Abstract
Objective
We evaluated the therapeutic effectiveness of a new, orally active epoxyeicosatrienoic acid analog (EET-A) in rats with angiotensin II (ANG II)-dependent malignant hypertension.
Methods
Malignant hypertension was induced in Cyp1a1-Ren-2 transgenic rats by activation of the renin gene using indole-3-carbinol (I3C), a natural xenobiotic. EET-A treatment was started either simultaneously with I3C induction process (early treatment) or 10 days later during established hypertension (late treatment). Blood pressure (BP) (radiotelemetry), indices of renal and cardiac injury, and plasma and kidney levels of the components of the renin–angiotensin system (RAS) were determined.
Results
In I3C-induced hypertensive rats, early EET-A treatment attenuated BP increase (to 175 ± 3 versus 193 ± 4 mmHg, P < 0.05, on day 13), reduced albuminuria (15 ± 1 versus 28 ± 2 mg/24 h, P < 0.05), and cardiac hypertrophy as compared with untreated I3C-induced rats. This was associated with suppression of plasma and kidney ANG II levels (48 ± 6 versus 106 ± 9 and 122 ± 19 versus 346 ± 11 fmol/ml− or g, respectively, P < 0.05) and increases in plasma and kidney angiotensin (1–7) concentrations (84 ± 9 versus 37 ± 6 and 199 ± 12 versus 68 ± 9 fmol/ml or g, respectively, P < 0.05). Remarkably, late EET-A treatment did not lower BP or improve renal and cardiac injury; indices of RAS activity were not affected.
Conclusion
The new, orally active EET-A attenuated the development of experimental ANG II-dependent malignant hypertension, likely via suppression of the hypertensiogenic axis and augmentation of the vasodilatory/natriuretic axis of RAS.
Keywords: epoxyeicosatrienoic acid analog, malignant hypertension, renal blood flow, renal blood flow autoregulation, renin–angiotensin system, sodium excretion
INTRODUCTION
Malignant hypertension is the most severe and, if untreated, fatal form of human hypertension. As originally described by Volhard and Fahr [1] and confirmed by other clinical investigators, its hallmark is acute elevation of blood pressure (BP) to extremely high levels, accompanied by vascular injury in many tissues, especially the kidney [2–7]. Although it affects only 1–2% of all hypertensive patients, the incidence and prevalence of this life-threatening condition do not decrease over time [8], even though the advances in antihypertensive treatment regimens have dramatically improved the prognosis [7,9]. As recognized in the latest guidelines for the management of arterial hypertension published by the European Society of Hypertension and European Society of Cardiology, malignant hypertension remains a serious economic burden on public health services [10]. Therefore, new anti-hypertensive strategies are needed to combat both severe hypertension and the associated organ damage. However, the prerequisite for success of such attempts is comprehensive knowledge of the pathophysiology of malignant hypertension which is still incompletely understood.
In this regard, it is important to emphasize that the seminal studies of Laragh et al. showed that abnormal activation of the renin–angiotensin system (RAS) plays a critical role in the pathophysiology of malignant hypertension. Depending upon the defect in the mechanism(s) controlling renin release, increases in renin release by the kidney results in increased generation of angiotensin II (ANG II) and more tissue vascular injury leading, again, to increases in renin release establishing a vicious circle that is eventually fatal to the patient [5,11–14]. The notion that RAS plays a critical role in the pathophysiology of malignant hypertension was subsequently supported by other investigators, and it is now proposed that malignant hypertension is a renin-dependent form of hypertension [5,6,14,15]. However, the exact nature of the alterations in RAS activity which result in the transition to malignant hypertension is not completely understood. It has been proposed that instead of mere activation, disturbances in the interactions of RAS with other vasoactive systems may underlie the development of malignant hypertension [5,14,15].
Within this concept, particular attention has been focused on epoxyeicosatrienoic acids (EETs), cytochrome P-450 (CYP)-dependent metabolites of arachidonic acid, because an increasing body of evidence indicates that EETs participate in the regulation of cardiovascular and renal function [16–19]. Recent studies have shown that increased EET bioavailability is associated with lowered BP and attenuation of the concurrent renal damage [19–26], hence, an obvious rationale for an attempt to reproduce these beneficial actions in malignant hypertension. Abnormalities in EET production and/or action have been found in various animal models of cardiovascular diseases [16–19,21,23]. More relevantly, human clinical studies have shown that EET deficiency has a role in the pathophysiology of renovascular hypertension and preeclampsia [27,28], whereas abnormally low EET levels and/or actions were detected in patients with essential hypertension, coronary artery disease, and renal diseases [19,29–31]. Therefore, elevation of EET levels is considered a promising therapeutic objective [15–18]. In most studies, tissue EET bioavailability was increased by blocking soluble epoxide hydrolase (sEH) to prevent the otherwise fast degradation of EETs to relatively inactive dihydroxyeicosatrienoic acids (DHETEs). However, the strategy to indirectly increase EET levels by inhibition of sEH might prove less successful whenever endogenous EET biosynthesis is compromised (e.g., as a consequence of inflammatory disorders, treatment with drugs that inhibit CYP activity, etc.). An alternative approach to utilize EETs in the treatment of cardiovascular diseases that circumvents the limitations of compromised EET biosynthesis is based upon the application of EET-agonistic analogs that are designed to exhibit good aqueous solubility as well as have improved metabolic and chemical stability [32]. Recently, a newly developed 14,15-EET-A [disodium (S)-2-(13-(3-pentyl)ureido)-tridec-8(Z)-enamido)succinate, EET-A] was found to be suitable for in-vivo studies [33], for example, in the study by Hye Khan et al. [34], EET-A attenuated the development of hypertension in ANG II-infused rats. Admittedly, attenuation of subsequent hypertension was observed only when EET-A treatment and ANG II infusion were started at the same time (‘early treatment protocol’) [34]. On the other hand, we recently found that in two-kidney, one-clip Goldblatt hypertensive rats, EET-A treatment did not attenuate hypertension or end-organ damage when applied in the phase of sustained hypertension (‘late treatment’ protocol) [35]. It should be emphasized that the effects of EET-A treatment have not yet been examined in malignant hypertension.
A promising model for studies of the role of the RAS in pathophysiology of hypertension is provided by a recently generated inbred transgenic rat with inducible hypertension (strain name: Cyp1a1-Ren-2); these animals express the mouse Ren-2 renin gene under control of the Cyp1a1 promoter. After exposure of the Cyp1a1-Ren-2 rat to oral indole-3-carbinol (I3C), expression of the Cyp1a1 promoter is rapidly enhanced, with a marked increase of Ren-2 renin gene expression in the liver. This is equivalent to infusion of renin, with a subsequent increase in plasma ANG II, and development of ANG II-dependent malignant hypertension [36–43].
Notably, this model displays the features of primarily renin-dependent hypertension, which accords well with the nature of malignant hypertension as originally proposed by Laragh [5]. Using the same model, we recently showed that development of typical malignant hypertension is not simply the result of RAS overactivity, but also of its perturbed interactions with other vasoactive systems. In particular, we found that the development of ANG II-dependent malignant hypertension in Cyp1a1-Ren-2 transgenic rats is accompanied by decreased intrarenal EET concentrations [26,38,41]. Based on the above considerations, we first decided to employ the model in an attempt to examine if chronic oral administration of EET-A would attenuate the development of ANG II-dependent malignant hypertension.
Second, to make the study more relevant to patients already in the malignant phase, we examined in Cyp1a1-Ren-2 transgenic rats if EET-A treatment would modify established malignant hypertension and end-organ damage.
Third, to gain more insight into the role of intrarenal interactions of CYP-derived eicosanoids and the RAS in ANG II-dependent malignant hypertension, we determined the effects of EET-A treatment on intrarenal concentrations of EETs and DHETEs, urinary excretion of nitrate/nitrite (UNOx V) and 8-isoprostane (UISOV), and the expression/activity of individual components of the RAS.
Fourth, to explore in more depth the possible role of interactions between CYP-dependent metabolites and the RAS in the potential antihypertensive potency of EET-A, we determined the effects of angiotensin-converting enzyme inhibition (ACEi) on the aforementioned parameters.
Fifth, as our recent studies have shown that improvement of deranged pressure-natriuresis relationship is likely a critical mechanism responsible for the antihypertensive effects of sEH inhibition in this ANG II-dependent malignant form of hypertension [26,38], and in view of the evidence that EET-A inhibits epithelial sodium channel (ENaC) activity and expression in ANG II-infused rats [34], we also examined the effects of EET-A treatment on the renal blood flow (RBF) autoregulatory capacity.
METHODS
The studies were performed in accordance with guidelines and practices established by the Committee for Animal Care and Use at the Institute of Clinical and Experimental Medicine.
Animals, diets, and chemicals
As a model of malignant hypertension, a recently generated inbred transgenic rat with inducible hypertension [strain name: TGR (Cyp1a1Ren-2)] was used as described in the introduction section. The fact that the transgene is inserted on the Y chromosome [36] limits the study of pathophysiology of ANG II-dependent malignant hypertension to males; nevertheless, the model has wide applicability. All animals used in the present study were bred at the Center for Experimental Medicine of the Institute of Clinical and Experimental Medicine from stock animals supplied from the Center for Cardiovascular Science, University of Edinburgh, UK (we acknowledge the generous gift of Professor Mullins). Rats were fed either a rat chow without I3C (noninduced groups) or a rat chow containing 0.3% I3C (I3C-induced groups).
EET-A was given in drinking water, and its concentration was adjusted in a way that the daily intake was 10 mg kg−1 body weight. The employed dosage was validated by pharmacokinetic and recent in-vivo studies of the drug [32–34]. In addition, our preliminary studies have shown that plasma EET-A concentrations in analog-treated noninduced or IC3-induced Cyp1a1-Ren-2 transgenic rats exceeded the IC50 level. EET-A was designed and synthesized in J.R.F.’s laboratory. Trandolapril (Gopten; Abbot, Prague, Czech Republic), an ACEi, was used at a dose of 6 mg/l in drinking water; we recently showed that this dose completely blocked the development of hypertension in Cyp1a1-Ren-2 transgenic rats after induction of the renin gene [42].
Experimental design
Series 1: Effects of starting EET-A and ACEi treatments together with induction of renin gene (‘early treatment’ protocol) on BP (radiotelemetry), albuminuria, urinary sodium excretion, UNOx V and UISO V, and cardiac hypertrophy and renal morphology
In accordance with the recommendation for BP measurement in experimental animals, we employed a radiotelemetry system for direct BP measurements [44]. Rats were anesthetized with a combination of tiletamine, zolazepam (Zoletil; Virbac SA, Carros Cedex, France; 8 mg/kg) and xylazine (Rometar, Spofa, Czech Republic; 4 mg/kg) intramuscularly, and TA11PA-C40 radiotelemetric probes (Data Sciences International, St. Paul, Minnesota, USA) were implanted for direct BP measurements as described previously [24,26,38,41]. The rats were allowed 10 days to recover before basal BP was recorded, and only animals with stable BP records at the end of this recovery period were used for experiments. Basal BP was determined for 4 days, and then induction of renin gene was started and continued until the end of the experiment that lasted 13 days. Treatment with EET-A or ACEi was started simultaneously with initiation of dietary administration of I3C. In the animals implanted with radiotransmitters, 24-h urine collections were performed in metabolic cages prior (day 2) and during (days 2, 6, and 12) I3C, EET-A, and ACEi administration to assess daily sodium excretion, albuminuria, and UNOx V and UISO V by methods described previously [22,26,41]. At the end of experiments, all rats were killed by an overdose of thiopental sodium (Sandoz, Basel, Switzerland), and the kidneys were removed for assessment of renal injury. Renal glomerular damage and tubulointerstial injury were assessed by methods described in our previous studies, thereby allowing comparison of the present results with those of our previous studies of pathophysiology of hypertension-associated end-organ damage [22–26,38,39]. We used the ratio of left ventricle weight to tibia length to evaluate the degree of cardiac hypertrophy, as we and others [39] have previously demonstrated that this is the most suitable index to assess cardiac hypertrophy when accompanied by a significant loss of body weight, which was the case in our experimental animals.
The following groups of Cyp1a1-Ren-2 transgenic rats were examined:
I3C-induced/untreated (n = 8)
I3C-induced/EET-A (n = 9)
I3C-induced/ACEi (n = 7)
Noninduced/untreated (n = 6)
Noninduced/EET-A (n = 6)
Noninduced/ACEi (n = 6)
Series 2: Effects of starting EET-A and ACEi treatments together with induction of renin gene (‘early treatment’ protocol) on plasma and kidney activity/expression of individual components of the RAS and kidney concentrations of EETs and DHETEs
Experimental groups, as in series 1, were employed (n = 7 in each group). It is now generally recognized that plasma and tissue ANG II concentrations in anesthetized animals are higher than those from decapitated conscious rats and that normotensive animals exhibit a greater increase in renin secretion in response to anesthesia and surgery than observed in ANG II-induced hypertensive intrarenal renin-depleted animals [37,45,46]. Therefore, in this study we determined ANG II levels in separate groups of conscious rats that were killed by decapitation. Plasma and whole-kidney angiotensin I (ANG I) and ANG II concentrations were assessed by radioimmunoassay (RIA) as described in detail in our previous studies [37,45,47]. In this study, measurement of plasma renin activity used a commercially available RIA kit (Cisbio Bioassays, Paris, France), and quantification of plasma and kidney ACE activities used the ACE kinetic assay (Bühlmann Laboratories, Allschwil, Switzerland). In this protocol, one unit of angiotensin-converting enzyme (ACE) activity was defined as the amount of enzyme required to release 1 μmol of hippuric acid/minute/liter of serum at 37 °C. In this study, ACE activity was also estimated as the ANG II to ANG I ratio, because we have recently found that in this ANG II-dependent model of hypertension, in accordance with one original study in ANG II-infused hypertensive rats, the ratio is a very reliable index for assessment of circulating and especially tissue ACE activity [47–50]. Plasma and kidney tissue renin, angiotensin-converting enzyme type 2 (ACE2) activities, and angiotensin-(1–7) (ANG 1–7) levels were measured as described previously [47–50]. In addition, expression of liver Ren-2 renin gene, of ANG II type 1 (AT1) receptor and G-protein-coupled receptor Mas gene (as a functional receptor for ANG 1–7) in the kidney, and of ACE and ACE2 gene in the kidney were determined as detailed previously [48–50]. The levels of the arachidonic acid metabolites, EETs, and DHETEs were measured in the kidney cortex. Samples were extracted, separated by reverse-phase HPLC, and analyzed by negative-mode electrospray ionization and tandem mass spectroscopy as described previously [47–50]. The procedures for blood sampling, the assays of individual components of RAS, and the EET and DHETE assays are routinely employed in our laboratory, and this standardized approach allows us to compare the results with those of our previous studies evaluating the role of the RAS and CYP-dependent metabolites in the pathophysiology of ANG II-dependent form of hypertension [24,26,35].
Series 3: Effects of starting EET-A treatment together with induction of renin gene (‘early treatment’ protocol) on RBF and glomerular filtration rate (GFR) responses to reduction of renal arterial pressure (RAP)
Experimental groups were exposed to the same chronic protocol as in series 1. On the day of acute experiment, rats were anesthetized with thiopental sodium (60 mg/kg, i.p.) and placed on a thermoregulated surgical table to maintain body temperature at 37 °C. Tracheostomy was performed, and a PE-240 tube (Smiths Medical Int. Ltd, Ashford, Kent, UK) was inserted to maintain a patent airway. The exterior end of the tracheal cannula was placed inside a small plastic chamber into which a humidified 95% oxygen/5% carbon dioxide mixture was continuously delivered, which has been shown to improve the stability of arterial BP of thiopental-anesthetized rats. The right jugular vein was catheterized for fluid infusion and anesthetic administration as needed. The left femoral artery was cannulated with a PE-50 catheter (Smiths Medical Int. Ltd) to allow continuous monitoring of arterial BP and blood sampling. Mean arterial pressure (MAP) was monitored with a pressure transducer (model MLT 1050) and recorded using a computerized data-acquisition system (PowerLab/4SP; AD Instruments, Oxford, UK). The left kidney was exposed via a flank incision, isolated from the surrounding tissue and placed in a lucite cup, and the ureter was cannulated with a PE-10 catheter (Smiths Medical Int. Ltd). The aortic clamp was placed on the aorta above the junction of the left renal artery to control the level of RAP. An ultrasonic transient-time flow probe (1RB; Transonic Systems, Altron Medical Electronic GmbH, Berlin, Germany) connected to a Transonic flowmeter was placed on the left renal artery to record RBF. During surgery, an isotonic saline solution containing bovine serum albumin (6%; Sigma Chemical Co., Prague, Czech Republic) was infused at a rate of 40 μl/min. After the surgery, isotonic saline solution containing albumin (1%) and polyfructosan inulin (7.5%; Inutest, Laevosan, Linz, Austria) was infused at the same volume infusion rate. After completion of the surgical procedures, an equilibration period of 50 min was allowed for the animals to establish steady state before initiating one 30-min control urine collection at an initial level of RAP. In the control protocol groups, another 30-min urine collection period at the initial level of RAP was obtained. In the experimental protocol groups, the first 30-min urine collection at the initial RAP level was followed by two 30-min urine collections at reduced RAP values of 110 and 95 mmHg. Five minutes of equilibration time was allowed after each step of RAP reduction. Blood samples were collected after the second and third urine collections to allow determination of GFR and fractional sodium excretion. This experimental procedure is similar to that used by Wang et al. [51] in ANG II-infused hypertensive rats and to that recently employed in our lab in Cyp1a1-Ren-2 transgenic rats [22,38,40]. Urine volume was measured gravimetrically. Urinary sodium concentration was determined by flame photometry. Polyfructosan clearance was used as an estimate of GFR. Plasma and urine polyfructosan was measured colorimetrically, and the values were corrected to gram of kidney weight. Sodium excretion was calculated using the standard formula. The following experimental groups of Cyp1a1-Ren-2 transgenic rats were examined:
I3C-induced/untreated + control protocol (n = 6)
I3C-induced/untreated + experimental protocol (n = 9)
I3C-induced/EET-A + control protocol (n = 6)
I3C-induced/EET-A + experimental protocol (n = 10)
Noninduced/untreated + control protocol (n = 6)
Noninduced/untreated + experimental protocol (n = 8)
Noninduced/EET-A + control protocol (n = 6)
Noninduced/EET-A + experimental protocol (n = 8)
Series 4: Effects of EET-A treatment in Cyp1a1-Ren-2 transgenic rats with established hypertension (‘late treatment’ protocol) on BP (radiotelemetry), albuminuria, urinary sodium excretion, NOX and UISO, and renal morphology
The animals were exposed to the same methodological approach as described for series 1, except that EET-A treatment was applied after 10 days of induction with I3C, in the phase of established hypertension. Three groups of Cyp1a1-Ren-2 transgenic rats were examined:
Noninduced/untreated (n = 7)
I3C-induced/untreated (n = 9)
I3C-induced/EET-A (n = 11)
Series 5: Renal concentrations of EETs and DHETEs and kidney/plasma ANG II and ANG 1–7 levels in the ‘late treatment’ protocol
Experimental groups exposed to the same experimental protocol as in series 4 were used (n = 8 in each group). The rats were euthanized by decapitation and concentrations of ANG II, ANG 1–7, EETs, and DHETEs were determined.
Statistical analyses
All values are expressed as mean ± SEM. Using the Graph-Pad Prism software (Graph Pad Software, San Diego, California, USA), statistical analyses were performed by Student’s t test, Wilcoxon’s signed-rank test for unpaired data, or one-way analysis of variance (ANOVA), when appropriate. ANOVA for repeated measurements, followed by Student–Newman–Keuls test were performed for the analyses within groups (e.g. analysis of autoregulation capacity of RBF and GFR). Values exceeding the 95% probability limits (P < 0.05) were considered statistically significant.
RESULTS
‘Early treatment’ protocol
Series 1: Effects of starting EET-A and ACEi treatment together with induction of renin gene (‘early treatment’ protocol) on BP (radiotelemetry), albuminuria, urinary sodium excretion, UNOx V and UISO V, cardiac hypertrophy, and renal morphology
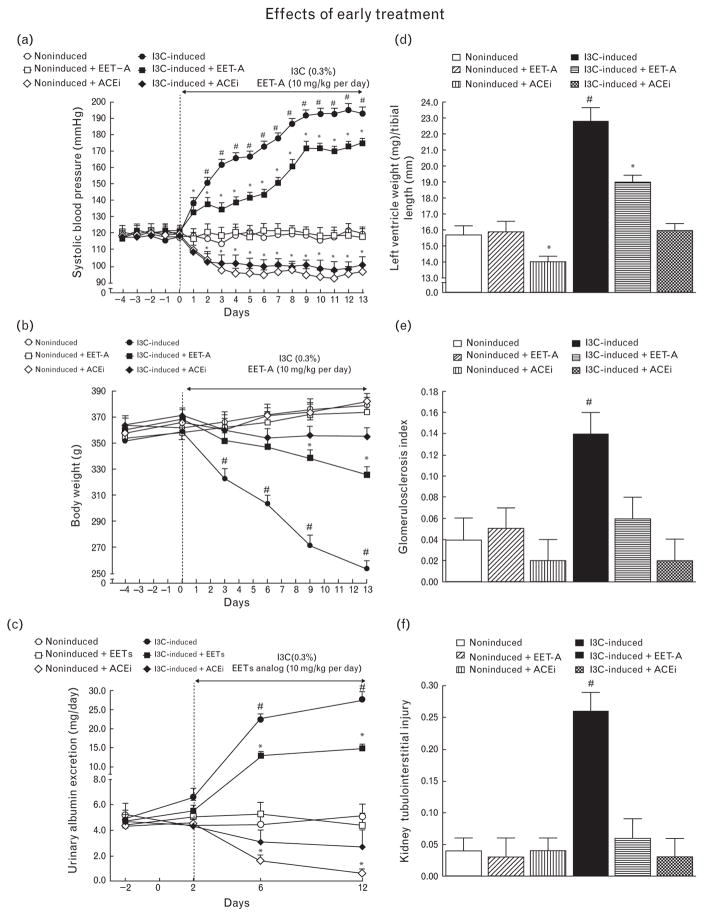
As shown in Fig. 1a, SBP in noninduced rats remained within the normotensive range throughout the experiment and was not altered by EET-A treatment. However, use of ACEi resulted in a significant decrease in SBP as compared with untreated noninduced rats (97 ± 4 versus 120 ± 4 mmHg, P < 0.05, on day 13). I3C induction resulted in severe hypertension, which was significantly attenuated by EET-A treatment: SBP was reduced by about 20 mmHg. Administration of ACEi in I3C-induced rats prevented the increase in SBP which at the end of the experiment was even below the level observed in untreated noninduced rats (Fig. 1a). The profound loss in body weight, as seen in untreated I3C-induced rats, was also greatly attenuated by EET-A treatment. Administration of ACEi to I3C-induced rats abolished body weight loss (Fig. 1b).
FIGURE 1.
Time course of SBP (a), body weight (b), albuminuria (c), data on left ventricle weights normalized by tibial length (d), glomerulosclerosis index (e), and kidney tubulointerstitial injury index (f) in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats, and effects of epoxyeicosatrienoic acid analog and angiotensin-converting enzyme inhibition treatment. *P <.0.05 versus basal values (or versus noninduced rats in the case of left ventricle weights). #P < 0.05 versus *marked values at the same time point (or versus all other groups of rats in the case of left ventricle weights).
As shown in Fig. 1c, untreated noninduced rats exhibited minimal albuminuria throughout the experiment which was not altered by EET-A treatment, whereas administration of ACEi caused reduction in albuminuria as compared with untreated noninduced rats (0.66 ± 0.32 versus 5.1 ± 0.95 mg/24, P < 0.05, on day 12). Untreated I3C-induced rats displayed pronounced albuminuria which was distinctly attenuated in rats treated with EET-A. Administration of ACEi in I3C-induced rats completely prevented the increase in albuminuria which remained at levels not significantly different from those observed in untreated noninduced rats (Fig. 1c).
As shown in Fig. 1d, untreated I3C-induced rats revealed marked cardiac hypertrophy as compared with noninduced rats. Treatment with EET-A attenuated the hypertrophy, whereas ACEi abolished it.
As shown in Fig. 1e and f, noninduced rats exhibited very low indices of kidney glomerulosclerosis and tubulointerstitial injury that were not altered by EET-A or ACEi treatment. In contrast, these indices were markedly elevated in untreated I3C-induced rats; the elevation was prevented by treatment with EET-A as well as with ACEi.
It is important to notice that untreated I3C-induced rats not only rapidly developed hypertension accompanied by profound body weight loss, but also showed extreme lethargy, polyuria, polydipsia, hunched posture, and piloerection. On one hand, these severe phenotype changes in the model closely resemble the characteristics of malignant hypertension observed in untreated patients, as originally described by Volhard and Fahr and confirmed by others [1–7]. On the other hand, the severe phenotype alterations and overall deterioration of the animals’ health limited the duration of experiments and, thus, interpretation as well as potential clinical implications of our experimental findings. Possibly, the described severe phenotype changes are not merely the consequence of BP elevation. Mitchell et al. [42] showed that administration of lower I3C doses retarded the development of hypertension without lowering the final BP levels; however, the normal phenotypical signs of malignant hypertension were reduced. This suggests that the basic protocol for induction of malignant hypertension could be modified to develop some optimal variant. Nevertheless, we are convinced that Cyp1a1-Ren-2 transgenic rats, as applied in the present study, provide a valuable model for studies of malignant hypertension pathophysiology.
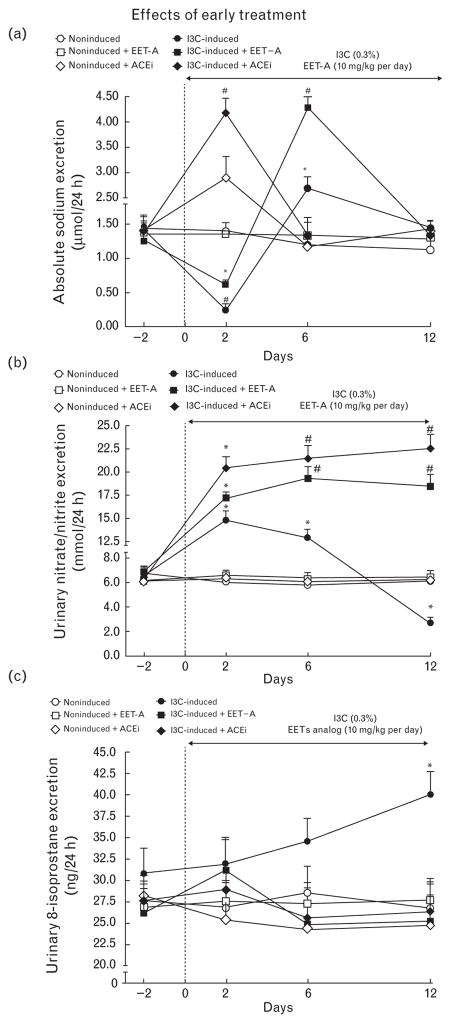
As shown in Fig. 2a, daily sodium excretion (UNa V) in noninduced rats remained unchanged throughout the experiment and was not altered by EET-A treatment. In noninduced rats, ACEi treatment elicited an initial increasein UNa V (day 2), which then returned to values similar to those observed in untreated noninduced rats. In untreated I3C-induced rats, UNa V initially decreased on day 2 after induction, but was above control values on day 6 and returned to control (i.e., day 2) values on day 12. EET-A treatment of I3C-induced rats significantly reduced the initial decrease in UNa V, but also enhanced the subsequent UNa V increase on day 6 to values 3.5-fold higher than without treatment (P < 0.05). Thereafter, UNa V returned to levels observed in untreated noninduced rats. Interestingly, ACEi treatment of I3C-induced rats not only prevented the initial decrease in UNa V, but even elicited a marked increase in UNa V (<3-fold, measured on day 2), thereafter UNa V returned to levels observed in untreated noninduced rats (Fig. 2a).
FIGURE 2.
Time course of daily sodium excretion (a), urinary excretion of nitrate/nitrite (b), and urinary 8-isoprostane excretion (c) in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats and effects of epoxyeicosatrienoic acid analog and angiotensin-converting enzyme inhibition treatment during ‘early treatment’ protocol. *P < 0.05 versus basal values. #P < 0.05 versus *marked values at the same time point.
As shown in Fig. 2b, UNOx V in untreated noninduced rats was stable throughout the experiment and was not altered either by EET-A or ACEi treatment. In I3C-induced rats, UNOx V initially increased (day 2 after induction) and then progressively and significantly decreased below values observed in noninduced rats. Treatment with either EET-A or ACEi prevented this late decrease and, at the end of the experiments, UNOx V in both groups was more than 3-fold higher than in noninduced rats (P <0.05).
As shown in Fig. 2c, UISO V in noninduced rats was stable throughout the experiment and was not altered by EET-A or ACEi treatment. In I3C-induced rats, UISO V gradually increased and on day 12 was 46% higher than in untreated noninduced rats (P < 0.05). Treatment with either EET-A or ACEi prevented this increase.
Series 2: Effects of starting EET-A and ACEi treatment together with induction of renin gene (‘early treatment’ protocol) on plasma and kidney activity/expression of individual components of the RAS and kidney concentrations of EETs and DHETEs
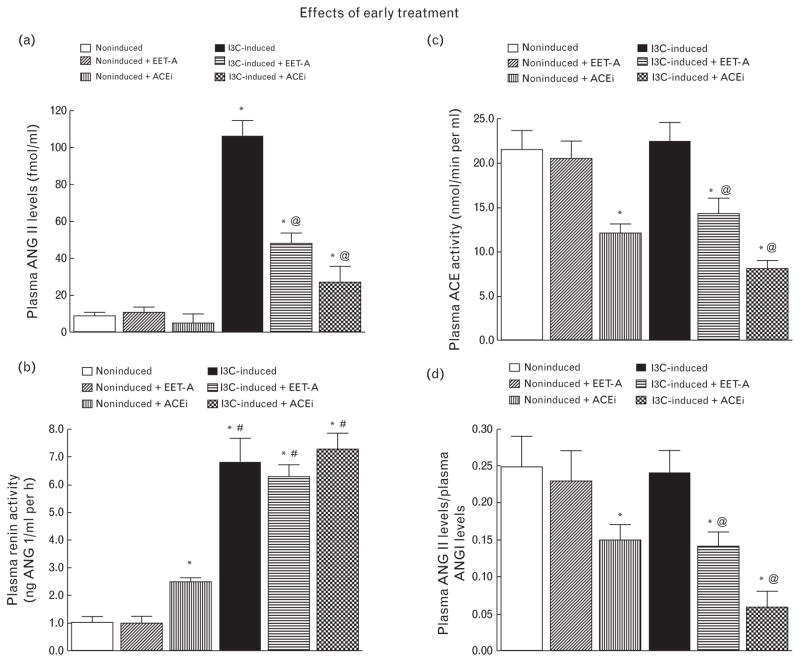
As shown in Fig. 3a, plasma ANG II levels were markedly higher in untreated I3C-induced rats than in untreated noninduced rats (106 ± 9 versus 9 ± 2 fmol/ml, P < 0.05). Neither EET-A nor ACEi treatment altered the plasma ANG II level in noninduced rats, but they did decrease it approximately to one-half in I3C-induced rats. ACEi treatment lowered plasma ANG II levels to one-third in I3C-induced rats, but they still remained significantly elevated as compared with noninduced rats (27 ± 9 versus 9 ± 2 fmol/ml, P < 0.05).
FIGURE 3.
Plasma angiotensin II levels (a), plasma renin activity (b), plasma angiotensin-converting enzyme activity (c), and the ratio of plasma angiotensin II to angiotensin I levels (d) in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats. Effects of epoxyeicosatrienoic acid analog and angiotensin-converting enzyme inhibition treatment are also shown. *P < 0.05 versus untreated noninduced rats #P < 0.05 versus *marked values. @P < 0.05 versus untreated indole-3-carbinol induced rats.
As shown in Fig. 3b, plasma renin activity was significantly higher (almost 7-fold) in untreated I3C-induced than in untreated noninduced rats. The application of EET-A did not alter plasma renin activity in noninduced rats. In contrast, administration of ACEi elicited a 2.5-fold increase in plasma renin activity in noninduced rats compared with untreated noninduced rats (P < 0.05). Neither EET-A nor ACEi changed plasma renin activity in I3C-induced rats.
As shown in Fig. 3c and d, plasma ACE activity measured either directly or estimated as the ratio of ANG II to ANG I did not significantly differ between untreated noninduced and I3C-induced rats. In contrast with ACEi treatment which significantly reduced plasma ACE activity in noninduced rats, EET-A treatment had no effect. Remarkably, the EET-A regien significantly reduced plasma ACE activity in I3C-induced rats and ACEi treatment caused a further decrease in plasma ACE activity in I3C-induced rats.
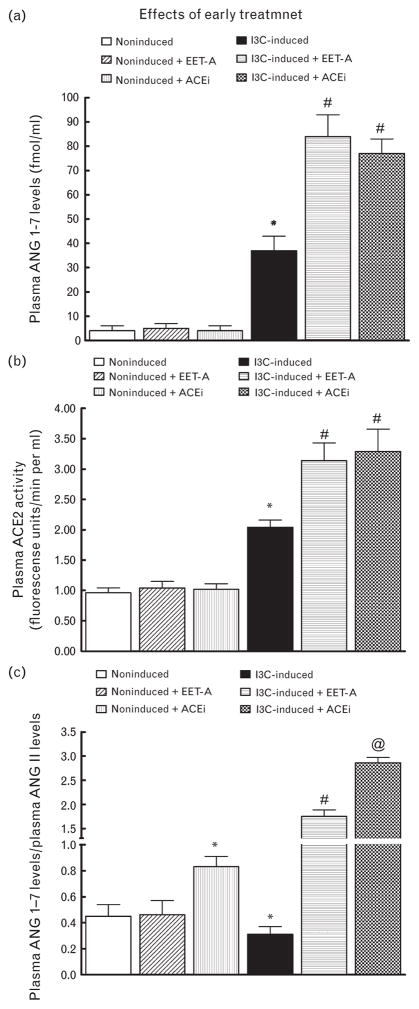
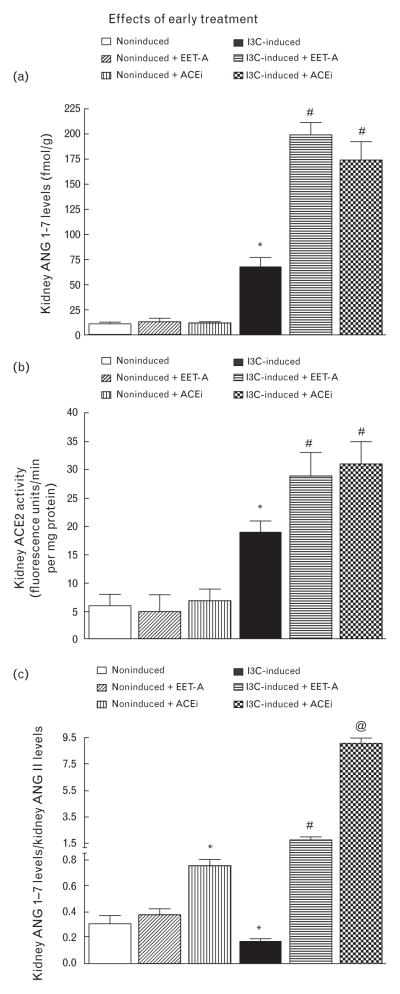
As shown in Fig. 4a, plasma ANG 1–7 levels were significantly higher in untreated I3C-induced than in untreated noninduced rats (37 ± 6 versus 4 ± 2 fmol/ml, P < 0.05). Administration of EET-A or ACEi did not change plasma ANG 1–7 levels in noninduced rats. Remarkably, when dosed with either EET-A or ACEi, plasma ANG 1–7 levels increased further in I3C-induced rats.
FIGURE 4.
Plasma angiotensin 1–7 levels (a), plasma angiotensin-converting enzyme type 2 activity (b) and the ratio of plasma angiotensin-1–7 to angiotensin II in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats. Effects of epoxyeicosatrienoic acid analog and angiotensin-converting enzyme inhibition treatment. *P < 0.05 versus noninduced rats. #P < 0.05 versus *marked values. @P < 0.05 versus untreated indole-3-carbinol induced rats.
As shown in Fig. 4b, plasma ACE2 activity, when measured directly, showed a similar plasma activity pattern in noninduced and I3C-induced rats as also observed with plasma ANG 1–7 levels.
Figure 4c demonstrates the balance between vasodilator and vasoconstrictor axes of the RAS in the circulation expressed as the ratio of ANG 1–7 to ANG II (the biologically most important peptides of both axes). This ratio has been validated as a reliable marker for evaluation of the activity of the ACE2/ANG 1–7 axis [50,52]. Our data show that in untreated I3C-induced rats, its value was only one-half of that in untreated noninduced rats. Employment of EET-A did not alter this ratio in noninduced rats, but administration of ACEi caused about a 2-fold increase in this ratio. In contrast, EET-A dosing elicited about a 6-fold increase in this ratio in I3C-induced rats as compared with untreated I3C-induced rats; treatment with ACEi caused even further increases in this ratio in I3C-induced rats (about 9-fold).
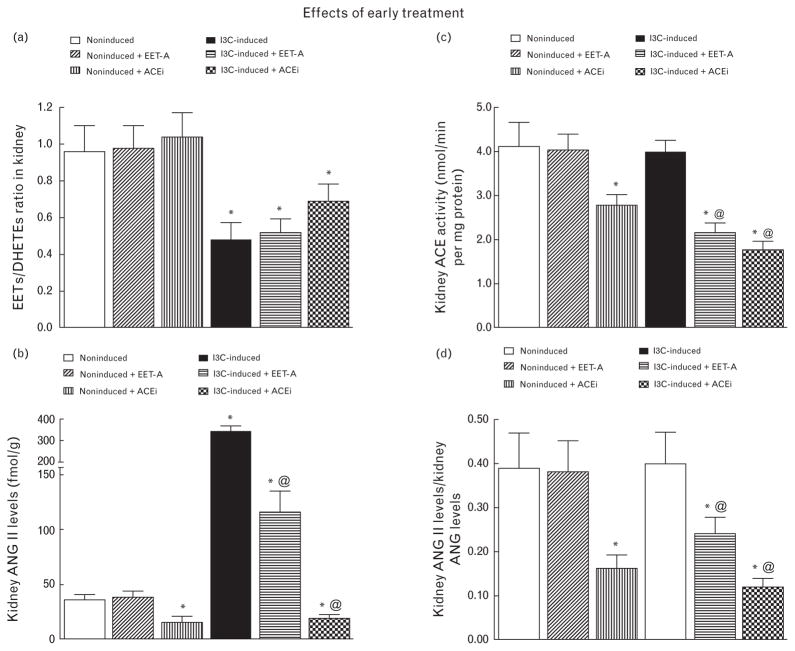
Figure 5a shows the intrarenal availability of biologically active epoxygenase metabolites expressed as the ratio of biologically active EETs to the almost inactive DHETEs. This ratio was significantly lower in I3C-induced rats compared with noninduced rats. Treatment either with EET-A or with ACEi did not alter it in either group.
FIGURE 5.
Kidney epoxyeicosatrienoic acids to dihydroxyeicosatrienoic acids ratio (a), kidney angiotensin II levels (b), kidney angiotensin-converting enzyme activity (c), the ratio of kidney angiotensin II to angiotensin I levels (d), in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats. Effects of epoxyeicosatrienoic acid analog and angiotensin-converting enzyme inhibition treatment. *P < 0.05 versus noninduced rat @P < 0.05 versus untreated indole-3-carbinol induced rats.
Figure 5b shows that untreated I3C-induced rats exhibited almost 10-fold higher kidney ANG II concentrations compared with untreated noninduced rats (P < 0.05). EET-A application did not alter kidney ANG II concentrations in noninduced rats, but decreased it more than 3-fold in I3C-induced rats. On the other hand, treatment with ACEi decreased kidney ANG II concentration in noninduced as well as in I3C-induced rats, even below levels observed in untreated noninduced rats (P < 0.05).
As shown in Fig. 5c and d, kidney ACE activity and the renal ANG II/ANG I ratio (index of ACE activity) did not significantly differ between untreated noninduced and I3C-induced rats. EET-A treatment did not alter kidney ACE activity in noninduced rats, whereas utilization of ACEi significantly reduced this activity. In I3C-induced rats, both EET-A and ACEi significantly reduced kidney ACE activity.
As shown in Fig. 6a–c, kidney ANG 1–7 concentrations, kidney ACE2 activity, and the ANG 1–7 to ANG II ratio all displayed the same value distribution pattern as observed in circulating blood.
FIGURE 6.
Kidney angiotensin 1–7 (a), kidney angiotensin-converting enzyme type 2 activity (b), the ratio of kidney angiotensin-1–7 to angiotensin II in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats. Effects of epoxyeicosatrienoic acid analog and angiotensin-converting enzyme inhibition treatment are also shown. *P < 0.05 versus noninduced rat. #P < 0.05 versus *marked values. @P < 0.05 versus all the other groups.
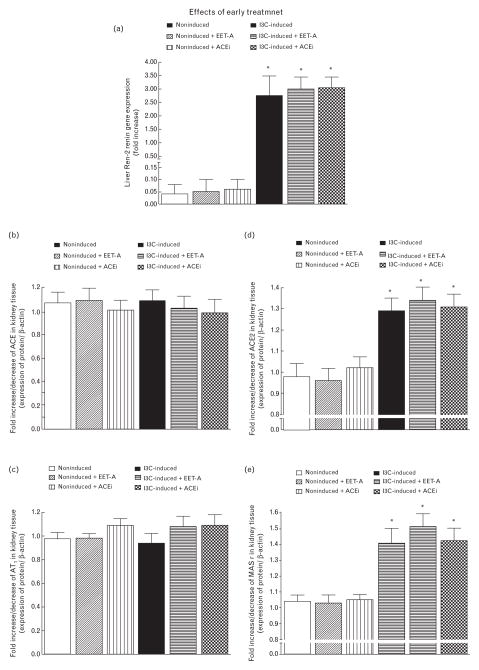
As shown in Fig. 7a, liver expression of the Ren-2 renin gene in noninduced rats was virtually undetectable and was not altered by EET-A or ACEi treatment. Dietary administration of I3C resulted in marked liver Ren-2 renin gene expression which was not significantly altered by EET-A or by ACEi treatment.
FIGURE 7.
Liver Ren-2 renin gene expression (a), kidney angiotensin-converting enzyme gene expression (b), kidney angiotensin II type 1 receptor gene expression (c), kidney angiotensin-converting enzyme type 2 (d), and kidney Mas receptor gene expression (e). Effects of epoxyeicosatrienoic acid analog and angiotensin-converting enzyme inhibition treatment are also shown. *P < 0.05 versus noninduced rat.
As shown in Fig. 7b and c, there were no significant differences in kidney ACE and AT1 receptor gene expressions between untreated noninduced rats and untreated I3C-induced rats. Exposure to either EET-A or ACEi did not alter kidney ACE and AT1 receptor gene expression in both noninduced and I3C-induced rats.
Figure 7d and e shows that kidney ACE2 and Mas receptor gene expressions were significantly higher in untreated I3C-induced than in untreated noninduced rats. Treatment with EET-A or with ACEi did not alter them in either group.
Series 3: Effects of starting EET-A treatment together with induction of renin gene (‘early treatment’ protocol) on RBF and GFR responses to reduction of RAP
Basal values of MAP, renal hemodynamics and electrolyte excretion (average values obtained from the first clearance periods obtained at an initial level of RAP were pooled from groups under control and experimental protocols) are summarized in Table 1.
TABLE 1.
Basal values of arterial blood pressure, renal blood flow, and renal excretory function
| Group | N | MAP (mmHg) | GFR (ml/min per g) | RBF (ml/min per g) | UNaV (μmol/min per g) | FENa (%) | V (μl/min per g) |
|---|---|---|---|---|---|---|---|
| I3C-induced rats/untreated | 15 | 179 ± 3* | 1.06 ± 0.28 | 4.59 ± 0.24* | 1.19 ± 0.28 | 1.01 ± 0.24 | 16.18 ± 2.61* |
| I3C-induced rats/EET-A | 16 | 156 ± 4** | 1.17 ± 0.22 | 7.02 ± 0.61 | 1.07 ± 0.24 | 0.98 ± 0.26 | 16.02 ± 2.12* |
| Noninduced rats/untreated | 14 | 114 ± 3 | 1.22 ± 0.25 | 7.81 ± 0.54 | 0.76 ± 0.27 | 0.81 ± 0.22 | 8.92 ± 0.71 |
| Noninduced rats/EET-A | 14 | 115 ± 4 | 1.16 ± 0.19 | 8.04 ± 0.47 | 0.79 ± 0.26 | 0.84 ± 0.23 | 9.22 ± 0.61 |
The pooled data derived from groups under control and experimental protocol. Values are means ± SEM. EET-A, group treated with orally active epoxyeicosatrienoic acid analog; FENa, fractional sodium excretion; GFR, glomerular filtration rate; I3C-induced rats, Cyp1a1-Ren-2 transgenic rats fed the diet containing 0.3% indole-3-carbinol; MAP, mean arterial pressure; Noninduced rats, Cyp1a1-Ren-2 transgenic rats fed the diet without indole-3-carbinol; RBF, renal blood flow; UNa V, sodium excretion; V, urine flow.
P < 0.05 versus noninduced rat group.
P < 0.05 versus untreated I3C-induced rats.
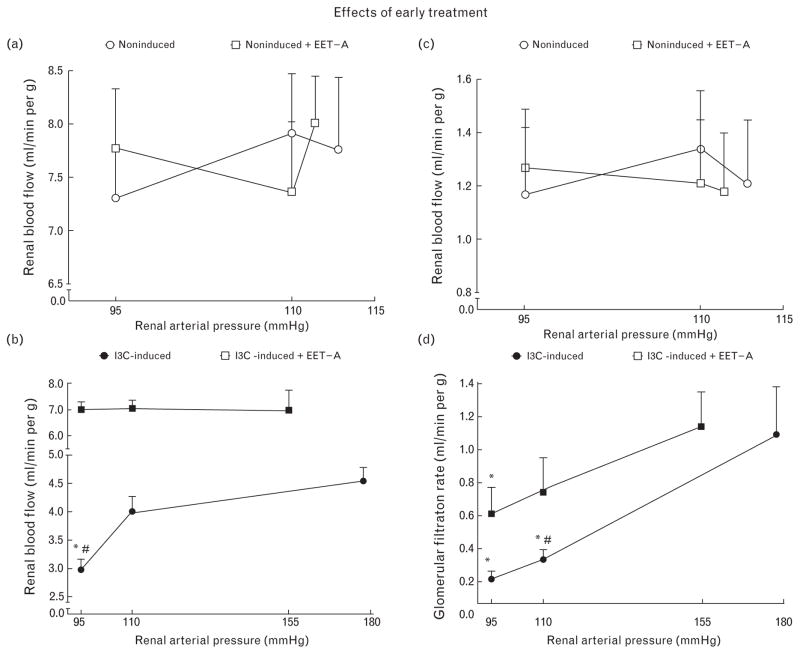
As shown in Fig. 8a and c, reductions of RAP in untreated noninduced rats did not significantly reduce RBF and GFR, which indicates that their autoregulatory capacity was maintained; this was also the case after EET-A treatment.
FIGURE 8.
The relationship between renal arterial pressure and renal blood flow (a and b), and glomerular filtration rate (c and d). The data for indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats and effects of epoxyeicosatrienoic acid analog treatment are shown. *P < 0.05 versus basal values. #P < 0.05 versus the corresponding value (at the same time point) in the other group.
As shown in Fig. 8b, at basal levels of RAP, RBF was distinctly lower in untreated I3C-induced rats as compared with untreated noninduced rats. In the untreated group, a reduction of RAP to 95 mmHg resulted in a profound decrease in RBF. Exposure of I3C-induced rats to EET-A not only resulted in a much higher RBF at the basal level of RAP, but also prevented the RBF decrease after BP reduction to 95 mmHg so that the response was comparable with that in noninduced rats (Fig. 8a).
As shown in Fig. 8d, at basal RAP levels, which were distinctly higher in the untreated compared with the treated group, GFR values were comparable. At pressure levels of 110 and 95 mmHg, GFR values were significantly higher during EET-A treatment.
‘Late treatment’ protocol
Series 4: Effects of EET-A treatment in Cyp1a1-Ren-2 transgenic rats with established hypertension (‘late treatment’ protocol) on BP (radiotelemetry), albuminuria, urinary excretion of sodium, NOX and UISO, and renal morphology
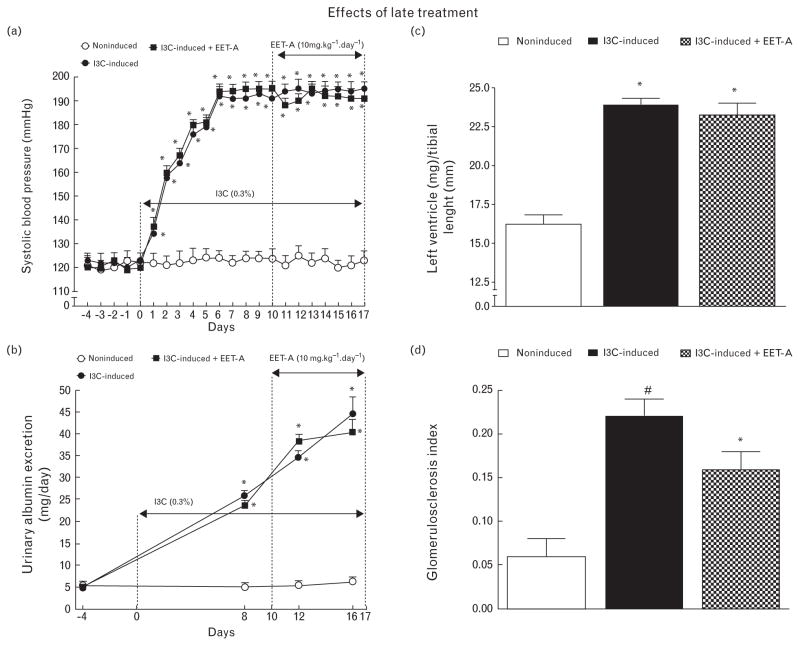
Figure 9a shows that I3C induction for 10 days resulted in severe hypertension (195 ± 3 mmHg); SBP elevation was not significantly altered by EET-A given during the following 7 days.
FIGURE 9.
Time course of SBP (a) and albuminuria (b), data for left ventricle weights normalized by tibial length (c), and glomerulosclerosis index (d) in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats, and effects of epoxyeicosatrienoic acid analog treatment started during established hypertension (‘late treatment’). *P < 0.05 versus basal values (or versus noninduced rats in the case of left ventricle weights and glomerulosclerosis index). #P < 0.05 versus all the other groups (in the case of glomerulosclerosis index).
Figure 9b shows that in untreated I3C-induced rats, albuminuria progressively increased almost 10-fold (P < 0.05), and that the increase was not influenced by EET-A treatment.
As shown in Fig. 9c, application of EET-A did not attenuate cardiac hypertrophy in I3C-induced rats with established hypertension. Fig. 9d shows that untreated I3C-induced rats exhibited pronounced renal glomerular damage that was moderately, but significantly reduced by usage of EET-A.
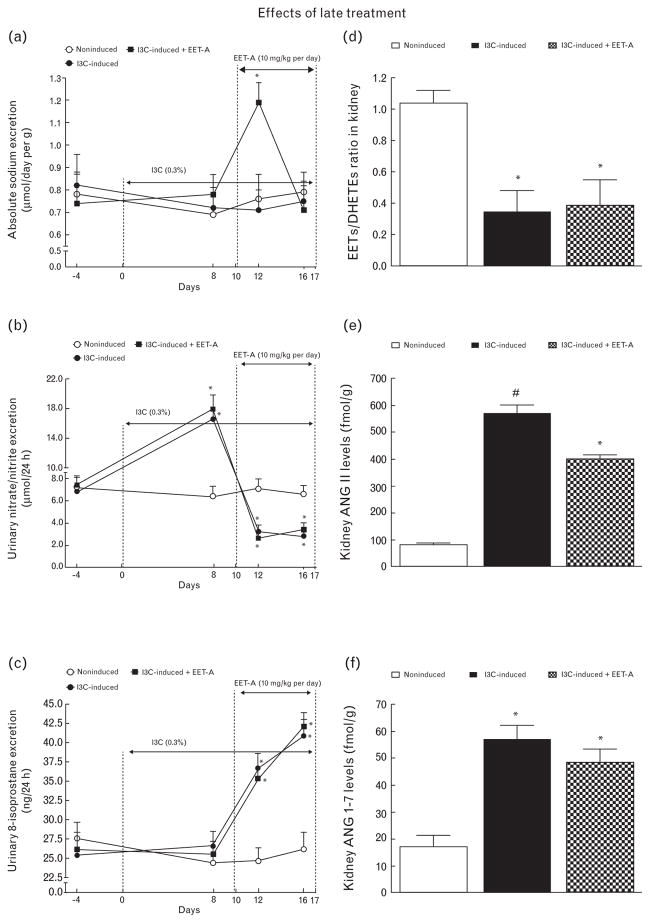
As shown in Fig. 10a, 2 days after initiation of EET-A therapy in I3C-induced rats, a 50% increase in UNa V was seen (P < 0.05), followed by a recovery to levels observed in untreated I3C-induced and noninduced rats.
FIGURE 10.
Time course of daily sodium excretion (a), urinary excretion of nitrate/nitrite (b) and urinary 8-isoprostane excretion (c), and the data for kidney epoxyeicosatrienoic acids to dihydroxyeicosatrienoic acids ratio (d), kidney angiotensin II levels (e), and kidney angiotensin 1–7 levels (f) in indole-3-carbinol induced and noninduced Cyp1a1-Ren-2 transgenic rats, and effects of epoxyeicosatrienoic acid analog treatment (‘late treatment’). *P < 0.05 versus basal values (or versus noninduced rats in the case angiotensin II levels). #P < 0.05 versus all the other groups (in the case of angiotensin II levels).
Figure 10b shows that, similarly as with the ‘early treatment’ protocol, in I3C-induced rats initially, UNOx V was significantly increased (day 8 after induction of renin gene), but on days 12 and 16, it was significantly below values measured in noninduced rats. EET-A did not alter these UNOx V changes.
Figure 10c shows that, in I3C-induced rats, UISO V was markedly elevated on days 12 and 16 of induction compared with the values for noninduced rats; EET-A did not affect this elevation.
Figure 10d shows that the EETs/DHETEs ratio was significantly lower in I3C-induced compared with noninduced rats and that this pattern was not affected by EET-A treatment.
Figure 10e shows that I3C-induced rats exhibited about 7-fold higher kidney ANG II levels compared with noninduced rats (P < 0.05) and that EET-A treatment significantly lowered these elevated values.
Figure 10f shows that untreated I3C-induced rats displayed about 4-fold higher kidney ANG 1–7 levels compared with noninduced rats (P < 0.05) and that EET-A treatment did not significantly alter them.
DISCUSSION
The first major finding
The first major finding of this study is that EET-A treatment in Cyp1a1-Ren-2 rats attenuated the development of malignant hypertension. It also prevented the usual decrease in RBF and simultaneously attenuated albuminuria, kidney tubulointerstitial injury, and glomerulosclerosis, as well as cardiac hypertrophy. Moreover, EET-A treatment not only prevented the decrease in RBF, but also helped maintain its autoregulation. Furthermore, the treatment attenuated the initial decrease in UNa V (day 2) and enhanced the later natriuresis observed in untreated I3C-induced rats (day 6).
To throw light on the possible mechanisms of the protective action of EET-A, we provided detailed information on alterations in NO activity, oxidative stress, and the RAS, including its ACE2-ANG 1–7-Mas receptor axis. We found that UNOx V increased early in untreated I3C-induced rats during the development of hypertension and subsequently decreased in its established phase. On the contrary, UISO V progressively increased in untreated I3C-induced rats throughout the experiment and ultimately was significantly higher than in noninduced rats. Remarkably, EET-A treatment of I3C-induced rats prevented the final decrease in UNOx V and abolished the increase in UISO V. It is noteworthy that ACEi treatment exhibited very similar (if not almost identical) actions on UNOx V and UISO V in I3C-induced rats. Collectively, these observations indicate the EET-A as well as ACEi treatment increased NO bioavailability and decreased superoxide (O2−) concentrations in I3C-induced rats. Unfortunatel y, the present data do not allow a determination of the mechanism(s) underlying these actions. Regardless of the exact mechanism(s) involved, it is obvious that increased NO bioavailability and decreased O2− production might significantly contribute to the antihypertensive actions of EET-A as well as ACEi in I3C-induced rats.
We found also that EET-A treatment of I3C-induced rats suppressed plasma and kidney ANG II levels and, yet, increased plasma and kidney ANG 1–7 concentrations and, especially, intrarenal availability of ANG 1–7 as well as plasma and kidney ACE2 activities. As suggested above, the mechanisms underlying the BP-lowering and end-organ protective effects of EET-A could involve direct action of the analog on renal tubular sodium transport, mediation of the effects by increased NO bioavailability, and suppression of O2− production, which might alter both vascular tone and renal tubular sodium reabsorption, and suppression of the circulating and intrarenal hypertensiogenic RAS axis combined with augmentation of the systemic and intrarenal vasodilator RAS axis.
Ad 1
It was reported that endogenous EETs inhibit sodium reabsorption in the proximal tubule by blocking the sodium–hydrogen exchanger [53] and in the cortical collecting duct by blocking ENaCs [34,54]. These detailed data support the growing evidence that the antihypertensive effect of pharmacologically enhanced tissue EETs is related to their ability to increase renal sodium excretion [17–24]. Therefore, it is plausible to assume the inhibitory action of EETs on sodium transport would act in concert with reduction of the known stimulatory effects of ANG II on direct transport; the latter is a consequence of decreasing plasma and tissue levels of the peptide caused by EET-A treatment as observed in the present study.
Ad 2
Our finding that, in the early phase of hypertension, I3C-induced rats exhibited markedly elevated UNOx V further supports the concept that in this ANG II-dependent model, increased intrarenal NO synthase (NOS) activity and enhanced NO synthesis have a renoprotective role and help compensate for the vasoconstrictor actions of elevated circulating and intrarenal ANG II levels [55,56]. Moreover, our recent study demonstrated that renal NO critically counteracts the enhanced vasoconstrictor action of ANG II after induction of malignant hypertension in Cyp1a1-Ren-2 transgenic rats [38]. Furthermore, it was shown that increased O2− production and the consequent reduced NO bioavailability contributed to the enhanced vascular responsiveness of the ‘slow’ pressure effects of ANG II in ANG II-dependent hypertension [57–59]. In addition, endogenous O2− is known to directly stimulate renal tubular reabsorption leading to sodium retention and, thus, may contribute to BP elevation in various forms of hypertension, including the ANG II-dependent model [55–60]. Furthermore, Hercule et al. [61] found that CYP-derived eicosanoids induced vasodilatation largely via activation of endothelial NOS (eNOS) with a subsequent increase in NO bioavailability. Finally, Patterson et al. [62] demonstrated that elevated O2− levels contributed to increased renal vascular resistance (RVR) and BP elevation in Cyp1a1-Ren-2 transgenic rats with ANG II-dependent malignant hypertension. To summarize, substantially increased NO bioavailability during the developmental as well as maintenance phases of hypertension, combined with abolishment of an increase in O2− production in the maintenance phase, as observed in this study, might have significantly contributed to the BP-lowering and renoprotective actions of chronic EET-A treatment in the ANG II-dependent malignant form of hypertension.
Ad 3
Our complex analysis of the RAS revealed that EET-A inhibited ANG II formation in the circulation and in the kidney at the ACE level. Notably, in ACEi treated rats plasma, ANG II and kidney ACE activities changed along the same pattern as observed in rats treated with EET-A. The striking suppression by EET-A of RAS activity, especially its intrarenal component, is critically important because it is now well recognized that inappropriate activation of kidney RAS and its local action is the main hypertensiogenic mechanism responsible for the development of ANG II-dependent hypertension [63]. Of special interest is our finding that EET-A treatment blocked the activity of the RAS at the ACE level. This is in line with recent findings by Gonzalez-Villalobos et al. [64] that ACE-mediated ANG II formation in the kidney is an independent factor involved in the development of hypertension and that elimination of kidney ACE markedly reduced the hypertension developing in response to ANG II infusion, again, largely by renal mechanisms.
Of special interest are our findings regarding the ACE2-ANG 1–7-Mas receptor axis. This is thought to counteract the classical vasoconstrictor ACE-ANG II-AT1 receptor axis, especially under conditions of enhanced RAS activity [47–50,65–67]. We found that chronic EET-A treatment in I3C-induced rats increased plasma and kidney ANG 1–7 levels and ACE2 activities.
The role of enhanced ACE2 activity should be emphasized because it increases the synthesis of ANG 1–7 from ANG I and from ANG II. The former process would diminish the pool of ANG I available for synthesis of ANG II, and the latter would deplete ANG II directly, as recently proposed by Ferrario [67]. The result would be a reduction of ANG II and an enhancement of ANG 1–7 bioavailability, as reflected in the increased ANG 1–7/ANG II ratio. Setting aside the observed decrease in kidney and plasma activity of ACE, it becomes obvious that EET-A treatment shifts the entire complex of pathways involved in angiotensin peptide synthesis toward generation of ANG 1–7, that is, fosters the prevalence of the vasodilator/natriuretic RAS axis. This is probably the background for the antihypertensive action of EET-A, which supports the view that counteracting the hypertensiogenic RAS axis is the main role of the ACE2-ANG 1–7-Mas pathway [47,65–67]. Remarkably, Gonzalez-Villalobos et al. [64] noted an important limitation of their study, namely, by not analyzing for the effects of elimination of kidney ACE on local expression of ACE2 and ANG 1–7 production, they did not resolve uncertainty about the possible protective contribution of the ANG 1–7-Mas receptor pathway against effects of ANG II infusion or NOS inhibition in mice lacking kidney ACE.
On the whole, our data suggest that suppression of circulating and especially intrarenal ANG II levels combined with activation of the intrarenal ACE2-ANG 1–7-Mas receptor axis are the most likely major mechanisms responsible for the antihypertensive and renoprotective effects of chronic EET-A treatment in Cyp1a1-Ren-2 transgenic rats. In this regard, it is important to remember that, in our newest study, we found that isolated alterations of the intrarenal ACE2-ANG 1–7 complex of the RAS did not significantly modify the course of hypertension in I3C-induced rats. This is because the malignant hypertension in this model clearly depends on the inappropriately increased activity of the ACE-ANG II-AT1 receptor axis [50]. Thus, it is likely that suppression of intrarenal ANG II concentrations is the most important mechanism for the antihypertensive effects arising from chronic EET-A treatment in I3C-induced rats. Most probably, the effects of axis activity shifting within the RAS are modified by changes in the bioavailability of NO depending, at least in part, on the rate of generation of reactive oxygen species (ROS). The status of both systems codetermines renal sodium excretion and peripheral and renal vascular morphology and function, with obvious consequences for BP level and end-organ condition.
The second major finding
The second major finding of this study is that no decrease in BP or substantial reduction of cardiac hypertrophy or renal glomerular and interstitial damage occurred when chronic EET-A treatment of Cyp1a1-Ren-2 transgenic rats was started in the phase of established malignant hypertension. However, it is noteworthy that even though EET-A decreased kidney ANG II during this ‘late treatment’ protocol, the level of ANG II still remained markedly elevated and close to that observed in untreated I3C-induced rats. Nor was there any evidence of an increase in intrarenal ANG 1–7 availability, prevention of oxidative stress, or diminishment of NO bioavailability. All of this contrasts with the ‘early treatment’ experiments.
Thus, in the ‘late treatment’ studies, unlike the ‘early treatment’ studies, the hypertensiogenic axis of RAS remained inappropriately elevated and EET-A treatment did not effectively activate the counteracting vasodilatory axis. Moreover, ‘late treatment’ did not prevent the usual decreases in UNOx V and increases in UISO V.
To summarize, a combination of minimal suppression of enhanced ANG II levels, no elevation of ANG 1–7, and persistent reduction of intrarenal NO bioavailability and enhanced oxidative stress explain the absence of antihypertensive and end-organ protective effects of EET-A when applied in Cyp1a1-Ren-2 transgenic rats with established malignant hypertension.
After setting aside the results from ‘early’ and ‘late’ applications of EET-A in an attempt to treat malignant hypertension in our rat model, a coherent interpretation can be offered. Recall that early treatment improved but did not normalize the indices of the RAS hypertensiogenic axis, body and kidney NO and oxidative stress status, and the rate of sodium excretion. Hence, attenuation, but not reversal of hypertension and end-organ damage was seen. It could be expected that, when treatment is initiated in the established malignant hypertension phase, alterations of vascular function and morphology (both intrarenal and extrarenal), the renal tubules, and the myocardium and tissue structure of multiple other organs are already irreversible. This was confirmed by the absence of lasting substantial improvement of the crucial indices of the RAS, NO system, oxidative stress status, and sodium excretion. Therefore, late EET-A treatment failed to lower BP, improve kidney function, or heart morphological status. However, it is not unlikely that prolonged EET-A treatment could bring improvement even if applied late.
In conclusion, when applied in the initial phase of BP increase, chronic treatment of I3C-induced Cyp1a1-Ren-2 transgenic rats with a new orally active EETs-A substantially attenuated the development of malignant hypertension and hypertension-induced end-organ damage. The treatment suppressed the activity of the ACE-ANG II-AT1 receptor (hypertensiogenic) axis of RAS and increased the activity of its ACE2-ANG 1–7-Mas receptor (vasodilatory/natriuretic) axis. This is probably the essential mechanism of antihypertensive and organ-protecting action of EET-A treatment. On the other hand, when EET-A was applied in the phase of established malignant hypertension, it did not lower BP or attenuate end-organ damage. These findings should be considered in attempts to develop new pharmacologic strategies for treatment of malignant hypertension and suggest that the phase of transition to malignant form in which the treatment is applied may determine the therapeutical effectiveness.
Acknowledgments
Previous presentations: None.
Sources of funding: This study was primarily supported by grant No. P303-15-07544S awarded by the Czech Science Foundation to Z.H. Š. J. is supported the Grant Agency of Charles University No. 266213. J.R.F. is supported by the Robert A. Welch Foundation (I-00110) and NIH (HL034300; HL111392). L.K. is supported by Ministry of Health of the Czech Republic within the project for the development of research organization 00023001 (IKEM) – institutional support. EET-A has been jointly patented by the Medical College of Wisconsin and UT Southwestern.
Abbreviations
- ACE
angiotensin-converting enzyme
- ACE2
angiotensin-converting enzyme type 2
- ACEi
angiotensin-converting enzyme inhibitor
- ANG 1–7
angiotensin-1–7
- ANG I
angiotensin I
- ANG II
angiotensin II
- AT1
receptors for angiotensin II, type 1
- BP
blood pressure
- BW
body weight
- CYP
cytochrome P-450
- DHETEs
dihydroxyeicosatrienoic acids
- EET-A
epoxyeicosatrienoic acid analog
- EETs
epoxyeicosatrienoic acids
- ENaC
epithelial sodium channel
- GFR
glomerular filtration rate
- GSI
glomerulosclerosis index
- I3C
indole-3-carbinol
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOX
nitrate/nitrite
- RAS
renin–angiotensin system
- RBF
renal blood flow
- sEH
soluble epoxide hydrolase
- UISO
8-isoprostane
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Volhard F, Fahr T. Die Brightsche Nierenkrankeit: Klinik, Pathologie und Atlas. Berlin: Springer; 1914. [Google Scholar]
- 2.Byrom FB. The hypertensive vascular crisis. London: William Heinemann Medical Books Ltd; 1969. [Google Scholar]
- 3.Giese J. The renin–angiotensin system and the pathogenesis of vascular disease in malignant hypertension. Clin Sci Mol Med. 1976;51:19–21. [PubMed] [Google Scholar]
- 4.Kawazo N, Eto T, Abe I, Takishita S, Ueno M, Kobayashi K, et al. Pathophysiology in malignant hypertension: with special reference to the renin–angiotensin system. Clin Cardiol. 1987;10:513–518. doi: 10.1002/clc.4960100911. [DOI] [PubMed] [Google Scholar]
- 5.Laragh JH. Laragh’s lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens. 2001;14:186–194. doi: 10.1016/s0895-7061(00)01317-0. [DOI] [PubMed] [Google Scholar]
- 6.Van den Born BJ, Koopmans RP, van Montfrans GA. The renin–angiotensin system in malignant hypertension revisited: plasma renin activity, microangiopathic hemolysis, and renal failure in malignant hypertension. Am J Hypertens. 2007;20:900–906. doi: 10.1016/j.amjhyper.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Guerin C, Gonthier R, Berthoux FC. Long-term prognosis in malignant or accelerated hypertension. Nephrol Dial Transplant. 1988;3:33–37. [PubMed] [Google Scholar]
- 8.Lane DA, Lip GYH, Beevers DG. Improving survival of malignant hypertension patients over 40 years. Am J Hypertens. 2009;22:1199–1204. doi: 10.1038/ajh.2009.153. [DOI] [PubMed] [Google Scholar]
- 9.Shantsila A, Lane D, Beevers DG, Lip GYH. Lack of impact of pulse pressure on outcomes in patients with malignant phase hypertension: the West Birmingham Malignant Hypertension study. J Hypertens. 2012;30:974–979. doi: 10.1097/HJH.0b013e3283526e47. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Fagard R, Narkiewicz K, Redán J, Zanchetti A, Böhm M, et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 11.Laragh JH, Baer L, Brunner HR, Buhler FR, Sealey JE, Vaughan ED., Jr Renin, angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med. 1972;52:633–652. doi: 10.1016/0002-9343(72)90054-x. [DOI] [PubMed] [Google Scholar]
- 12.Laragh JH, Sealey JE, Atlas SA. The renin system for understanding human hypertension: evidence for blood pressure control by a bipolar vasoconstriction-volume mechanism. Prorenin as a determinant of renin secretion. Clin Exp Hypertens. 1982;4:2303–2337. doi: 10.3109/10641968209062392. [DOI] [PubMed] [Google Scholar]
- 13.Laragh JH, Sealey JE. Renin system understanding for analysis and treatment of hypertensive patients: a means to quantify the vasoconstrictor elements, diagnose curable renal and adrenal causes, assess risk of cardiovascular morbidity, and find the best-fit drug regimen. In: Laragh JH, Brenner BM, editors. Hypertension: pathophysiology, diagnosis and management. New York, NY: Raven Press, Publishers; 1990. pp. 1813–1836. [Google Scholar]
- 14.Kincaid-Smith P. Malignant hypertension. J Hypertens. 1991;9:893–899. [PubMed] [Google Scholar]
- 15.Fleming S. Malignant hypertension – the role of the paracrine renin–angiotensin system. J Pathol. 2000;192:135–139. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH674>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev. 2014;66:1106–1140. doi: 10.1124/pr.113.007781. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65:476–482. doi: 10.1161/HYPERTENSIONAHA.114.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmarakby AA. Reno-protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease. Am J Physiol. 2012;302:R321–R330. doi: 10.1152/ajpregu.00606.2011. [DOI] [PubMed] [Google Scholar]
- 19.Fan F, Muoya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24:37–46. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, Morisseau C, Wang JF, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol. 2007;293:F342–F349. doi: 10.1152/ajprenal.00004.2007. [DOI] [PubMed] [Google Scholar]
- 21.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sporková A, Kopkan L, Varcabová A, Husková Z, Hwang SH, Hammock BD, et al. Role of cytochrome P450 metabolites in the regulation of renal function and blood pressure in 2-kidney, 1-clip hypertensive rats. Am J Physiol. 2011;300:R1468–R1475. doi: 10.1152/ajpregu.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neckář J, Kopkan L, Husková Z, Kolář F, Papoušek F, Kramer HJ, et al. Inhibition of soluble epoxide hydrolase by cis-4-[4-(3-adamantan-I-ylureido)cyclohexy-loxy]benzoic acid exhibits antihypertensive and cardioprotective actions in transgenic rats with angiotensin II-dependent hypertension. Clin Sci. 2012;122:513–525. doi: 10.1042/CS20110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honetschlägerová Z, Husková Z, Vaňourková Z, Sporková A, Kramer HJ, Hwang SH, et al. Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J Physiol. 2011;589:207–219. doi: 10.1113/jphysiol.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varcabová Š, Husková Z, Kramer HJ, Hwang SH, Hammock BD, Imig JD, et al. Antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats is mediated by suppression of the intrarenal renin–angiotensin system. Clin Exp Pharmacol Physiol. 2013;40:273–281. doi: 10.1111/1440-1681.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honetschlägerová Z, Sporková A, Kopkan L, Husková Z, Hwang SH, Hammock BD, et al. Inhibition of soluble epoxide hydrolase improves the impaired pressure-natriuresis relationship and attenuates the development of hypertension and hypertension-associated end-organ damage in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2011;29:1590–1601. doi: 10.1097/HJH.0b013e328349062f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, et al. Altered release of cytochrome P450 metabolites of arachidonic acid in renovascular disease. Hypertension. 2008;51:1379–1385. doi: 10.1161/HYPERTENSIONAHA.107.105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catella F, Lawson JA, Fitzgerald DJ, FitzGerald GA. Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci U S A. 1990;87:5893–5897. doi: 10.1073/pnas.87.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuck RN, Theken KN, Edin ML, Caughey M, Bass A, Ellis K, et al. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis. 2013;227:442–448. doi: 10.1016/j.atherosclerosis.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellien J, Iacob M, Remy-Jouet I, Lucas D, Monteil C, Gutierrez L, et al. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation. 2012;125:1266–1275. doi: 10.1161/CIRCULATIONAHA.111.070680. [DOI] [PubMed] [Google Scholar]
- 31.Bellien J, Joannides R. Epoxyeicosatrienoic acid pathway in human health and diseases. J Cardiovasc Pharmacol. 2013;61:188–196. doi: 10.1097/FJC.0b013e318273b007. [DOI] [PubMed] [Google Scholar]
- 32.Falck JR, Kodela R, Manne R, Atcha R, Puli N, Dubasi N, et al. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–5075. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki RV, et al. Development of epoxyeiocastrienoic acids analogs with in vivo antihypertensive actions. Front Physiol. 2010;1:157. doi: 10.3389/fphys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hye Khan MA, Pavlov TS, Christain SV, Neckář J, Staruschenko A, Gauthier KM, et al. Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilatation and sodium channel inhibition. Clin Sci. 2014;127:463–474. doi: 10.1042/CS20130479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alánová P, Husková Z, Kopkan L, Sporková A, Jíchová Š, Neckář J, et al. Orally active epoxyeicosatrienoic acid analog does not exhibit anti-hypertensive and reno- or cardioprotective actions in two-kidney, one-clip Goldblatt hypertensive rats. Vascul Pharmacol. 2015;73:45–56. doi: 10.1016/j.vph.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, et al. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 37.Husková Z, Vaňourková Z, Erbanová M, Thumová M, Opočenský M, Mullins JJ, et al. Inappropriately high circulating and intrarenal angiotensin II levels during dietary salt loading exacerbate hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2010;28:495–509. doi: 10.1097/HJH.0b013e3283345d69. [DOI] [PubMed] [Google Scholar]
- 38.Honetschlägerová Z, Kitada K, Husková Z, Sporková A, Kopkan L, Bürgelová M, et al. Antihypertensive and renoprotective actions of soluble epoxide hydrolase inhibition in ANG II-dependent malignant hypertension are abolished by pretreatment with L-NAME. J Hypertens. 2013;31:321–332. doi: 10.1097/HJH.0b013e32835b50aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaňourková Z, Kramer HJ, Husková Z, Vaněčková I, Opočenský M, Čertíková Chábová V, et al. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens. 2006;24:2465–2472. doi: 10.1097/01.hjh.0000251909.00923.22. [DOI] [PubMed] [Google Scholar]
- 40.Erbanová M, Thumová M, Husková Z, Vaněčková I, Vaňourková Z, Mullins JJ, et al. Impairment of the autoregulation of renal hemodynamics and of the pressure-natriuresis relationship precedes the development of hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2009;27:575–586. doi: 10.1097/hjh.0b013e32831cbd5a. [DOI] [PubMed] [Google Scholar]
- 41.Sporková A, Jíchová Š, Husková Z, Kopkan L, Nishiyama A, Hwang SH, et al. Different mechanisms of acute versus long-term antihypertensive effects of soluble epoxide hydrolase inhibition: Studies in Cyp1a1-Ren-2 transgenic rats. Clin Exp Pharmacol Physiol. 2014;41:1003–1013. doi: 10.1111/1440-1681.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal angiotensin II levels of graded severity in Cyp1a1-Ren2 transgenic rats. JRAAS. 2006;7:74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 43.Peters B, Grisk O, Becher B, Wanka H, Kuttler B, Ludemann J, et al. Dose-dependent titration of prorenin and blood pressure in Cyp1a1-Ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J Hypertens. 2008;26:102–109. doi: 10.1097/HJH.0b013e3282f0ab66. [DOI] [PubMed] [Google Scholar]
- 44.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurements in humans and experimental animals. Part 2: Blood pressure measurements in experimental animals. Hypertension. 2005;45:299–310. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- 45.Husková Z, Kramer HJ, Vaňourková Z, Červenka L. Effects of changes in sodium balance on plasma and kidney angiotensin II levels in anesthetized and conscious Ren-2 transgenic rats. J Hypertens. 2006;24:517–527. doi: 10.1097/01.hjh.0000209988.51606.c7. [DOI] [PubMed] [Google Scholar]
- 46.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol. 1992;262:F902–F909. doi: 10.1152/ajprenal.1992.262.5.F902. [DOI] [PubMed] [Google Scholar]
- 47.Červenka L, Bíbová J, Husková Z, Vaňourková Z, Kramer HJ, Herget J, et al. Combined suppression of the intrarenal and circulating vasoconstrictor renin-ACE-ANG II axis and augmentation of the vasodilator ACE2-ANG 1-7-Mas axis attenuates the systemic hypertension in Ren-2 transgenic rats exposed to chronic hypoxia. Physiol Res. 2015;64:11–24. doi: 10.33549/physiolres.932842. [DOI] [PubMed] [Google Scholar]
- 48.Bürgelová M, Vaňourková Z, Thumová M, Dvořák P, Opočenský M, Kramer HJ, et al. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1–7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens. 2009;27:1988–2000. doi: 10.1097/HJH.0b013e32832f0d06. [DOI] [PubMed] [Google Scholar]
- 49.Hampl V, Herget J, Bíbová J, Baňasová A, Husková Z, Vaňourková Z, et al. Intrapulmonary activation of the angiotensin-converting enzyme type 2/angiotensin 1–7/G-protein-coupled mas receptor axis attenuates pulmonary hypertension in Ren-2 transgenic rats exposed to chronic hypoxia. Physiol Res. 2015;64:25–38. doi: 10.33549/physiolres.932861. [DOI] [PubMed] [Google Scholar]
- 50.Husková Z, Kopkan L, Červenková L, Doleželová Š, Vaňourková Z, Škaroupková P, et al. Intrarenal alterations of the angiotensin-converting enzyme type 2/angiotensin 1–7 complex of the renin–angiotensin system do not alter the course of malignant hypertension in Cyp1a1-Ren-2 transgenic rats. Clin Exp Pharmacol Physiol. 2016;43:438–449. doi: 10.1111/1440-1681.12553. [DOI] [PubMed] [Google Scholar]
- 51.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 52.Elased KM, Cunha TS, Marcondes FK, Morris M. Brain angiotensin-converting enzyme 2 in processing angiotensin II in mice. Exp Physiol. 2008;93:665–675. doi: 10.1113/expphysiol.2007.040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madhun ZT, Goldthwait DA, McKay D, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J Clin Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakairi Y, Jacobson HR, Noland DT, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am J Physiol. 1995;268:F931–F939. doi: 10.1152/ajprenal.1995.268.5.F931. [DOI] [PubMed] [Google Scholar]
- 55.Sigmon DH, Beierwaltes WH. Renal nitric oxide and angiotensin II interaction in renovascular hypertension. Hypertension. 1993;22:237–242. doi: 10.1161/01.hyp.22.2.237. [DOI] [PubMed] [Google Scholar]
- 56.Navar LG, Ichihara A, Chin SY, Imig JD. Nitric oxide–angiotensin II interactions in angiotensin II-dependent hypertension. Acta Physiol Scand. 2000;168:139–147. doi: 10.1046/j.1365-201x.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 57.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 58.Kawada N, Imai E, Karber A, Welch W, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 59.Kopkan L, Husková Z, Vaňourková Z, Thumová M, Škaroupková P, Červenka L, Majid DSW. Superoxide and its interaction with nitric oxide modulates renal function in prehypertensive Ren-2 transgenic rats. J Hypertens. 2007;25:2257–2265. doi: 10.1097/HJH.0b013e3282efb195. [DOI] [PubMed] [Google Scholar]
- 60.Majid DSW, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–952. doi: 10.1111/j.1440-1681.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 61.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, et al. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- 62.Patterson ME, Mullins JJ, Mitchell KD. Renoprotective effects of neuronal NOS-derived nitric oxide and cyclooxygenase-2 metabolites in transgenic rats with inducible malignant hypertension. Am J Physiol. 2008;294:F205–F211. doi: 10.1152/ajprenal.00150.2007. [DOI] [PubMed] [Google Scholar]
- 63.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin–angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MTX, Riquier-Brison AD, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrario CM, Santos RA, Brosnihan KB, Block CH, Schiavone MT, Khosla MC, et al. A hypothesis regarding the function of angiotensin peptides in the brain. Clin Exp Hypertens. 1988;10(Suppl 1):107–121. doi: 10.3109/10641968809075966. [DOI] [PubMed] [Google Scholar]
- 66.Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A, et al. Angiotensin-(1–7): a new hormone of the angiotensin system. Hypertension. 1991;18(Suppl 5):126–133. doi: 10.1161/01.hyp.18.5_suppl.iii126. [DOI] [PubMed] [Google Scholar]
- 67.Ferrario CM. ACE2: more Ang-(1–7) or less Ang II? Curr Opin Nephrol Hypertens. 2011;20:1–6. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]