Abstract
Objective: Executive function (EF) deficits in patients with autism spectrum disorder (ASD) are ubiquitous and understudied. Further, there are no effective, neuroscience-based treatments to address this impairing feature of ASD. Repetitive transcranial magnetic stimulation (rTMS) has demonstrated promise in addressing EF deficits in adult neuropsychiatric disorders. This article will outline the design of a novel randomized-controlled trial of bilateral, 20 Hz, rTMS applied to the dorsolateral prefrontal cortex (DLPFC) for treatment of EF deficits in ASD that is currently ongoing. We describe prior therapeutic rTMS research for ASD and prior rTMS trials targeting EFs in adult neuropsychiatric disorders. A neurophysiological rationale for rTMS treatment of EF deficits in ASD is presented.
Methods: An ongoing protocol will enroll participants aged 16–35 with ASD and no intellectual disability. Psychotropic medications will be continued during the 4-week trial of active 20 Hz versus sham rTMS applied to the DLPFC. Twenty, active treatment sessions consisting of 25 stimulation trains at a 90% motor threshold will be administered. The primary outcome measure is the Cambridge Neuropsychological Test Automated Battery (CANTAB) spatial working memory task. At present, recruitment, enrollment, and treatment within the described clinical trial are ongoing.
Conclusions: EF deficits are common and impairing symptoms of ASD. There are no evidence-based treatments for EF deficits in ASD. The protocol described here will provide important preliminary data on the feasibility and efficacy of 20 Hz rTMS to DLPFC for EF deficits in ASD.
Keywords: : Asperger's disorder, autistic disorder, repetitive transcranial magnetic stimulation, executive function deficits, treatment
Introduction
Autism spectrum disorder (ASD) is a lifelong neuropsychiatric disorder with potentially poor outcomes that affects ∼1% of children (Lazoff et al. 2010). In the past decade, ASD administrative prevalence rates rose across several world regions, with disproportionate increases seen for diagnoses among individuals without comorbid intellectual disability (CDC 2012). As prevalence rates for childhood ASD continue to grow, the need for effective interventions that improve long-term outcome is becoming increasingly important. A systematic review of intervention research for the past 30 years recently indicated that the evidence base supporting interventions that can optimize the transition of teens with ASD to adulthood is of poor quality (Lounds Taylor et al. 2012). For individuals aged 13–30 with ASD, there are few treatment options beyond medications with evidence of efficacy for treatment of aggressive behaviors, and limited evidence for nonpharmacological interventions targeting social skills and functional behavior based on small, short-term studies (Lounds Taylor et al. 2012).
Although individuals with ASD without intellectual disability can have good long-term outcomes, longitudinal data indicate that less than 25% achieve functional independence, with respect to independent living, employment, and maintaining relationships, as adults (Howlin and Moss 2012). The years after the exit from high school are a critical time for all young people when a negative transition may pave the way for poor subsequent social and occupational functioning (Rutter 1989). In individuals with ASD, the exit from high school is a time of particular vulnerability when easily available daily structured activity may be lost along with school-based supports/resources, despite ongoing challenges with social communication and reliance on others to help navigate unstructured environments (Shattuck et al. 2011).
Perhaps unsurprisingly, the exit from high school coincides with a time when improvements in ASD symptoms and problem behaviors may slow down (Seltzer et al. 2004). Although individuals with ASD without intellectual comorbidity are more likely to be competitively employed than those with intellectual disability, they are also at a greater risk of having no daytime activities after high school, highlighting the inadequacy of our current system to cultivate the successful transition to adulthood for individuals with ASD without intellectual disability (Taylor and Seltzer 2011).
Executive functions (EF) refer to the high-order cognitive functions that are necessary to shift flexibly from one focus to another (i.e., set-shifting/cognitive flexibility), control or regulate behavior (i.e., response inhibition), and maintain and manipulate information over a short period (i.e., working memory) (Pellicano 2012). Convergent findings indicate that individuals with ASD without intellectual disability feature marked impairments on lab-based measures of working memory and cognitive flexibility (Luna et al. 2007; Eack et al. 2013). Measures of the effects of EF impairments in everyday life indicate that individuals with ASD without intellectual disability also have significant difficulties in initiating, planning, organizing, following through, and monitoring task performance in the real world (Gilotty et al. 2002; Rosenthal et al. 2013).
EF impairments in ASD are strong predictors for poor adaptive functioning in socialization and communication domains over childhood and adolescence (Gilotty et al. 2002), and of poor overall adaptive functioning in adulthood (Szatmari et al. 1989). Therefore, EF deficits may be considered an important target for interventions that are aimed at improving overall function and independence in older youth and young adults with ASD without intellectual disability as they transition to adulthood.
Repetitive transcranial magnetic stimulation applied to the dorsolateral prefrontal cortex as a promising treatment intervention for EF deficits
Transcranial Magnetic Stimulation (TMS) is a unique tool that allows for noninvasive stimulation of the cortex, with broad applications for research and therapeutic interventions in neuropsychiatric populations (Barker et al. 1985; Croarkin et al. 2011). TMS applied repetitively (i.e., repetitive TMS [rTMS]) involves the stimulation of the cortex by a train of magnetic pulses in contrast to single-pulse TMS in which the frequency of stimulation is less than 1 Hz (Wassermann et al. 1998). TMS can selectively activate or inhibit the cortex by stimulating interneurons (Rothwell 1997) at different frequencies, producing minimal discomfort (Croarkin et al. 2011), and treatment effects. A number of rTMS protocols are commonly implemented, including 1, 10, and 20 Hz, theta burst stimulation, and paired associative stimulation protocols.
Central executive control is highly dependent on structural and functional integrity of the dorsolateral prefrontal cortex (DLPFC) (Brodmann Area, BA 9/46). A number of preliminary studies indicate that high-frequency rTMS applied to DLPFC improves EF performance measures of working memory, processing speed, and cognitive flexibility, both in healthy participants and in individuals with neuropsychiatric disorders (e.g., major depressive disorder [MDD] and schizophrenia) (Vanderhasselt et al. 2006a, 2006b; Vanderhasselt et al. 2007; Guse et al. 2010; Huang et al. 2012).
Recently, a randomized, double-blind, sham-controlled pilot study in 27 adults with chronic schizophrenia (average duration of illness = 18 years) found that high-frequency rTMS applied to DLPFC substantially improved working memory performance with a large effect size (Cohen's d = 0.91) (Barr et al. 2013). In this pilot study, participants with schizophrenia were randomized to receive either active (20 Hz) or sham rTMS applied to the DLPFC for 4 weeks (5 days/week). Treatment was applied bilaterally in sequential order to DLPFC (Rusjan et al. 2010). The primary outcome variable was change in performance accuracy on the verbal working memory N-back task completed before and after rTMS treatment. Working memory performance in individuals with chronic schizophrenia was similar to that of healthy controls after 4 weeks of active rTMS (Barr et al. 2013). No significant adverse events were reported during or after treatment.
Can rTMS be applied for treatment of EF deficits in ASD?
Review of rTMS applications in ASD
Thus far, 10 published studies have explored TMS as a therapeutic tool in small samples of individuals with ASD without intellectual disability, including seven open-label studies (Sokhadze et al. 2009, 2012, 2014; Baruth et al. 2010; E. Sokhadze et al. 2010; Casanova et al. 2014; Sokhadze et al. 2014). Of the open-label TMS studies, reported primary outcomes focused on change in event-related potentials (Sokhadze et al. 2009, 2012; Baruth et al. 2010; E. Sokhadze et al. 2010; Casanova et al. 2014). Two of the seven open-label studies found improved cognitive performance (reduced errors and increased error monitoring on a selective attention task) as a secondary outcome after weekly sessions of low-frequency (1 Hz) rTMS to DLPFC for 12–18 weeks in individuals aged 9–21 years with ASD, compared with a non-intervention waitlist group (Sokhadze et al. 2014a, b).
The other three small TMS studies in ASD that have used a randomized controlled design did not target cognitive impairment (Fecteau et al. 2011; Enticott et al. 2012, 2014). Of these, one clinical trial administered a course of deep rTMS (i.e., >2 treatment sessions) to 30 adults (18–59 years) with ASD and IQ >80 and found, after active 5 Hz rTMS to the dorsomedial prefrontal cortex at a resting motor threshold over 5 days per week for 2 weeks, that treatment was tolerable (one dropout from sham and one from the active condition) and social relatedness improved in the active group compared with the sham-controlled group (Enticott et al. 2014).
rTMS safety, efficacy, and tolerability considerations
rTMS is generally regarded as safe, without lasting adverse effects. Inadvertent induction of a seizure is the most important potential neurophysiologic safety concern (Rossi et al. 2009). With the adoption and widespread use of recommended safety guidelines, the risk for rTMS-induced seizures is estimated at ∼0.01%–0.1%, compared with 0.7%–0.9% for spontaneous seizures in the general population, and 0.1%–0.6% for those with antidepressant treatment (Milev et al. 2016). Positive efficacy and safety profiles for rTMS applied to DLPFC have led to its approval for treatment of MDD by Health Canada and treatment-resistant depression by the U.S. Food and Drug Administration (FDA). A number of studies have now extended applications of rTMS to youth (<18 years) with attention-deficit/hyperactivity disorder, Tourette's syndrome, mood disorders, schizophrenia, and ASD (Croarkin et al. 2011). A recent review of rTMS studies conducted in 9–26 year-olds indicates that rTMS has a favorable treatment profile with respect to safety, efficacy, and tolerability, highlighting that: (i) rTMS was very well tolerated, with few adverse effects reported; (ii) in those who did report side effects, transient headaches and scalp discomfort were the most common and were mild to moderate in intensity; (iii) treatment discontinuation rates were low; and (iv) no patients experienced seizures after treatment (Croarkin et al. 2011).
Though relatively few patients with ASD have participated in TMS protocols (<500), no seizures have been reported in any published study and the frequency and quality of reported side effects (transient and mild overall) are consistent with those seen in adolescents and adults with other neuropsychiatric disorders (Oberman et al. 2015).
Rationale for extending success by using rTMS for EF deficits in schizophrenia to ASD
High-order cognitive functions, such as EFs, are reliant on neural network oscillations in the gamma (γ) frequency (30–80 Hz) band (Lewis et al. 2005). It has been proposed that GABA inhibitory interneurons in the DLPFC contribute to the synchronization of pyramidal neurons that is necessary for EF performance. Impaired GABAergic neurotransmission within the DLPFC has been linked with altered gamma oscillatory activity and working memory deficits in schizophrenia (Farzan et al. 2012). Active rTMS treatment has been shown to modulate gamma oscillatory activity within the DLPFC in both healthy individuals (Barr et al. 2009) and patients with schizophrenia (Barr et al. 2011; Farzan et al. 2012) who have demonstrated improved EF performance after rTMS. These results indicate that rTMS effects on EF performance are likely mediated through modulation of gamma oscillations within the DLPFC.
GABAergic impairment, altered DLPFC activation and connectivity, and potential to target DLPFC to treat EF impairments in ASD
Direct comparisons have found similar degrees of EF impairment across adults with ASD without intellectual disability, as found in schizophrenia (Schneider and Asarnow 1987; Goldstein et al. 2002). As in schizophrenia, postmortem data showing reductions in GABA receptor subunit expression (Casanova et al. 2003; Fatemi et al. 2009a, 2009b), and SPECT studies showing reduced GABAergic neurotransmission (Harada et al. 2011; Mori et al. 2012) have indicated that the prefrontal GABAergic system is suppressed in ASD. Further, as in schizophrenia, neuroimaging studies have found an altered DLPFC structure and reduced DLPFC activation and connectivity during EF tasks in individuals with ASD who feature EF deficits (Koshino et al. 2005; Just et al. 2007). Preliminary neurophysiological studies using combined TMS-electromyography (TMS-EMG) or TMS-electroencephalography (TMS-EEG) have further indicated that impaired cortical inhibition (Enticott et al. 2010) and altered gamma oscillatory activity are present during EF task performance in ASD (Sokhadze et al. 2009; Baruth et al. 2010) in a pattern that is very similar to schizophrenia (Rogasch et al. 2014).
Although rTMS has yet to be used specifically to treat EF deficits in ASD, preliminary work in ASD indicates that rTMS applied to DLPFC can modulate gamma oscillatory activity (Baruth et al. 2010) and improve performance monitoring on a visual attention task (Sokhadze et al. 2012). As EF impairments in ASD are similar to those found in schizophrenia, and are linked to alterations in DLPFC function and connectivity (Koshino et al. 2005; Just et al. 2007), the same biological treatments targeting the DLPFC may improve EF performance in both conditions.
Here, we present the protocol for an ongoing randomized, double-blind, sham-controlled study of rTMS applied bilaterally to DLPFC for 4 weeks (5 days/week) for treatment of EF deficits in older youth and young adults with ASD. We use the same rTMS protocol that brought about EF improvements in adults with schizophrenia (Barr et al. 2013). We will also leverage our controlled study design to improve our understanding of the biological mechanisms of rTMS treatment by conducting high-resolution magnetic resonance imaging (MRI) both immediately before the treatment trial and immediately after the completion of treatment (“pre/post” treatment imaging).
The objectives of our ongoing rTMS clinical trial are to: evaluate the efficacy of rTMS as a treatment for EF deficits in ASD; to determine whether rTMS results in structural and functional brain changes within regions that are important for EF performance compared with sham rTMS. We hypothesize that rTMS will be well tolerated in individuals with ASD, that DLPFC targeted treatment will bring about improvements in spatial working memory performance in our active compared with the sham-controlled group, and that changes in DLPFC structure/function will be seen after rTMS, including changes to DLPFC thickness and functional activation (at rest and during completion of a visuospatial N-back fMRI task), white matter anisotropy in tracts that connect the DLPFC, and magnetic resonance spectroscopy-measured GABA concentrations within the DLPFC.
Methods
Our ongoing clinical trial examining the efficacy of rTMS for treatment of EF deficits in ASD uses a randomized, double-blind, sham-controlled study design. Participants are recruited from Child, Youth and Family and Adult ASD services at the Centre for Addiction and Mental Health (CAMH), community clinics and agencies providing services for individuals with ASD, and through advertisements at local colleges and universities. Interested participants are initially provided information about the study and prescreened for eligibility based on age, presence of ASD diagnosis, perceived EF difficulties, verbal fluency, absence of any personal history of seizures, or history of seizure disorder in first-degree relatives. After an initial screening, participants are invited to attend an in-person assessment to determine/confirm eligibility for the study.
The overall recruitment goal for the ongoing rTMS clinical trial is N = 40 participants, randomized to active versus sham rTMS (N = 20 participants/arm). Participants are individuals with ASD without intellectual disability (confirmed by the Wechsler Adult Intelligence Scale-Fourth Edition [WAIS-IV]) (age 16–35 years) (Benson et al. 2010) demonstrating significant EF deficits in everyday functioning. A previous diagnosis of ASD is confirmed by a psychiatrist with specialty training in child and youth psychiatry (SA) and extensive clinical and research training in the evaluation, care, and application of clinical-research methods in individuals with ASD. Confirmation of the presence of an ASD diagnosis in interested participants is supported by review of prior clinical assessments, confirmation of the presence of symptoms that are significant for an ASD diagnosis based on evaluation using the Autism Diagnostic Observation Schedule-II (ADOS-II) (Module 4) (Lord et al. 2000), and information gathered during a clinical interview/assessment that also confirms clinical stability (i.e., no active risk of harm to self or others based on the absence of active suicidal or homicidal ideation and absence of history indicative of significant risk for aggression).
Participants are fluent in the English language, are verbal, competent to consent based on ability to provide a spontaneous narrative description of the key elements of the study, and have no prior history of seizures. Participants who are excluded from the rTMS clinical trial include: individuals with a history of substance abuse or dependence in the past 6 months or a positive urine toxicology screen; those who have a concomitant major medical or neurologic illness, have had a seizure in the past, or first-degree relative with epilepsy, are pregnant or likely to get pregnant during the 4-week trial, are on benzodiazepines >2 mg equivalent of Lorazepam or anticonvulsant medication (Hoffman et al. 2003), have had a history of rTMS treatment, or are not able to commit to the rTMS study protocol. Participants are withdrawn from the rTMS study if they: demonstrate a failure to tolerate the procedure, develop any significant adverse events (e.g., seizure or seizure-like activity), withdraw consent, miss any more than two rTMS treatment sessions in a row or miss any more than four sessions in total, become clinically unstable and the principal investigator believes that for safety reasons (i.e., potential for harm to self or others) the patient should be withdrawn from the study protocol.
All psychotropic medications are continued during the trial; however, no changes are allowed from 4-weeks before randomization until the end of the trial. All participants provide written, informed consent before participation. The study has the approval of the local institutional review board for human subject research protection (CAMH, Toronto, Canada).
Baseline clinical, functional, and cognitive assessment before rTMS treatment
The ADOS-II (Lord et al. 2000) is administered to all participants to confirm diagnosis of ASD. The WAIS-IV is used to assess cognitive level (Benson et al. 2010). The Behavior Rating Inventory for Executive Function (BRIEF for participants 16–18, or BRIEF-A, adult version for participants ≥18) is used to confirm the presence of clinically significant impairment on everyday tasks requiring executive functioning skills, based on a T score above 65 on any sub-scale. The Vineland Adaptive Behavior Scale-II (VABS-II), which can be used in very young individuals and up to those aged 90 (Sparrow and Cicchetti 1985), is used to assess adaptive functioning. Comorbid psychiatric disorders are assessed by using the Mini International Neuropsychiatric Interview (MINI) (≥18 years) or the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) (16–18 years).
The Cambridge Neuropsychological Test Automated Battery (CANTAB, www.cambridgecognition.com) Spatial Working Memory task, for use in individuals 4–90 years of age, is a computer-administered cognitive battery that has been reliably used to assess EFs in ASD (Ozonoff et al. 2004). This measure was chosen as the primary outcome measure for the ongoing rTMS clinical trial as: (1) impairment in spatial working memory assessed using this measure appears to be stable across children and youth with ASD without intellectual disability (Chen et al. 2016), (2) spatial working memory is associated with bilateral DLPFC activation using rTMS (Owen et al. 2005), and (3) working memory has been shown to be responsive to rTMS in healthy controls and other clinical groups, including the rTMS study in schizophrenia using the same treatment protocol (Barr et al. 2013) as administered here in ASD.
The CANTAB Spatial Working Memory task (∼8 minutes) is a self-ordered search task where participants search for tokens hidden inside colored squares and must remember which boxes have already been searched. The number of items to be searched increases from two to eight, reflecting increased difficulty (spatial working memory load). Errors made in this task include touching boxes already found to be empty in the same trial, or boxes already found to contain tokens. Outcome measures include the number of search strategies used in 6- and 8-box trials (strategy utilization) and the total number of errors in 4-, 6-, and 8-box trials (total errors).
The BRIEF self-report was chosen as a secondary outcome measure for the ongoing rTMS clinical trial. The BRIEF (validated for use in those <18 years) and BRIEF-A (validated for use in those ≥18) has demonstrated evidence of reliability, validity, and clinical utility as an ecologically sensitive measure of EFs in healthy adolescents and adults and in a range of conditions, including in individuals with ASD (Gilotty et al. 2002). This self-report measure assesses EF behaviors in everyday (school/home/work) environments and provides eight scales of real-world executive functioning (inhibit, shift, emotional control, initiate, working memory, plan/organize, organization of materials, task monitor). Two broader indices are computed by using subsets of the eight scales: the behavioral regulation index (based on inhibit, shift, emotional control, self-monitor scales) and the meta-cognition index (working memory, plan/organize, organization of materials, monitor); an overall Global Executive Composite score is also provided. On the BRIEF, T scores between 50 and 65 are in the borderline range and T scores >65 are in the clinically significant range and indicative of impairment.
Additional CANTAB EF measures are also collected in the ongoing rTMS clinical trial, including the intradimensional/extradimensional set shift task of cognitive flexibility and the stop signal task measuring response inhibition. Trained raters that are blind to treatment allocation assess all outcome variables for the clinical trial.
Study procedures
The ongoing clinical trial utilizes a randomized, double-blind, sham-controlled design comparing active (20 Hz) and sham rTMS applied 5 days per week for 4 weeks bilaterally to DLPFC in older youth and young adults with ASD. Each rTMS treatment session lasts ∼30–45 minutes. EF performance measures, as described earlier (CANTAB and BRIEF), are administered at baseline and immediately after trial completion. All participants enrolled in our ongoing rTMS clinical trial are asked to return for repeat cognitive assessments at 1 month, 6 months, and 1 year after trial completion, to monitor for adverse effects and to determine whether there is any lasting effect of rTMS treatment on EF performance (Hoffman et al. 2003).
Participants in the ongoing clinical trial undergo MRI-based structural and functional neuroimaging at two time-points: before commencing rTMS treatment and immediately after the 4-week rTMS trial. Pre-/post-rTMS MRI acquisitions include: structural MRI, diffusion imaging, resting-state functional MRI, functional MRI during performance of a spatial working memory task, and a magnetic resonance spectroscopy sequence allowing for indirect measurement of GABA within the DLPFC immediately after the 4-week rTMS trial.
Before commencing the first rTMS treatment session, participants are randomized to active versus sham rTMS treatment by using a computer-generated list based on a stratified randomization scheme and using a permuted block method with a random number generator. The technician who administers the rTMS intervention is the only person aware of the group (active vs. sham) assignment (Schulz and Grimes 2002). Study investigators, clinical/cognitive raters, and participants are blind to the treatment condition. Integrity of our blinded treatment assignment is assessed after the first rTMS treatment session, at which time the participants are asked whether they think they received active versus sham stimulation. A semi-structured clinical interview is administered after each rTMS treatment session to assess for any adverse effects and the general experience of rTMS.
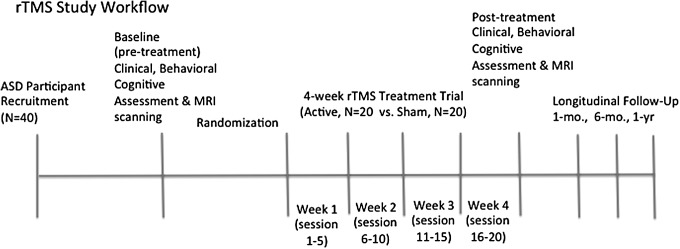
The ongoing clinical trial study protocol conforms to the international Consolidated Standards of Reporting Trials (CONSORT) guidelines and is registered in clinicaltrials.gov (ID: NCT02311751), an international clinical trial registry. See Figure 1 depicting rTMS study workflow and timing for study procedures.
FIG. 1.
rTMS study workflow. rTMS, repetitive transcranial magnetic stimulation.
rTMS treatment
Intensity and frequency
In the ongoing clinical trial, the resting motor threshold is determined according to previously published methods (Daskalakis et al. 2006) before the commencement of the treatment course. Active treatment is delivered at a 90% resting motor threshold intensity. Stimulation is administered at 20 Hz with 25 stimulation trains of 30 stimuli each with an inter-train interval of 30 seconds at equivalent stimulation parameters (Barr et al. 2013). These parameters are consistent with current safety guidelines (Wassermann 1998). Treatment is applied bilaterally to either left or right and then right or left DLPFC at each rTMS session, based on random sequence assignment. The order of bilateral stimulation is held constant for all 20 treatments. The stimulation of the subsequent hemisphere is administered immediately after the stimulation of the first hemisphere. Advanced neuronavigation methods are used to target rTMS to the DLPFC after a T1-weighted MRI scan with a stimulation site: Talairach (x, y, z) = (−) 50, 30, 36; that is, BA 9 and superior section BA 46, using the Ascension MINIBIRD/MRIcro/MRIreg package according to published methods (Rusjan et al. 2010).
In the active rTMS treatment arm, stimulation occurs between two points (posterior and anterior middle frontal gyrus, a stimulation area of ∼2 cm in diameter) at a high intensity to ensure stimulation over both regions (Cohen et al. 1990). To target this region (BA 9/46), seven vitamin E capsules are attached to the scalp surface before MRI acquisition (inion, nasion, right/left mastoid process, hairline, midpoint of a line from the tragus of the ear to the external canthus of the eye on right and left sides). These capsules serve as localizers for subsequent probe registration and coil placement based on a 3D reconstruction of the subject's MRI. The probe on the subject's scalp is tracked on the rendered brain volume with a 1 mm spatial resolution. These techniques have been effectively applied to locate sites of stimulation in rTMS investigation of cognitive function (Barr et al. 2009). In the sham rTMS treatment arm of our ongoing clinical trial, a single-wing tilt position producing contraction of scalp muscles with minimal direct brain effects (Barr et al. 2009) is used (same parameters and site as active condition).
Participants receive a total of 20 treatments in each condition (5 days per week, 4 weeks in total). TMS protocols vary based on: location, intensity and frequency of stimulation, and number and frequency of sessions. TMS parameters for the current trial were chosen to be identical to those found to improve working memory performance in the published pilot study in schizophrenia (Barr et al. 2013) and supported by a recent meta-analysis in >300 adults (Brunoni and Vanderhasselt 2014) and a study of depressed adolescents (14–18 years) (Wall et al. 2013), indicating that high-frequency rTMS to DLPFC has the potential to improve cognition across healthy controls, depressed adults/adolescents, and in schizophrenia.
Proposed analysis
After completion of our ongoing clinical trial, we will conduct an intent-to-treat (ITT) approach to assess the efficacy of rTMS in improving working memory. Our ITT analysis will include all randomized participants who complete at least one rTMS session. We will conduct additional analyses in participants who complete the study protocol. Baseline demographic and clinical characteristics will be compared for the active and sham rTMS groups. The change in CANTAB spatial working memory scores—that is, pre-/postscore differences—will be the main outcome measure in a mixed-effects regression model. Additional exploratory evaluations will examine treatment-attributable changes in BRIEF (everyday EF) scores and additional CANTAB EF measures (significance set at p < 0.05 for all). Adverse event rates will be compared between study groups.
The current sample size of the ongoing clinical trial was chosen based on the effect size of the mean change difference in working memory performance between active and sham groups set at Cohen's d = 0.9 based on the pilot study in individuals with schizophrenia that this study is modeled after (Barr et al. 2013). Power calculation shows that 16 subjects in each group would be required to achieve 80% power to detect a difference of this magnitude. With our proposed sample size of 40 subjects (n = 20 in each group), we will have >85% power to detect this size of difference between groups. We estimate a potential dropout rate of ∼20% based on dropout rates for the pilot study in schizophrenia utilizing the exact same rTMS parameters (Barr et al. 2013) and the prior double-blind sham-controlled 2 week rTMS study in adults with ASD, which had a very low (<5%) dropout rate (Enticott et al. 2014).
Discussion
There are currently no evidence-based interventions available to treat EF deficits in ASD. Recent reviews indicate that pharmacological treatment studies have largely focused on treating core ASD symptoms (e.g., repetitive behaviors) and co-morbid aggressive/self-injurious behaviors (Ameis et al. 2013). Atypical antipsychotics, such as risperidone and aripiprazole, the only FDA-approved medications for use in ASD, are often used to target these behaviors. These medications have many potentially serious side-effects and do not address EF impairments (Lounds Taylor et al. 2012; Ameis et al. 2013).
The present study is the first to assess the efficacy of using rTMS applied to DLPFC as a treatment intervention for EF deficits in ASD. It is anticipated that participants in our sample will display significant impairment in multiple domains of everyday functioning that require EF skills as well as adaptive function impairments. To our knowledge, the current study will be the largest randomized controlled trial testing rTMS in the ASD population.
In the current study, we will also combine our rTMS clinical trial with a comprehensive pre-/posttreatment neuroimaging protocol whereby neuroimaging is conducted at two time-points: immediately before (“pre”) rTMS treatment begins and immediately after (“post”) our 4-week rTMS trial. The advent of sophisticated neuroimaging techniques coupled with the growing realization of the brain's extraordinary plasticity, even in disease states, have led to several reports of structural brain changes after pharmacological, behavioral, and brain stimulation interventions over shorter, similar, and longer durations than the length of our intervention (i.e., 4-weeks) (Pajonk et al. 2010; Bezzola et al. 2011; Falkai et al. 2013). This approach can: (i) provide an explanatory neural mechanism for rTMS treatment efficacy and (ii) potentially identify biomarkers of treatment response.
Our current study provides us with a valuable opportunity to test the relationship between brain structure and function, EF performance, and EF improvement after rTMS and to explore whether changes in brain structure and function after active (vs. sham) rTMS may provide a biological mechanism for treatment efficacy in ASD.
Conclusion
At its conclusion, our rTMS clinical trial will be well positioned to determine whether rTMS treatment holds promise for improvement of EF deficits in ASD. A number of important factors need to be considered after the completion of our study and with respect to future rTMS clinical trial designs. First, we are using high-frequency stimulation, which may have sensory and tolerability implications, particularly in a population where hypersensitivity to sensory stimuli is often present. Second, our study includes the need for participants to be able to commit to a 5-day per week, 4-week treatment trial. This intense schedule may limit our recruitment to participants who are not working or attending school and may present practical challenges with respect to future efforts to scale up our research to change clinical practice. On the other hand, participation in the current trial involves contact with supportive research staff daily over 4 weeks; thus, the social aspects of participation in the current study will also need to be considered with respect to treatment adherence and unintended (and potentially) therapeutic factors.
Future studies will need to test whether shorter courses of rTMS may also improve EF deficits in this population. An important advantage of our study design is the incorporation of longitudinal follow-up at 1-month, 6-months, and 1-year after the 4-week rTMS trial. This design will provide valuable information regarding the potential duration of treatment effects and the need for future research to incorporate ongoing intervention in the form of rTMS booster sessions or combination treatments, such as rTMS paired with cognitive interventions, to improve treatment effects, duration of effects, and potential generalization of effects to everyday life.
Clinical Significance
There are few evidence-based interventions that have been proved to improve outcomes in young people with ASD. If our study indicates that rTMS applied to DLPFC has efficacy as a novel intervention for EF deficits in ASD, it will provide the rationale to undertake a large-scale efficacy trial in this population, a critical step for changing clinical practice. By leveraging our rTMS clinical trial to acquire pre-/posttreatment neuroimaging measures, we will be able to track the effects of active versus sham rTMS on brain structures that are essential for EF performance across the duration of our study and begin to examine the neural mechanisms for rTMS treatment efficacy. Incorporation of longitudinal follow-up will provide insights regarding the need for ongoing intervention to maintain treatment effects.
Acknowledgments
S.H.A. received financial support from the CAMH Foundation via the O'Brien Scholarship Fund, the University of Toronto, Faculty of Medicine, Dean's Fund New Staff Grant, and an Ontario Mental Health Foundation New Investigator Fellowship. D.M.B. received research support from the Canadian Institutes of Health Research (CIHR), National Institute of Health (NIH), Brain Canada, and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd., and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for an investigator-initiated study and medication supplies from Invidior for an investigator-initiated trial. In the past 3 years, Z.J.D. received research and in-kind equipment support from Brainsway Inc and Magventure, Inc for an investigator-initiated study. Z.J.D. has also served on the advisory board for Sunovion, Hoffmann-La Roche Limited and Merck and received speaker support from Eli Lilly. This work was supported by the Ontario Mental Health Foundation (OMHF), the Canadian Institutes of Health Research (CIHR), the Brain and Behaviour Research Foundation, and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute. P.E.C. reports research grant support from Pfizer, the National Institute of Mental Health (K23 MH100266), the Brain and Behavior Research Foundation, and Mayo Clinic Foundation. P.E.C. received equipment support (disposable Senstar shields) from Neuronetics for investigator-initiated studies; he received supplies and genotyping services from Assurex for an investigator-initiated study. P.D. is supported by the Innovation Fund from the Alternate Funding Plan of the Academic Health Sciences Centres of Ontario, Department of Psychiatry, University of Toronto Excellence Fund, Dean's Fund Faculty of Medicine, University of Toronto, and the Caskey/Francis Family Award in Clinical Research.
Disclosures
No competing financial interests exist.
References
- Ameis S, Corbett-Dick P, Cole L, Correll CU: Decision making and antipsychotic medication treatment for youth with autism spectrum disorders: Applying guidelines in the real world. J Clin Psychiatry 2013:74,1022–1024 [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL: Non-invasive magnetic stimulation of human motor cortex [letter]. Lancet 1:1106–1107, 1985 [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Arenovich T, Chen R, Fitzgerald PB, Daskalakis ZJ: The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS One 6:e22627, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. : Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry 73:510–517, 2013 [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Rusjan PM, Chen R, Fitzgerald PB, Daskalakis ZJ: Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology 34:2359–2367, 2009 [DOI] [PubMed] [Google Scholar]
- Baruth JM, Casanova MF, El-Baz A, Horrell T, Mathai G, Sears L, et al. : Low-frequency Repetitive Transcranial Magnetic Stimulation (rTMS) modulates evoked-gamma frequency oscillations in Autism Spectrum Disorder (ASD). J Neurother 14:179–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N, Hulac DM, Kranzler JH: Independent examination of the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV): What does the WAIS-IV measure? Psychol Assess 22:121–130, 2010 [DOI] [PubMed] [Google Scholar]
- Bezzola L, Merillat S, Gaser C, Jancke L: Training-induced neural plasticity in golf novices. J Neurosci 31:12444–12448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt MA: Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn 86:1–9, 2014 [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J: Disruption in the inhibitory architecture of the cell minicolumn: Implications for autism. Neuroscientist 9:496–507, 2003 [DOI] [PubMed] [Google Scholar]
- Casanova MF, Hensley MK, Sokhadze EM, El-Baz AS, Wang Y, Li X, et al. : Effects of weekly low-frequency rTMS on autonomic measures in children with autism spectrum disorder. Front Hum Neurosci 8:851, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC: Prevalence of Autism Spectrum Disorders Among Multiple Areas of the United States in 2008. Community Report From the Autism and Developmental Disabilities Monitoring (ADDM) Network. 2012. www.cdc.gov/mmwr (Accessed December1, 2016)
- Chen SF, Chien YL, Wu CT, Shang CY, Wu YY, Gau SS: Deficits in executive functions among youths with autism spectrum disorders: An age-stratified analysis. Psychol Med 46:1625–1638, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Roth BJ, Nilsson J, Dang N, Panizza M, Bandinelli S, et al. : Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr Clin Neurophysiol 75:350–357, 1990 [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Wall CA, Lee J: Applications of transcranial magnetic stimulation (TMS) in child and adolescent psychiatry. Int Rev Psychiatry 23:445–453, 2011 [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R: The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res 174:403–412, 2006 [DOI] [PubMed] [Google Scholar]
- Eack SM, Bahorik AL, Hogarty SS, Greenwald DP, Litschge MY, Mazefsky CA, et al. : Brief report: Is cognitive rehabilitation needed in verbal adults with autism? Insights from initial enrollment in a trial of cognitive enhancement therapy. J Autism Dev Disord 43:2233–2237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Fitzgibbon BM, Kennedy HA, Arnold SL, Elliot D, Peachey A, et al. : A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul 7:206–211, 2014 [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB: A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev Med Child Neurol 52:e179–e183, 2010 [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB: Repetitive transcranial magnetic stimulation (rTMS) improves movement-related cortical potentials in autism spectrum disorders. Brain Stimul 5:30–37, 2012 [DOI] [PubMed] [Google Scholar]
- Falkai P, Malchow B, Wobrock T, Gruber O, Schmitt A, Honer WG, et al. : The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: A randomized controlled MRI study. Eur Arch Psychiatry Clin Neurosci 263:469–473, 2013 [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Sun Y, Fitzgerald PB, Daskalakis ZJ: Transcranial magnetic stimulation on the modulation of gamma oscillations in schizophrenia. Ann N Y Acad Sci 1265:25–35, 2012 [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD: Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum 8:64–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD: GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord 39:223–230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Oberman L, Pascual-Leone A: Brain stimulation over Broca's area differentially modulates naming skills in neurotypical adults and individuals with Asperger's syndrome. Eur J Neurosci 34:158–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE: Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychol 8:241–248, 2002 [DOI] [PubMed] [Google Scholar]
- Goldstein G, Minshew NJ, Allen DN, Seaton BE: High-functioning autism and schizophrenia: A comparison of an early and late onset neurodevelopmental disorder. Arch Clin Neuropsychol 17:461–475, 2002 [PubMed] [Google Scholar]
- Guse B, Falkai P, Wobrock T: Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: A systematic review. J Neural Transm 117:105–122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, et al. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord 41:447–454, 2011 [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, et al. : Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry 60:49–56, 2003 [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P: Adults with autism spectrum disorders. Can J Psychiatry 57:275–283, 2012 [DOI] [PubMed] [Google Scholar]
- Huang ML, Luo BY, Hu JB, Wang SS, Zhou WH, Wei N, et al. : Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: A double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry 46:257–264, 2012 [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ: Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex 17:951–961, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA: Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage 24:810–821, 2005 [DOI] [PubMed] [Google Scholar]
- Krings T, Naujokat C, von Keyserlingk DG: Representation of cortical motor function as revealed by stereotactic transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 109:85–93, 1998 [DOI] [PubMed] [Google Scholar]
- Lazoff T, Zhong L, Piperni T, Fombonne E: Prevalence of pervasive developmental disorders among children at the English Montreal School Board. Can J Psychiatry 55:715–720, 2010 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW: Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324, 2005 [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. : The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223, 2000 [PubMed] [Google Scholar]
- Lounds Taylor J, Dove D, Veenstra-VanderWeele J, Sathe NA, McPheeters ML, et al. : Interventions for Adolescents and Young Adults with Autism Spectrum Disorders Comparative Effectiveness Review. Rockville: Agency for Healthcare Research and Quality; pp. 1–374, 2012 [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA: Maturation of executive function in autism. Biol Psychiatry 61:474–481, 2007 [DOI] [PubMed] [Google Scholar]
- Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al. : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 4. Neurostimulation treatments. Can J Psychiatry 61:561–575, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Mori K, Fujii E, Toda Y, Miyazaki M, Harada M, et al. : Evaluation of the GABAergic nervous system in autistic brain: (123)I-iomazenil SPECT study. Brain Dev 34:648–654, 2012 [DOI] [PubMed] [Google Scholar]
- Oberman LM, Rotenberg A, Pascual-Leone A: Use of transcranial magnetic stimulation in autism spectrum disorders. J Autism Dev Disord 45:524–536, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E: N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, et al. : Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the collaborative programs of excellence in autism network. J Autism Dev Disord 34:139–150, 2004 [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, et al. : Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry 67:133–143, 2010 [DOI] [PubMed] [Google Scholar]
- Pellicano E: The development of executive function in autism. Autism Res Treat 2012:146132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Daskalakis ZJ, Fitzgerald PB: Cortical inhibition, excitation, and connectivity in schizophrenia: A review of insights from transcranial magnetic stimulation. Schizophr Bull 40:685–696, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, et al. : Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology 27:13–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A: Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC: Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods 74:113–122, 1997 [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, et al. : Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp 31:1643–1652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M: Pathways from childhood to adult life. J Child Psychol Psychiatry 30:23–51, 1989 [DOI] [PubMed] [Google Scholar]
- Schneider SG, Asarnow RF: A comparison of cognitive/neuropsychological impairments of nonretarded autistic and schizophrenic children. J Abnorm Child Psychol 15:29–45, 1987 [DOI] [PubMed] [Google Scholar]
- Schulz KF, Grimes DA: Allocation concealment in randomised trials: Defending against deciphering. Lancet 359:614–618, 2002 [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS: Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 10:234–247, 2004 [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Wagner M, Narendorf S, Sterzing P, Hensley M: Post-high school service use among young adults with an autism spectrum disorder. Arch Pediatr Adolesc Med 165:141–146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, Tasman A, Mansoor M, Ramaswamy R, Sears L, et al. : Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl Psychophysiol Biofeedback 35:147–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze EM, Baruth JM, Sears L, Sokhadze GE, El-Baz AS, Casanova MF: Prefrontal neuromodulation using rTMS improves error monitoring and correction function in autism. Appl Psychophysiol Biofeedback 37:91–102, 2012 [DOI] [PubMed] [Google Scholar]
- Sokhadze EM, El-Baz A, Baruth J, Mathai G, Sears L, Casanova MF: Effects of low frequency repetitive transcranial magnetic stimulation (rTMS) on gamma frequency oscillations and event-related potentials during processing of illusory figures in autism. J Autism Dev Disord 39:619–634, 2009 [DOI] [PubMed] [Google Scholar]
- Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF: rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci 8:134, 2014a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze EM, El-Baz AS, Tasman A, Sears LL, Wang Y, Lamina EV, et al. : Neuromodulation integrating rTMS and neurofeedback for the treatment of autism spectrum disorder: An exploratory study. Appl Psychophysiol Biofeedback 39:237–257, 2014b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV: Diagnostic uses of the vineland adaptive behavior scales. J Pediatr Psychol 10:215–225, 1985 [DOI] [PubMed] [Google Scholar]
- Szatmari P, Bartolucci G, Bremner R, Bond S, Rich S: A follow-up study of high-functioning autistic children. J Autism Dev Disord 19:213–225, 1989 [DOI] [PubMed] [Google Scholar]
- Taylor JL, Seltzer MM: Employment and post-secondary educational activities for young adults with autism spectrum disorders during the transition to adulthood. J Autism Dev Disord 41:566–574, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Baeken C, Leyman L, Clerinx P, D'Haenen H: The influence of rTMS over the right dorsolateral prefrontal cortex on top-down attentional processes. Brain Res 1137:111–116, 2007 [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Baeken C, Leyman L, D'Haenen H: The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Exp Brain Res 169:279–282, 2006a [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Baeken C, Leyman L, D'Haenen H: The influence of s over the right dorsolateral prefrontal cortex on intentional set switching. Exp Brain Res 172:561–565, 2006b [DOI] [PubMed] [Google Scholar]
- Wall CA, Croarkin PE, McClintock SM, Murphy LL, Bandel LA, Sim LA, et al. : Neurocognitive effects of repetitive transcranial magnetic stimulation in adolescents with major depressive disorder. Front Psychiatry 4:165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM: Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108:1–16, 1998 [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R: Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett 250:141–144, 1998 [DOI] [PubMed] [Google Scholar]