemogenic potential at E9.5, E10.5 and E11.5 and quantitative analysis of phenotypically distinct hematopoietic populations generated by VE+CD45− hemogenic endothelial clones.
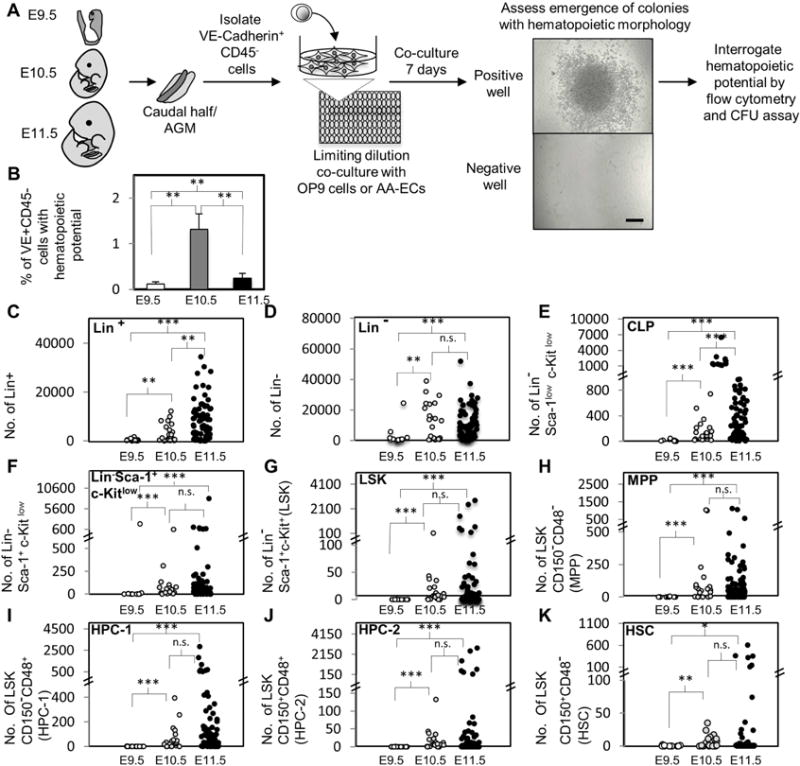
A) Experimental schematic. VE+CD45− cells were sorted to determine the frequency of hematopoietic potential. VE+CD45− were cultured at limiting dilution in 96 well or 384 well plates with OP9 cells or AA-ECs. Seven days later, each well was examined for hematopoietic colonies. A representative image of an emerging hematopoietic colony is shown. Scale bar: 250μm. Some colonies were then either re-plated into semi-solid media or analyzed by flow-cytometry. B) The frequency of E9.5, E10.5 and E11.5 VE+CD45− cells with hematopoietic potential after OP9 co-culture is shown. The average of ≥ three independent experiments is shown, two of which were performed in parallel with all three developmental stages (see Table 1 and Supplemental Table 1). Error bars represent standard deviation. C–K) Hematopoietic colonies generated by HE clones during OP9 co-culture were analyzed by flow cytometry for the following populations: Lin+, Lin−, Lin− Sca-1low c-Kitlow (CLP), Lin− Sca-1+ c-Kitlow (CLP), Lin− Sca-1+ c-Kitlow, lin− Sca-1+ c-Kit+ (LSK), LSK CD150− CD48− (MPP), LSK CD150− CD48+ (HPC-1), LSK CD150+CD48+(HPC-2) and LSK CD150+CD48− (HSC). Each circle represents the absolute number of cells yielded by individual hemogenic endothelial clones. (E9.5, n=12; E10.5, n=21; E11.5, n=77 clones). *, P < 0.1; **, P < 0.05; ***, P< 0.001; n.s.: not statistically significant.
Table 1.
Limiting dilution analysis of hemogenic potential in E9.5, E10.5 and E11.5 mouse endothelium
| Exp. | Stage | HE Frequency | HE Frequency (% of total) (95% Confidence Interval) | Chi-square (Pearson) P-value | Chi-square (Deviance) P-value |
|---|---|---|---|---|---|
| #1 | E9.5 | 1 in 573 | 0.17 (0.056–0.54) | 0.19** | 0.40** |
| E10.5 | 1 in 70 | 1.4 (1.1–1.9) | 0.03* | 0.013* | |
| E11.5 | 1 in 267 | 0.4 (0.2–0.6) | 0.06** | 0.09** | |
| #2 | E9.5 | 1 in 975 | 0.1 (0.05–0.2) | 0.97** | 0.91** |
| E11.5 | 1 in 486 | 0.2 (0.08–0.55) | 0.60** | 0.39** | |
| #3 | E9.5 | 1 in 1094 | 0.09 (0.02–0.36) | 0.62** | 0.50** |
| E10.5 | 1 in 64 | 1.6 (1.2–2.1) | 0.68** | 0.58** | |
| E11.5 | 1 in 436 | 0.2 (0.1–0.5) | 0.90** | 0.80** | |
| #4 | E10.5 | 1 in 105 | 0.95 (0.57–1.58) | 0.93** | 0.93** |
| E11.5 | 1 in 282 | 0.35 (0.27–0.46) | 0.35** | 0.33** |
LDA assumption does not hold (p<0.05)
LDA assumption holds well (p>0.05)
We next analyzed and quantified by flow cytometry the phenotype of the emerged hematopoietic colonies with respect to different hematopoietic markers (Figs. 1C–1K). Specifically, we quantitatively assessed the output of individual hemogenic endothelial cells regarding the following phenotypically defined mature and immature hematopoietic populations: Lineage+ (Lin+), Lin−, Lin− Sca-1lowc-Kitlow (enriched for common lymphoid progenitors, CLPs (Kondo et al., 1997)), Lin− Sca-1+c-Kit+ (LSK), Lin− Sca-1+c-Kitlow (this populations displays an intermediate CLP and LSK phenotype), LSK CD150−CD48− (multi-potent progenitors, MPPs), LSK CD150−CD48+ (HPC-1), LSK CD150+CD48+ (HPC-2), LSK CD150+CD48− (HSC) (Kiel et al., 2005; Kiel et al., 2008; Oguro et al., 2013) (Fig. 1). LSK cells are highly enriched for a heterogeneous mixture of HSPCs compared to whole bone marrow, including HPC-1, HPC-2, MPPs and HSCs. HPC-1 and HPC-2 represent heterogeneous restricted progenitors with limited in vivo repopulating potential (Kiel et al., 2005; Kiel et al., 2008; Oguro et al., 2013). MPPs are also heterogeneous with respect to lineage potential, but are generally considered to be the immediate downstream progeny of dHSCs and display multi-lineage short term in vivo repopulating activity (Kiel et al., 2005; Kiel et al., 2008, Oguro et al., 2013). Long-term repopulating dHSCs are LSK CD150+CD48− (Kiel et al., 2005; Kiel et al., 2008; Oguro et al., 2013). Remarkably, although we detected fewer functional HE at E11.5 than E10.5 (Fig. 1B), E11.5 HE trended towards an increased ability to produce large numbers hematopoietic cells, including phenotypic dHSCs (Fig. 1C–1K), although our previous studies indicate that these phenotypic dHSCs that emerge during OP9 co-culture do not transplant (Hadland et al. 2015).
AA-EC co-culture supports HE with superior hematopoietic potential relative to OP9 cell co-cultures
As the frequency of functional HE in the VE+CD45− compartment peaked at E10.5 (Fig. 1B), we chose this developmental time point for further study. Although OP9 cells support the emergence of hematopoietic colonies they fail to promote the specification of bona fide dHSC from E9–E11 embryos with robust transplantation activity (Hadland et al., 2015). In contrast, AA-ECs robustly support the maturation of dHSC precursors to functional long-term repopulating dHSCs from embryos as early as E9 (Hadland et al., 2015). Thus, these cells can be used as a platform to assess the functional heterogeneity of the hemogenic endothelium at the clonal level in a context in which bona fide dHSCs develop well. Sorted E10.5 VE+CD45− were co-cultured at limiting dilution with OP9 cells or AA-ECs. No differences in the frequency of functional hemogenic endothelial cells were detected in these co-cultures (Fig. 2A, Table 2, Supplemental Table 2). We next characterized the hematopoietic output of E10.5 HE from both OP9 and AA-EC co-cultures side-by-side by interrogating individual colonies seven days post-plating for primitive hematopoietic cell surface marker expression by flow cytometry (Figs. 2–3, Supplemental Figs. 2–3). Here, 140 and 143 hematopoietic colonies were examined from OP9 or AA-EC co-cultures, respectively. We arbitrarily defined a “large number of cells generated” as a value greater than that seen in 90% (90th percentile, P90) of colonies examined for a particular co-culture condition and hematopoietic population. OP9 cells supported the emergence of large numbers of Lin+ cells more often than AA-ECs (Fig. 2B). Indeed, 36% of E10.5 VE+CD45− cells generated >10,000 Lin+ cells on OP9 cells in contrast to 13% of those plated on AA-ECs (Fig. 2B, Fig. 2K). OP9 cells produced a slightly higher number of Lin− cells (Fig. 2C, Fig. 2K). About 14.7% of AA-EC co-cultures produced >2000 Lin− Sca-1low c-Kitlow (CLP) and/or 2000 Lin− Sca-1+ c-Kitlow cells, while less than 3% of cells plated on OP9s performed similarly with respect to these populations (Fig. 2D, Fig. 2E, Fig. 2K). Regarding HSPC production, although the absolute numbers of OP9 or AA-EC-derived HE capable of generating phenotypic LSK cells or MPPs was not significantly different (Figure 2F and G), AA-EC co-cultures gave rise to significantly more HE that could generate large numbers of these cells (>2000 LSK cells and >800 MPPs, Figure 2K). AA-EC co-cultures also generated significantly more HE with dHSC potential than OP9 cultures (Figure 2J). Thus, AA-ECs tend to support the emergence of E10.5 HE with primitive hematopoietic potential better than OP9 cells, which better support the emergence of Lineage+ cells from HE.
Figure 2. Ex vivo limiting dilution assay for hemogenic potential at E10.5 on OP9 or AA-EC cultures and quantitative analysis of phenotypically distinct hematopoietic populations generated by E10.5 VE+CD45− hemogenic endothelial clones.
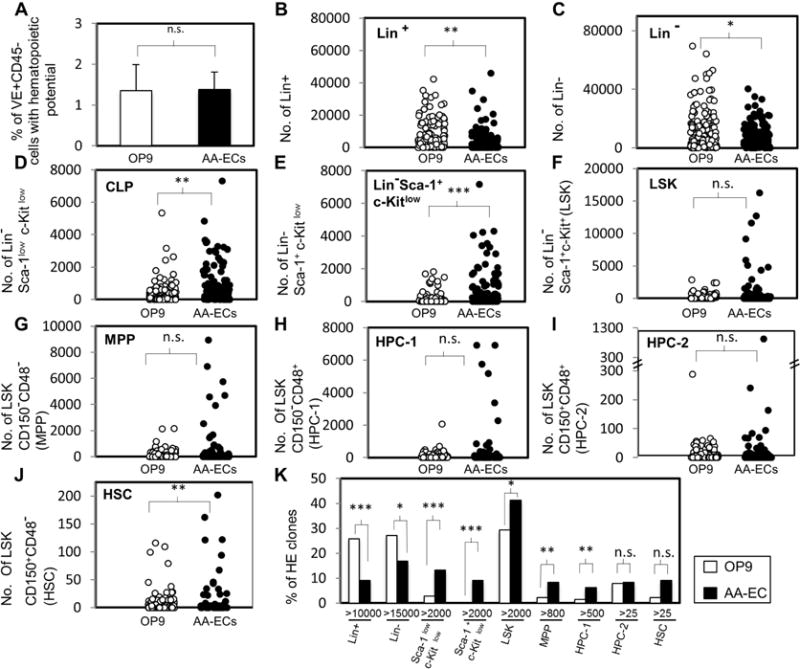
A) The frequency of El 0.5 VE+CD45_ cells with hematopoietic potential In OP9 cell and AA-EC co-cultures is shown. See Supplemental Table 2 for further details. Bars represent the averages of at least four independent experiments. Four of them were performed in parallel. Standard deviations are indicated. B–J) Hematopoietic colonies generated by HE clones during OP9 cell or AA-EC co-culture were analyzed by flow cytometry similarly as in Fig.1. Each circle represents the absolute number of cells yielded by individual hemogenic endothelial clones (OP9/HE clones: n=140; AA-EC/HE clones: n=143). K) Graph indicates the % of clones that generated a “large numbers of cells”, which was defined as a value greater than that seen in 90% (90th percentile, P90) of colonies examined for a particular co-culture condition and hematopoietic population. This value was >10000 Lin+ cells, 15000 Lin− cells, 100 Lin− Sca-llow c-Kitlow cells (Sca-llowc-Kitlow), 400 Lim Sca-1+ c-Kitlow cells (Sca-1+ c-Kitlow), 2000 LSK cells, 800 MPP cells, 500 HPC-1 cells, 25 HPC-2 cells, or 25 HSC. A–J) White-filled circles or bars account for endothelial clones plated and co-cultured with OP9 stromal cells. Black-filled circles or bars represent clones plated and co-cultured on AA-ECs. *, P < 0.1; **, P < 0.05; ***, P< 0.001; n.s.: not statistically significant.
Table 2.
Limiting dilution analysis of hemogenic potential in E10.5 mouse endothelium (AA-EC co-cultures and OP9 co-cultures)
| Experiment | Co-culture | Frequency of hemogenic endothelium | Frequency of hemogenic endothelium (% of total) (95% Confidence Interval) | Chi-square (Pearson P-values | Chi-square (Deviance) P-value |
|---|---|---|---|---|---|
| #3 | OP9 | 1 in 64 | 1.57 (1.2–2.1) | 0.68** | 0.58** |
| AA-EC | 1 in 50 | 1.98 (1.59–2.46) | 0.80** | 0.80** | |
| #4 | OP9 | 1 in 105 | 0.95 (0.57–1.58) | 0.93** | 0.93** |
| AA-EC | 1 in 101 | 1.0 (0.58–1.7) | 0.64** | 0.62** | |
| #5 | OP9 | 1 in 100 | 1 (0.67–1.5) | 0.43** | 0.43** |
| AA-EC | 1 in 82 | 1.2 (0.8–1.8) | 0.79* | 0.79* | |
| #6 | OP9 | 1 in 42 | 2.37 (2.07–2.7) | 0.16** | 0.12** |
| AA-EC | 1 in 74 | 1.4 (1.2–1.6) | 0.1** | 0.1** | |
| #7 | OP9 | 1 in 118 | 0.85 (0.6–1.2) | 0.024* | 0.035* |
LDA assumption does not hold (p<0.05)
LDA assumption holds well (p>0.05)
Please note that experiments #3 and #4 are part of the same experiment as in Table 1.
Figure 3. Hemogenic endothelial clones classified by phenotypic hematopoietic output.
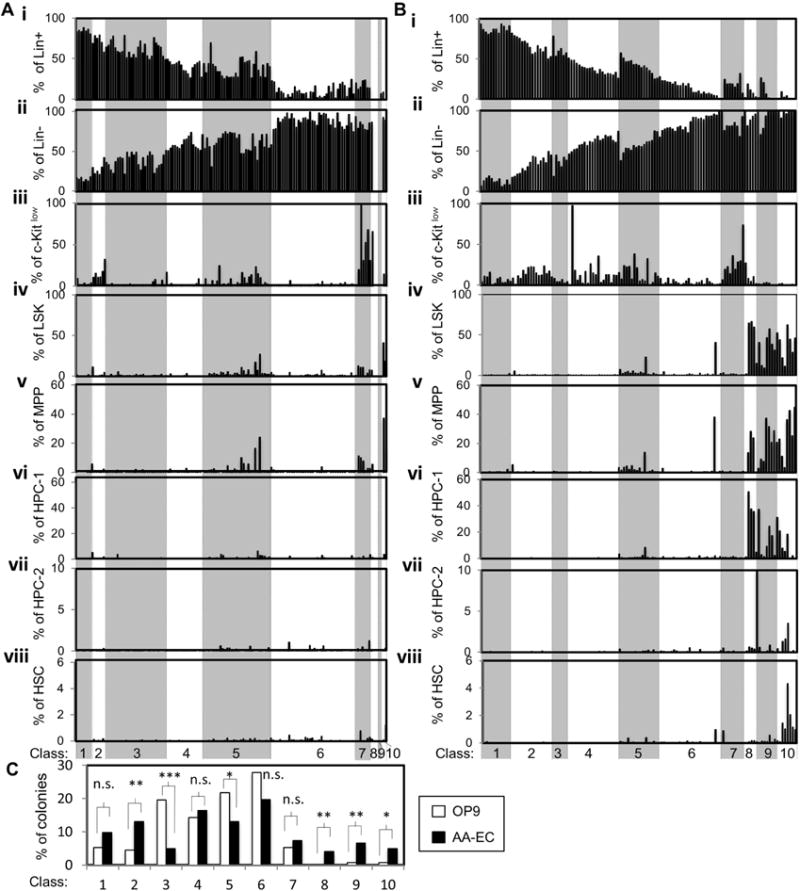
The phenotypic hematopoietic output of individual colonies from OP9/ or AA-EC/ HE co-cultures shown on Fig. 2 were assigned to one of ten classes (Class 1–10) that progress from more differentiated (Class 1) to more multi-potent (Class 10). See Suppl. Figs. 2–3 for precise Class definitions and representative flow cytometry plots. A) Classification of colonies emerging from HE clones co-cultured with OP9 cells. B) Classification of colonies emerging from HE clones co-cultured with AA-ECs. For (A) and (B), each bar represents a single hematopoietic colony. Bars in the same position in each panel (i–viii) refer to the same colony. C) Summary of the % of HE clones belonging to each Class. White bars represent clones collected from OP9 co-cultures and black bars represent clones collected from AA-EC co-cultures. *, P < 0.1; **, P < 0.05; ***, P< 0.001; n.s.: not statistically significant.
The relatively few number of HE generating large numbers of phenotypic MPP and dHSC during OP9 co-culture agrees with our previous finding that this niche fails to robustly support the specification and maintenance of cells with long-term repopulating activity (Hadland et al., 2015). Our data also suggest dramatic heterogeneity in hematopoietic potential between individual E10.5 HE cells. To further illustrate this finding, we classified each colony generated by individual VE+CD45− endothelial cells according to the relative composition of the hematopoietic populations described above (Fig. 3, Supplemental Fig. 2–3). We stratified them into 10 classes (1–10) that progress from more differentiated (e.g Class 1 are composed of >80% Lineage+ cells) to more multi-potent (e.g. Class 10 contain >1% phenotypic dHSC) (Fig. 3). E10.5 HE clones co-cultured on OP9 cells mainly generated Class 1–7 colonies (98.5%), the more developmentally primitive Class 8–10 colonies were barely detected, only 0.75% of the colonies analyzed belonged to Class 9 or 10 (Fig. 3A, Fig. 3C). In contrast, 15% of colonies from AA-EC co-cultures were Class 8–10 colonies (Fig. 3Aiv–viii, Fig. 3Biv–viii, Fig. 3C). In particular 4%, 7% and 5% of the colonies from AA-EC co-cultures belonged to Classes 8, 9 and 10, respectively (Fig. 3C). This was significantly more than was seen in OP9 co-cultures. Class 10 colonies are the only Class containing phenotypic dHSCs (Fig. 3Bviii, Fig. 3C).
In sum, globally, HE cultured on OP9 cells tend to produce hematopoietic colonies comprised mainly of mature cells and very few phenotypically primitive progenitors. HE cultured on AA-ECs generate hematopoietic colonies that often contain phenotypic HSPCs. These data confirm that AA-ECs are a superior ex vivo niche for the specification and/or expansion of HSPCs from HE relative to OP9 cells (Hadland et al., 2015). Importantly, these data also illustrate the tremendous diversity of hematopoietic potential of individual E10.5 HE, both qualitatively and quantitatively.
E10.5 hemogenic endothelial precursors are functionally heterogeneous
To explore further the heterogeneity of E10.5 HE, 86 and 59 colonies expanded from individual VE+CD45− cells plated on OP9 cells or AA-ECs, respectively, were analyzed for their ability to form colony-forming-units (CFUs) when re-plated in semi-solid media supplemented with hematopoiesis-promoting cytokines (Fig. 1A, Fig. 4, Fig. 5). 78% of both OP9 and AA-EC co-culture-derived colonies generated CFUs (Fig. 4A). CFUs generated by both OP9 and AA-EC derived co-cultures were highly heterogeneous with respect to the total number of CFU colonies generated (Fig. 4B), the distribution of eythroid-myeloid potential (Fig. 4C, Fig. 4D, Fig. 4F, Fig. 5), and their ability to generate multi-potent CFU-GEMM colonies (Fig. 4E, Fig. 5). CFU-GEMMs are mixed colonies composed of granulocytes, erythroid cells, megakaryocytes, and monocytes that presumably derive from multi-potent HSPCs.
Figure 4. CFU output of hemogenic endothelial clones.
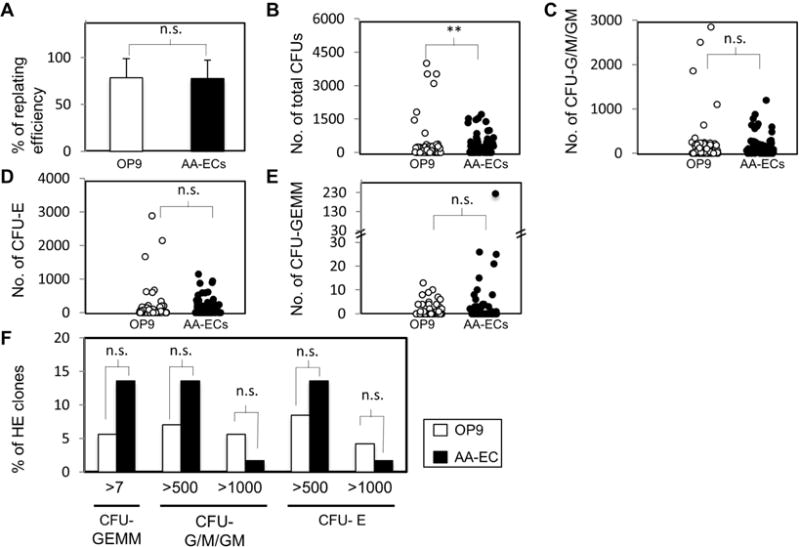
A) % of individual colonies from OP9/ or AA-EC/ HE co-cultures capable of re-plating into semi-solid media supplemented with hematopoiesis promoting cytokines. Bars represent the average from five independent experiments, three of them performed in parallel. A total of 86 and 59 colonies were plated respectively from OP9/ and AA-EC/ HE co-cultures. Error bars indicate standard deviation. B–F) Colonies with replating-ability were further analyzed for their CFU type composition. B) Total absolute number of CFUs generated by colonies collected from OP9 or AA-EC HE co-cultures. C) Total absolute number of CFU-G/M/GM (granulocyte and monocyte colonies) generated by colonies collected from OP9/ or AA-EC/ HE co-cultures. D) Total absolute number of CFU-Es (erythroid) generated by colonies collected from OP9/ or AA-EC/ HE co-cultures. E) Total absolute number of CFU-GEMMs (mixed colonies of granulocytes, erythrocytes, macrophages, and megakaryocytes) generated by colonies collected from OP9/ or AA-EC/ HE co-cultures. For B–E, each circle represents the output of an independent colony collected from OP9 (white circles) and AA-EC (black circles) co-cultures. F) % of primary colonies that generated >seven CFU-GEMMs, 500 or 1000 CFU-G/M/GMs, and more than 500 or 1000 CFU-Es. *, P < 0.1; **, P < 0.05; ***, P< 0.001; n.s.: not statistically significant.
Figure 5. Hemogenic endothelial clones classified by CFU potential.
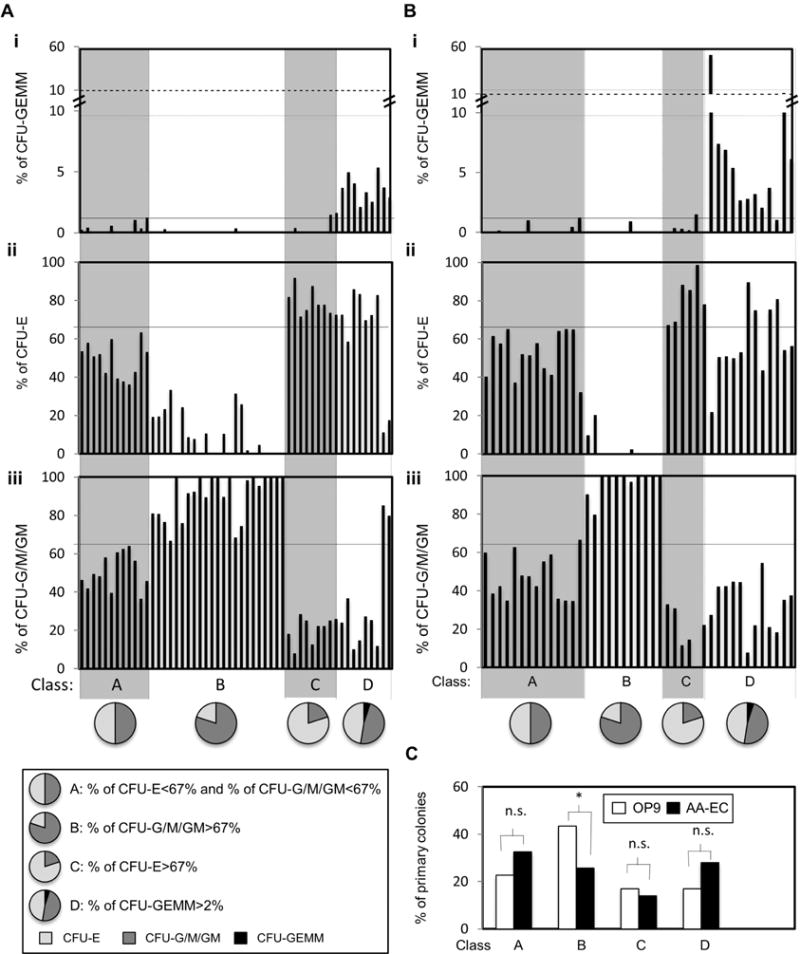
The CFU potential of individual colonies from OP9 (A) or AA-EC (B) co-cultures from Figure 4 were assigned to one of four classes (Class A–D), as defined in inset. Bars in the same position in each panel (i–iii) refer to the same colony. C) % of colonies assigned to each Class. *, P < 0.1; **, P < 0.05; ***, P< 0.001; n.s.: not statistically significant.
To facilitate analysis of these results, we categorized the observed CFU potential into four classes (Fig. 5). Class A colonies generated a balanced number of CFU-Erythroid (CFU-E) and myeloid colonies (CFU-G/M/GM) (i.e. CFU-E<67% and CFU-G/M/GM <67%). Class B colonies skewed towards myeloid potential (CFU-G/M/GM >67%) while Class C colonies were more erythroid (CFU-E >67%). Finally, Class D colonies were those that displayed multi-potent CFU-GEMM potential (Fig. 5). Although colonies expanded on AA-ECs that generated CFU after replating tended to generate more Class D colonies (i.e. primary colonies with multi-potent potential) than OP9 co-culture derived colonies, this difference was not significant (Fig. 5C). AA-EC and OP9 co-culture-derived colonies generated similar numbers of CFUs with a balanced erythroid-myeloid output (Class A), although AA-EC co-cultures yielded many fewer CFUs with skewed myeloid output (Class B) (Fig. 5C).
In sum, HE co-cultured on OP9 cells that were capable of re-plating in CFU assays tended to yield more total CFU activity (Fig. 4B). Although HE co-cultured with AA-ECs were enriched in multi-potent CFU-GEMM potential, these differences were not statistically significant (Fig. 4E, Fig. 4F, Fig. 5C).
Kinetic analysis of dHSC emergence in OP9 and AA-EC co-cultures
To examine the kinetics of dHSC emergence in these co-cultures, E10.5 VE+CD45− cells were co-cultured in bulk with OP9s or AA-ECs and then examined 5, 7 and 9 days post-plating by flow cytometry for phenotypic dHSCs. dHSCs were detected as early as day 5 and significantly increased over time (Supplemental Fig. 4). As seen in limiting dilution co-cultures, by day 7 and 9, AA-ECs were significantly more supportive of the development of phenotypic dHSCs than OP9s (Supplemental Fig. 4). Indeed, although the frequency of phenotypic dHSCs increased dramatically between day 7 and day 9 of both OP9 and AA-EC co-culture, this increase was much more dramatic in AA-EC co-cultures (Supplemental Fig. 4).
In total, these experiments confirm that AA-ECs are a superior ex vivo niche for the specification of dHSC relative to OP9 cells. It is also possible that AA-ECs support superior dHSC maintenance and/or expansion.
Discussion
Our results reveal that E9.5, E10.5 and E11.5 HE constitutes about 0.1%, 1.3% and 0.29% of VE+CD45− endothelium, respectively. These data suggests that the emergence of HE within the VE+CD45− compartment peaks at E10.5 but drops off at E11.5, possibly because many HE acquire a VE+CD45+ cell surface phenotype by this stage and are no longer found at high numbers in the VE+CD45− compartment, as suggested by previous work (Taoudi, et al., 2008). Interestingly, although the frequency of VE+CD45− HE cells is lower at E11.5 than E10.5, E11.5 VE+CD45− HE display a more robust hematopoietic output than E10.5 VE+CD45− HE (Figs. 1B–K). So, it is possible that both VE+CD45− and VE+CD45+ cell compartments contain HE that contribute to the hematopoietic landscape at this stage.
Our work is the first clonal analysis to demonstrate high heterogeneity in the hematopoietic potential and output of E9.5, E10.5 and E11.5 HE, including the output of phenotypic dHSCs. We found that individual HE clones displayed broad heterogeneity in the absolute numbers of phenotypic dHSCs produced (Fig. 1K). Our data suggest either distinct rates of maturation within the VE+CD45− HE compartment in this ex vivo setting or that hemogenic endothelium represents a developmental continuum of hematopoietic precursors with distinct hematopoietic potential, with some capable of giving rise to very primitive multi-potent HSPCs and others yielding primarily Lineage+ cells.
Previous studies have used transplantation to identify and estimate the number of dHSC or HSPCs present throughout ontogeny. Classically, E11.5 AGM is believed to harbor one dHSC (Kumaravelu et al., 2002). Indeed, Taoudi et al., reported that VE+CD45+ cells in the AGM contain all transplantation activity at E11.5 and that only 1 in 70 of these cells were functional dHSCs (Taoudi et al., 2005). More recently, a quantitative limiting dilution assay using OP9 co-aggregates estimated that there were only one or two pro- HSCs at E9.5 in the embryo, five pre-HSC by E10, 20–30 by E10.5 and 65 by E11 (Rybtsov et al., 2016). Altogether, these transplantation studies suggest that HSPCs and dHSCs are rare during development. Other studies have shown that 10% of E9.5 VE+CD31+ cells that incorporate acetylated LDL generate hematopoietic colonies in collagen gel containing SCF, IL-3, G-CSF and erythropoietin (EPO) (Nishikawa et al., 1998b). OP9 co-culture of E11.5 VE+CD45+ in α-MEM medium supplemented with EPO, IL3, pokeweed mitogen spleen-conditioned medium, and G-CSF conditioned medium found that 7.6% displayed hematopoietic potential, while the VE+CD45− fraction did not produce detectable hematopoietic cells (Taoudi et al., 2005). Although these previous studies employed different conditions and examined different developmental time-points, altogether they also suggest that HE constitutes a minor fraction of the endothelium. These studies, when combined with our data here, paint the picture of a dynamic embryonic endothelium, where between E9.5 and E10.5 more cells acquire the ability to generate hematopoietic progeny ex vivo. As development continues, the few VE+CD45− cells that retain this potential display increased hematopoietic output.
We found that at E10.5 HE precursors display important functional differences (Figs. 2–3) in terms of hematopoietic potential, with some giving rise to cultures of mostly mature hematopoietic cells and others generating cultures largely composed of phenotypic and functionally multi-potent HSPCs. These data suggest that HE is a heterogeneous mixture of precursors with distinct developmental potential or a mixture of different developmental maturation stages. It has been shown that a fraction of the E10 VE+CD45− compartment expresses B220, CD3e and Ter119 and different levels of the early hematopoietic marker CD41. By transplantation, the VE+CD45−CD41lowLin− population contained the majority of cells capable of maturing into dHSCs (Rybtsov et al., 2011). It would be interesting to determine if these or other cell surface markers could discriminate among different HE precursors with disparate hematopoietic potential. Such studies would facilitate efforts to determine if the disparate hematopoietic potential seen here truly reflects a developmental continuum or represents a heterogeneous pool of independently specified precursors with distinct inherent developmental fates. Indeed, a Cre recombinase transgene driven by CD41 promoter elements labeled only 35–60% of adult blood (Rybtsov et al., 2011). Although this incomplete labeling may be technical due to limited transgene expression, it is also formally possible that variable CD41 expression during early blood development may reflect distinct blood progenitors. Indeed, it has been shown that primitive and definitive erythroid progenitors can be discriminated based on the level of CD41 expression (Ferkowicz et al., 2003). It is interesting to consider if HE with distinct hematopoietic potential might contribute to distinct adult hematopoietic compartments during life-long hematopoiesis. This is of clinical relevance as distinct hematopoietic malignancies might have distinct developmental origins, especially those that emerge in children. In line with this and very intriguingly, a lympho-myeloid restricted progenitor that emerges from the yolk sac prior to dHSC emergence and that contributes transiently to adult blood has been identified (Boiers et al., 2013). Moreover, Beaudin and colleagues have recently shown the existence of a developmentally restricted dHSC with capacity to robustly generate innate-like B and T cells. (Beaudin et al., 2016).
In summary, our results show that HE is a small fraction of the total endothelium in the mid-gestation embryo. Whether this identity was acquired before cells migrated or coalesced to form the dorsal aorta or whether it was gained in situ in the arterial floor after exposure to specific Endothelial to Hematopoietic transition (EHT)-inducing factors remains an unresolved question in the field. Our work indicates that AA-ECs are a superior supportive niche for HSPC specification. Moreover, our study reveals the presence of large heterogeneity within the HE, suggesting the existence of distinct HE populations. Whether these populations constitute different types of long-lived HSPCs or are reflective of distinct maturation states warrants further investigation.
Supplementary Material
Highlights.
-
➢
Hemogenic endothelium (HE) constitutes a minor fraction of midgestation murine endothelium
-
➢
Midgestation HE is already highly heterogeneous in its hematopoietic potential
-
➢
AKT expressing AGM-endothelial cells are a superior hematopoietic supportive niche
Acknowledgments
We thank McKinney-Freeman laboratory and Department of Hematology at St. Jude Children’s Research Hospital (SJCRH) for critical discussions and reading of the manuscript; D. Ashmun and S. Schwemberger for FACS support; Cara Davis-Goodrum, and Krista Millican for help with timed pregnancies. This work was supported by the American Society of Hematology (S.M.-F.), the Hartwell Foundation (S.M.-F.), the NIDDK (K01DK080846 and R01DK104028, S.M.-F.), the American Lebanese Syrian Associated Charities (S.M.-F.), the NIH NHLBI UO1 grant #HL100395 (I.B.) and the Ancillary Collaborative Grant #HL099997 (I.B.). B.H. is supported by the Alex’s Lemonade Stand Foundation and Hyundai Hope on Wheels Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors contributions
M.G. designed the study, collected and analyzed data, and wrote the paper, A.C. collected FACS data, C.L. and G.K. performed statistical analyses, B.H. and I.B. generated AA-EC cells, S.M.-F. designed the study, analyzed data, and wrote the paper. All authors discussed the results and commented on the manuscript.
References
- Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J Cell Biol. 1994 Jul;126(1):247–58. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin AE, Boyer SW, Perez-Cunningham J, Hernandez GE, Derderian SC, Jujjavarapu C, Aaserude E, MacKenzie T, Forsberg EC. A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell. 2016 Sep;15 doi: 10.1016/j.stem.2016.08.013. pii: S1934-5909(16)30260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010 Mar 4;464(7285):108–11. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010 Mar 4;464(7285):116–20. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Böiers C, Carrelha J, Lutteropp M, Luc S, Green JC, Azzoni E, Woll PS, Mead AJ, Hultquist A, Swiers G, Perdiguero EG, Macaulay IC, Melchiori L, Luis TC, Kharazi S, Bouriez-Jones T, Deng Q, Pontén A, Atkinson D, Jensen CT, Sitnicka E, Geissmann F, Godin I, Sandberg R, de Bruijn MF, Jacobsen SE. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013 Nov 7;13(5):535–48. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009 Feb 12 10.1038/nature07619;457(7231):887–91. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998 Feb;125(4):725–32. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, Yoder MC. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003 Sep;130(18):4393–403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- Fraser ST, Ogawa M, Yu RT, Nishikawa S, Yoder MC, Nishikawa S. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin(+) population. Exp Hematol. 2002 Sep;30(9):1070–8. doi: 10.1016/s0301-472x(02)00887-1. [DOI] [PubMed] [Google Scholar]
- Hadland BK, Varnum-Finney B, Poulos MG, Moon RT, Butler JM, Rafii S, Bernstein ID. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J Clin Invest. 2015 May;125(5):2032–45. doi: 10.1172/JCI80137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lièvre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998 Nov;125(22):4575–83. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005 Jul 1;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007 Sep 13;449(7159):238–42. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997 Nov 28;91(5):661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002 Nov;129(21):4891–9. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- Matsuyoshi N, Toda K, Horiguchi Y, Tanaka T, Nakagawa S, Takeichi M, Imamura S. In vivo evidence of the critical role of cadherin-5 in murine vascular integrity. Proc Assoc Am Physicians. 1997 Jul;109(4):362–71. [PubMed] [Google Scholar]
- Maximow A. Untersuchengenuber blut und bindgewebe: 1. Diefruhesten entwicklungs-stadian der Blut- und Bindgewebszellen beim saugetierembryo, bis sum anfang der blutbildung in der leber. Arch Mikr Anat Entwicklungsgesch. 1909;4:159–166. [Google Scholar]
- McKinney-Freeman SL, Naveiras O, Yates F, Loewer S, Philitas M, Curran M, Park PJ, Daley GQ. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009 Jul 9;114(2):268–78. doi: 10.1182/blood-2008-12-193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996 Sep 20;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011 Mar;138(6):1017–31. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- Müller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994 Jul;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994 Aug 19;265(5175):1098–101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998a May;125(9):1747–57. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998b Jun;8(6):761–9. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013 Jul 3;13(1):102–16. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin FR. Studies on the origin of blood vessels and of red blood corpuscules as seen in the living blastoderm of chicks during the second day of incubation. Contrib Embryol. 1920;9:213–262. [Google Scholar]
- Smith RA, Glomski CA. Hemogenic endothelium of the embryonic aorta: Does it exist? Dev Comp Immunol. 1982 Spring;6(2):359–68. doi: 10.1016/s0145-305x(82)80019-0. [DOI] [PubMed] [Google Scholar]
- Swiers G, Speck NA, de Bruijn MF. Visualizing blood cell emergence from aortic endothelium. Cell Stem Cell. 2010 Apr 2;6(4):289–90. doi: 10.1016/j.stem.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Taoudi S, Morrison AM, Inoue H, Gribi R, Ure J, Medvinsky A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005 Sep;132(18):4179–91. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008 Jul 3;3(1):99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Tavian M, Coulombel L, Luton D, Clemente HS, Dièterlen-Lievre F, Péault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996 Jan 1;87(1):67–72. [PubMed] [Google Scholar]
- Rybtsov S, Sobiesiak M, Taoudi S, Souilhol C, Senserrich J, Liakhovitskaia A, Ivanovs A, Frampton J, Zhao S, Medvinsky A. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med. 2011 Jun 6;208(6):1305–15. doi: 10.1084/jem.20102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S, Batsivari A, Bilotkach K, Paruzina D, Senserrich J, Nerushev O, Medvinsky A. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Reports. 2014 Sep 9;3(3):489–501. doi: 10.1016/j.stemcr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S, Ivanovs A, Zhao S, Medvinsky A. Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development. 2016 Apr 15;143(8):1284–9. doi: 10.1242/dev.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6273–8. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997a Sep;7(3):335–44. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A. 1997b Jun 24;94(13):6776–80. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010 Nov;137(21):3651–61. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008 Dec 4;3(6):625–36. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


