Abstract
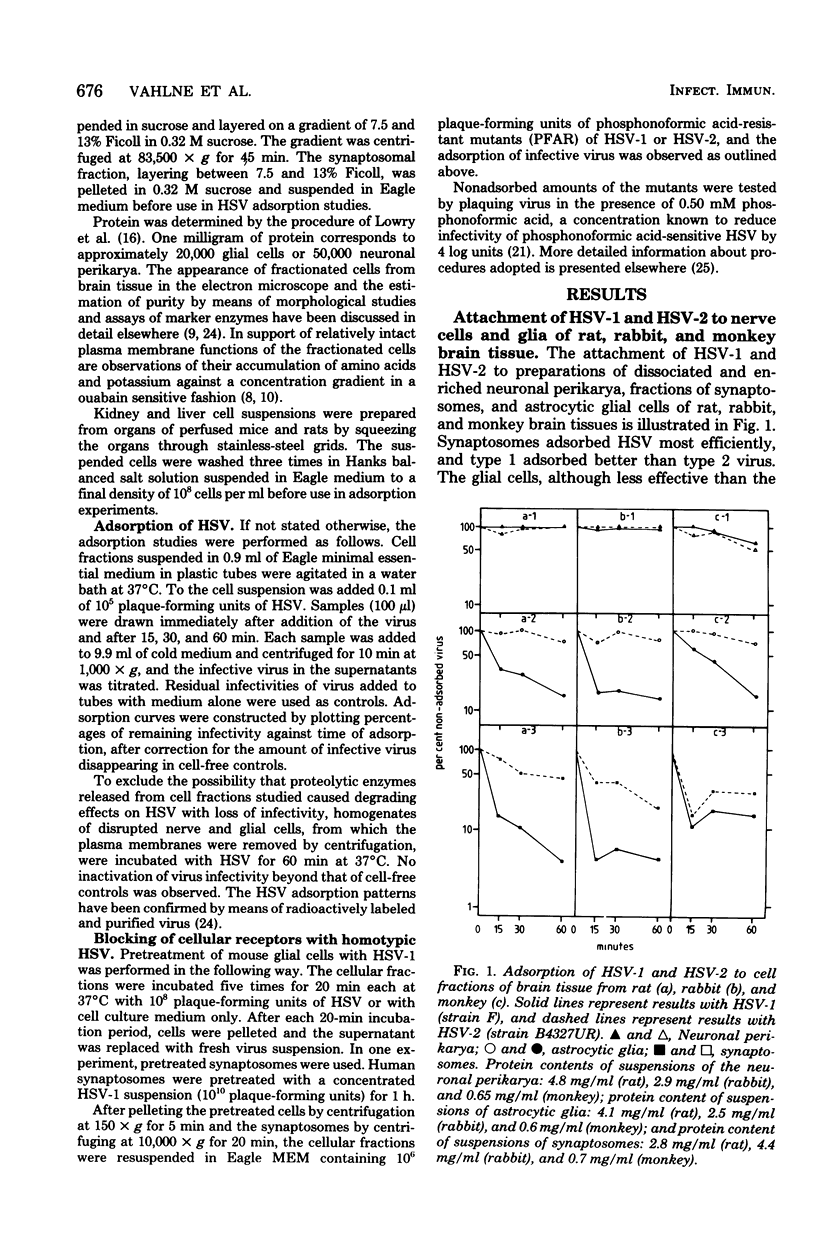
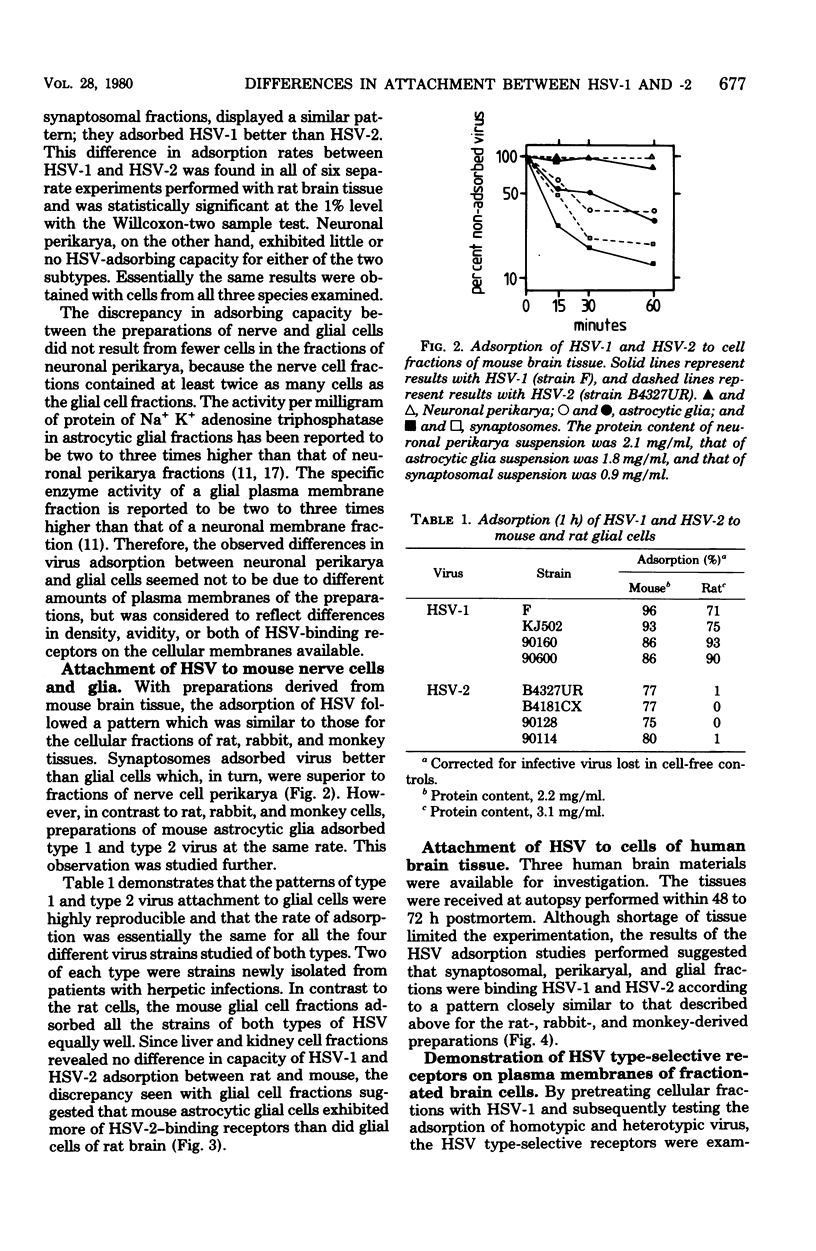
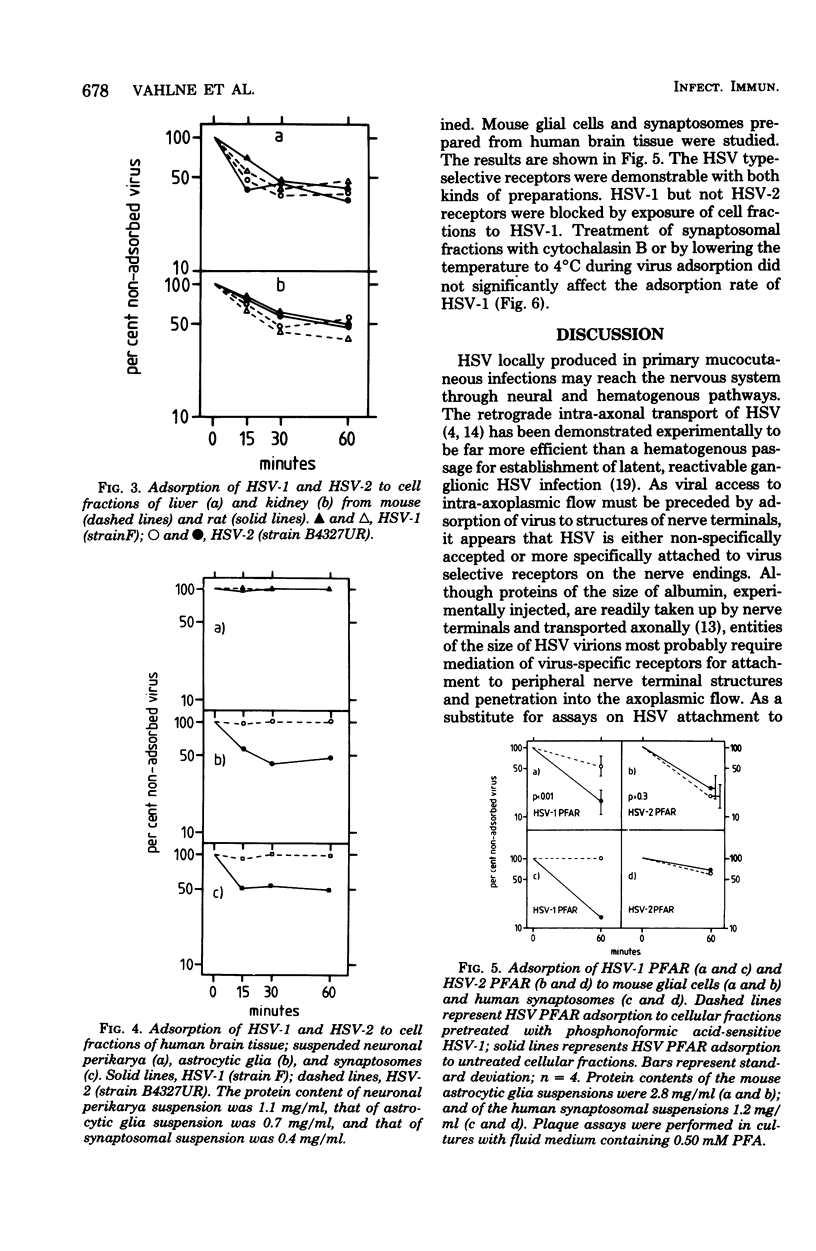
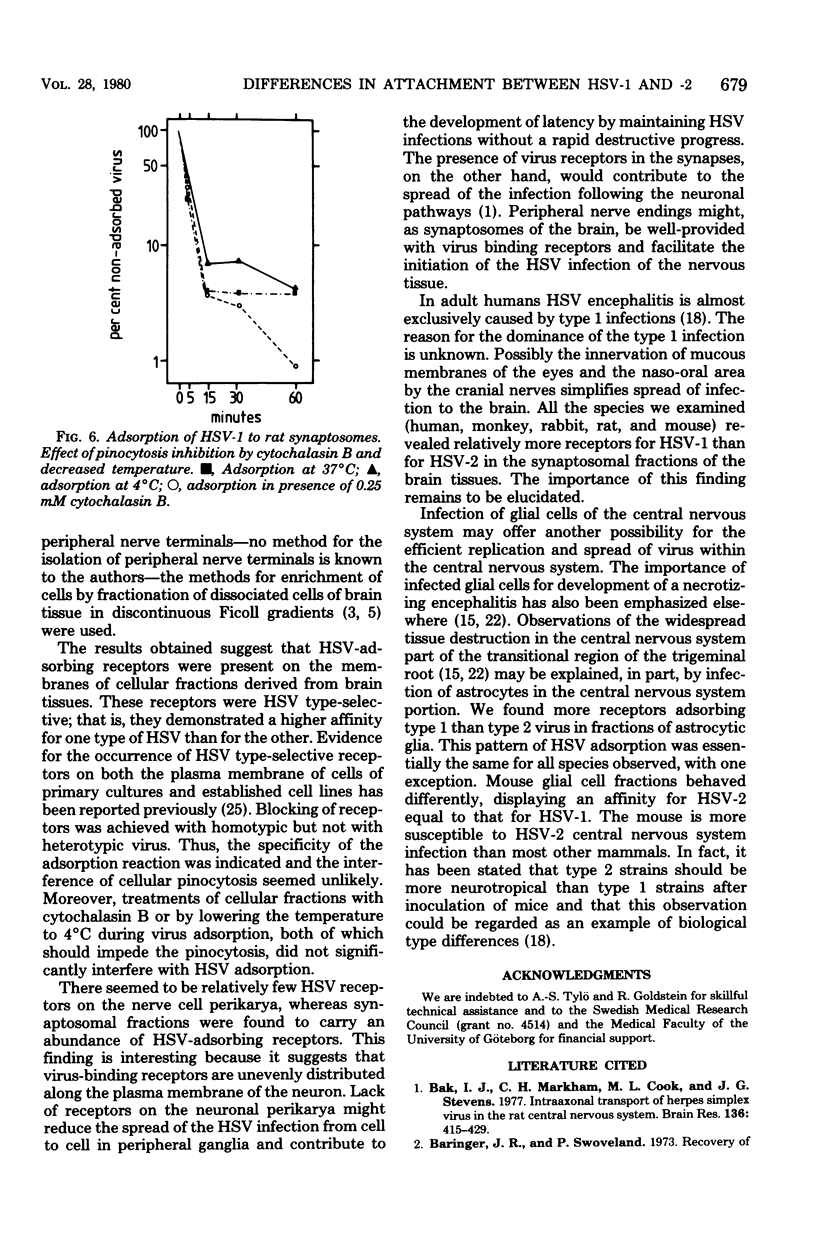
Fractions of nerve cell perikarya, synaptosomes, and astrocytic glia were prepared from human, monkey , rabbit, rat, and mouse brain tissue. The herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) binding capacity of these fractions was studied. Pretreatment of fractions with one type of HSV and the subsequent testing of adsorption of homotypic and heterotypic virus ws employed to reveal type selectivity of virus binding receptors. A higher density of HSV-1 than of HSV-2 selective receptors was found on synaptosomes and glial cells, except with mouse-derived preparations. Synaptosomal and glial cell preparations of mouse brains adsorbed both types of HSV well. Little or no adsorption was observed with HSV-1 and HSV-2 to neuronal perikarya. The type selectivity of HSV binding receptors on brain cells ws demonstrated on preparations of human synaptosomes and mouse glial cells. Some possible implications of the observations on the HSV infection of the nervous system are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak I. J., Markham C. H., Cook M. L., Stevens J. G. Intraaxonal transport of Herpes simplex virus in the rat central nervous system. Brain Res. 1977 Nov 18;136(3):415–429. doi: 10.1016/0006-8993(77)90067-1. [DOI] [PubMed] [Google Scholar]
- Blomstrand C., Hamberger A. Protein turnover in cell-enriched fractions from rabbit brain. J Neurochem. 1969 Sep;16(9):1401–1407. doi: 10.1111/j.1471-4159.1969.tb05992.x. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Matthews D. A. Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochim Biophys Acta. 1971 Dec 3;249(2):380–394. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- Forghani B., Klassen T., Baringer J. R. Radioimmunoassay of herpes simplex virus antibody: correlation with ganglionic infection. J Gen Virol. 1977 Sep;36(3):371–375. doi: 10.1099/0022-1317-36-3-371. [DOI] [PubMed] [Google Scholar]
- Haljamäe H., Hamberger A. Potassium accumulation by bulk prepared neuronal and glial cells. J Neurochem. 1971 Oct;18(10):1903–1912. doi: 10.1111/j.1471-4159.1971.tb09596.x. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Haljamäe H., Hamberger A. Glial cell function: active control of extracellular K + concentration. Brain Res. 1972 Aug 25;43(2):437–443. doi: 10.1016/0006-8993(72)90399-x. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Hamberger A. Glial cell function: uptake of transmitter substances. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2686–2690. doi: 10.1073/pnas.68.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeansson S. Differentiation between herpes simplex virus type 1 and type 2 strains by immunoelectroosmophoresis. Appl Microbiol. 1972 Jul;24(1):96–100. doi: 10.1128/am.24.1.96-100.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Lycke E., Sjöstrand J. Spread of herpes simplex virus in peripheral nerves. Acta Neuropathol. 1971;17(1):44–53. doi: 10.1007/BF00684740. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Vahlne A., Persson L. A., Lycke E. Neural spread of herpes simplex virus types 1 and 2 in mice after corneal or subcutaneous (footpad) inoculation. J Neurol Sci. 1978 Feb;35(2-3):331–340. doi: 10.1016/0022-510x(78)90013-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Medzihradsky F., Sellinger O. Z., Nandhasri P. S., Santiago J. C. ATPase activity in glial cells and in neuromal perikarya of rat cerebral cortex during early postnatal development. J Neurochem. 1972 Feb;19(2):543–545. doi: 10.1111/j.1471-4159.1972.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- Price R. W., Schmitz J. Route of infection, systemic host resistance, and integrity of ganglionic axons influence acute and latent herpes simplex virus infection of the superior cervical ganglion. Infect Immun. 1979 Feb;23(2):373–383. doi: 10.1128/iai.23.2.373-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Svennerholm B., Vahlne A., Lycke E. Inhibition of herpes simplex virus infection in tissue culture by trisodium phosphonoformate. Proc Soc Exp Biol Med. 1979 Jun;161(2):115–118. doi: 10.3181/00379727-161-40502. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Blomberg J., Olofsson S., Lycke E. Subtyping of herpes simplex virus. Acta Pathol Microbiol Scand B. 1975 Oct;83(5):506–512. doi: 10.1111/j.1699-0463.1975.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Nyström B., Sandberg M., Hamberger A., Lycke E. Attachment of herpes simplex virus to neurons and glial cells. J Gen Virol. 1978 Aug;40(2):359–371. doi: 10.1099/0022-1317-40-2-359. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Svennerholm B., Lycke E. Evidence for herpes simplex virus type-selective receptors on cellular plasma membranes. J Gen Virol. 1979 Jul;44(1):217–225. doi: 10.1099/0022-1317-44-1-217. [DOI] [PubMed] [Google Scholar]



