Abstract
Protein kinase CK2 (CK2) is a highly promising target for cancer therapy, and anti-CK2 gene expression therapy has shown effectiveness in rodent models of human head and neck cancer (HNC). To date, there has been no large-animal model of cancer in which to further explore anti-CK2 therapies. Feline oral squamous cell carcinoma (FOSCC) has been proposed as a large-animal model for human HNC, and we have previously shown that CK2 is a rational target in FOSCC. Here we have tested the hypothesis that a novel tenfibgen-coated tumor-specific nanocapsule carrying RNA interference (RNAi) oligonucleotides targeting feline CK2α and CK2α′ (TBG-RNAi-fCK2αα′) would be safe in cats with FOSCC; assessment of target inhibition and tumor response were secondary aims. Nine cats were enrolled and treated at two dose levels in a 3+3 escalation. Cats received a total of six treatments with TBG-RNAi-fCK2αα′. Pre- and posttreatment, tumor and normal oral mucosa biopsies were collected to assess CK2 expression, using immunohistochemistry (IHC) preparations evaluated by light microscopy. Toxicity and tumor response were assessed on the basis of standard criteria. The most common adverse events were grade 1 or 2 weight loss and anorexia. Grade 3 tissue necrosis was seen in association with tumor response in one cat, asymptomatic grade 4 elevations in aspartate transaminase and creatine phosphokinase in one cat, and asymptomatic grade 3 hypokalemia in one cat. Of six cats with evaluable biopsies, two had a reduction in CK2 IHC score in tumors after treatment. Four cats had progressive disease during the study period, three had stable disease, one had partial response, and response could not be evaluated in one cat. We conclude that the drug appeared safe and that there is some evidence of efficacy in FOSCC. Further investigation regarding dosing, schedule, target modulation, toxicity, and efficacy in a larger group of cats is warranted and may inform future clinical studies in human head and neck cancer.
Keywords: : disease models, cancer, CK2, head and neck cancer
Introduction
Protein kinase CK2 (CK2) is an intriguing emerging target for cancer therapy, but no data from a large-animal model with naturally occurring cancer currently exist to investigate CK2-targeted therapies. CK2 inhibition or molecular downregulation in rodent models of human head and neck cancer (HNC) has shown considerable promise.1,2 Feline oral squamous cell carcinoma (FOSCC) shares many clinical and molecular features with human HNC, and has been proposed as a naturally occurring, large-animal model of HNC.3,4 FOSCC is a devastating disease, which is typically advanced at diagnosis and poorly responsive to standard therapies.5 Although CK2 downregulation in rodent models of HNC appears promising,1,2 inclusion of animals with naturally occurring cancer in the assessment of new therapies represents an important opportunity in drug development. Dogs and cats with spontaneous tumors offer many advantages as models of human cancer. They have immune systems and environmental exposures similar to people with cancer; pharmacokinetic and pharmacodynamic studies that are not possible in rodent models can be performed; and drug trials can typically be completed more rapidly in animals than in humans, allowing rapid assessment of efficacy. In addition, the lack of standard-of-care treatment for many cancers in dogs and cats may allow assessment of new therapies in a less heavily pretreated population compared with many people in phase 1 clinical trials.3,6 We have shown that CK2 is expressed in FOSCC, and that downregulation of CK2 by feline-specific naked anti-CK2 RNA interference (RNAi) in feline SCC in vitro resulted in reduced viability and induction of apoptosis.7 Thus, feline oral SCC may be a model for CK2-targeted therapies as well as a general model for human HNC. As well as informing development of anti-CK2 therapies in humans, investigating CK2 downregulation in cats with oral SCC may lead to advances in treatment of this disease and others in companion animals.
The CK2 holoenzyme is a tetrameric complex composed of two catalytic subunits (α and/or α′) linked through two regulatory subunits (β). Expression of CK2α or CK2β is essential for mouse embryogenesis; the α and α′ subunits have largely overlapping substrate targets; and there is some evidence of in vivo compensation between the two catalytic subunits.8,9 CK2 is involved in normal cell growth, proliferation, and survival, as well as suppression of apoptosis. These functions, as well as increased expression of CK2 in a wide variety of human tumors, make it an attractive target for cancer therapy.8,10,11 CK2 plays diverse molecular roles in cancer; it promotes tumorigenesis by suppressing apoptosis, activating oncogenic pathways such as NF-κB, Wnt/β-catenin, and PI3K/Akt, and inactivating tumor suppressors, and is also implicated in chemoresistance.12–14 Elevated and/or dysregulated CK2 expression has been documented in many human malignancies, both solid and hematopoietic, and is frequently identified as a negative prognostic factor.15–26
Of the two catalytic subunits, CK2α is generally considered the more important for cell survival, and our work with feline SCC in vitro supports this, with induction of apoptosis being greatest when CK2α was maximally suppressed. Reduced viability was seen in cells treated with small interfering RNA (siRNA) targeting CK2α alone or in combination with siRNA targeting CK2α′ but not when CK2α′ alone was targeted.7 Given the possibility of compensation, it is considered ideal to inhibit both CK2α and CK2α′—we have followed this strategy in our work. Targeting CK2 with small molecules or by RNAi has shown promise in mouse models of human cancers, and CK2 inhibitors are in early-stage clinical trials in people.27–29 RNAi with naked oligonucleotides is an effective strategy for targeting CK2 in vitro; however, one of the significant challenges in translating RNAi in vivo is ensuring that the therapeutic agent is delivered in a protected manner to reach the target tissue where effective cellular uptake occurs. In the specific case of CK2, its ubiquity and normal cells' dependence on it for survival make enhancement of tumor-specific targeting particularly important. Nanoparticle technology is one way to address these challenges. A novel nanocapsule containing tenfibgen (TBG), the carboxy-terminal fibrinogen globe domain of tenascin C, has been shown to home to tumors and to deliver anti-CK2-targeted therapies preferentially to tumor cells in xenograft models of human prostate and head and neck cancer.30–33 In this paper, we have extended evaluation of this tumor-targeted anti-CK2 delivery system to feline oral squamous cell carcinoma.
Here we have tested the hypothesis that the novel TBG nanocapsule containing feline-specific anti-CK2α and anti-CK2α′ oligonucleotides (TBG-RNAi-fCK2αα′) would be safe in cats with naturally occurring oral SCC. We were able to safely treat nine cats to assess for safety of TBG-RNAi-fCK2αα′ in a phase 1 type trial. Our secondary aim was to assess for evidence of efficacy via target inhibition and/or tumor response, and we were able to identify objective tumor response in one cat, although consistent target inhibition could not be detected 3–4 days after treatment.
Research Design and Methods
Research design and methods are summarized in the supplementary information (supplementary data are available online at www.liebertpub.com/humc).
Results
Nanoencapsulated RNAi-CK2 inhibits cell growth in feline and human SCC cells
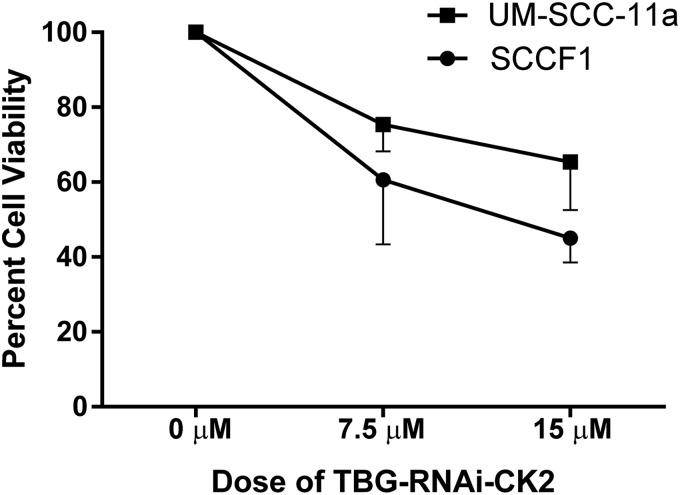
We previously showed that delivery of naked siRNA targeting CK2 caused loss of viability in feline SCC cells.7 Here, we specifically tested whether TBG nanocapsule-mediated delivery of single-stranded RNAi oligonucleotides targeting CK2α and α′ would have a similar impact on viability. Growth of human (UM-SCC-11a) and feline (SCCF1) SCC cells was significantly inhibited by TBG-RNAi-CK2 at both 7.5 μM (p = 0.008) and 15 μM (p = 0.016) concentrations compared with control treated cells (Fig. 1). Data were similar when cell lines were evaluated separately, although sample size was too small to run formal statistical analyses.
Figure 1.
Effect of TBG-RNAi-CK2 treatment on cell growth in feline and human laryngeal SCC cell lines, determined via neutral red uptake at 48 hr. Values are graphed relative to cells treated with TBG-sugar, labeled as a dose of 0 μM. Error bars, SEM; n = 4.
Characteristics of enrolled cats
Nine cats with oral squamous cell carcinoma were enrolled. An additional five cats were screened, but were not included because of owner declining enrollment (three cats), no macroscopic tumor (one cat), and lack of definitive diagnosis of oral tumor (one cat). Demographics, tumor characteristics, presenting clinical signs, concurrent medication, and adverse events are summarized in Table 1.
Table 1.
Characteristics of cats in each dose group at the time of enrollment, concurrent medications, and adverse events recorded during study
| Dose | ||
|---|---|---|
| 2 μg/kg (n = 6) | 20 μg/kg (n = 3) | |
| Median age, yr (range) | 13.5 (9–17) | 13 (10–15) |
| Median weight, kg (range) | 2.86 (2.45–6.06) | 4.41 (3.54–4.9) |
| Sex | ||
| Female spayed | 5 | 2 |
| Male neutered | 1 | 1 |
| Breed | ||
| DSH | 6 | 2 |
| DLH | 0 | 1 |
| Tumor stagea | ||
| I | 2 | 0 |
| II | 2 | 2 |
| III | 1 | 1 |
| Tumor location | ||
| Mandibular | 4 | 1 |
| Maxillary | 1 | 1 |
| Buccal | 0 | 1 |
| Sublingual/pharyngeal | 1 | 0 |
| Clinical signs | ||
| Reduced appetite | 3 | 2 |
| Weight loss | 3 | 1 |
| Pain | 3 | 1 |
| Visible mass | 2 | 2 |
| Concurrent disease | ||
| CKD | 2 | 1 |
| Hyperthyroidism | 2 | 0 |
| Ocular diseaseb | 0 | 1 |
| Concurrent medications | ||
| Analgesics | 6 | 3 |
| Systemic antibiotics | 3 | 2 |
| Antihistamine | 1 | 0 |
| Topical ocular medications | 0 | 1 |
| Adverse events | ||
| Anorexia | ||
| Grade 1 | 4 | 1 |
| Grade 2 | 2 | 1 |
| Weight loss | ||
| Grade 1 | 5 | 2 |
| Anemia | ||
| Grade 1 | 3 | 1 |
| Grade 2 | 0 | 1 |
| Lethargy | ||
| Grade 1 | 1 | 1 |
| Grade 2 | 1 | 0 |
| Vomiting | ||
| Grade 1 | 1 | 1 |
| Skin ulceration | 0 | 1 |
| Constipation | 0 | 1 |
| Elevated BUN, ALT, AST, CPK | 1 | 0 |
| Hypokalemia | 0 | 1 |
Tumor stage not evaluable in one cat as primary tumor was diffuse in nature, precluding repeatable measurements.
Cranial nerve III and V denervation left side (possibly tumor related), herpes keratitis, keratoconjunctivitis sicca.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CKD, chronic kidney disease; CPK, creatine phosphokinase; DSH, domestic short hair; DLH, domestic long hair.
No cat had had prior surgery, chemotherapy, or radiation therapy for oral SCC. Four cats had been treated with nonsteroidal antiinflammatory drugs (NSAIDs) before enrollment. In all cases, tumor progression had been noted and NSAIDs were discontinued at least 24 hr before study entry. All cats received some form of concurrent medication during the study (see Table 1). Analgesics (buprenorphine in nine cats, gabapentin in four, transdermal fentanyl in one) and antibiotics were the most commonly administered. These were prescribed for tumor-related pain and possible infection, respectively. Mirtazapine was used as an appetite stimulant in one cat for anorexia that could have been either tumor- or drug-related. In one cat that vomited during the first nanocapsule treatment, antihistamine (diphenhydramine, 2 mg/kg intramuscularly) was administered before subsequent treatments.
Systemic administration of nanoencapsulated RNAi is safe in cats with oral squamous cell carcinoma
Six cats were treated at 2 μg/kg and three cats were treated at 20 μg/kg. The first cohort was expanded because of a grade 4 adverse event in one cat (cat 3). Treatment was discontinued after five treatments in five cats (three in the 2-μg/kg group and two in the 20-μg/kg group) because of perceived progressive disease and/or worsening clinical signs.
Adverse events are summarized in Table 1. Most adverse events were grade 1–2 and considered acceptable. The most common were anorexia and weight loss, which were considered possibly related to the experimental drug in all cases. In the cats where vomiting was noted, it was considered likely to be related to the experimental drug in one cat, where it happened at the time of the first treatment, and unlikely to be related in one cat (probable reaction to sedation). Lethargy was considered unrelated to the experimental drug in one cat (related to a secondary infection that resolved with antibiotics) and possibly related in the remaining two. Anemia was considered possibly related to the experimental drug, and developed at various times during the study, from day 10 (at the time of third treatment) to posttreatment evaluation on days 23–24. Three cats experienced grade 3 or grade 4 adverse events. One of these cats had grade 4 elevations in aspartate transaminase (AST) and creatine phosphokinase (CPK) along with grade 2 elevations in alanine aminotransferase (ALT) and blood urea nitrogen (BUN) at the posttreatment evaluation. This cat had milder elevations on day 17 (i.e., after four treatments). The second of these cats had grade 3 hypokalemia (2.5 mmol/L) at the time of posttreatment evaluation, which had not been noted during the rest of the study. These were considered possibly related to the experimental drug. There were no clinical signs associated with these metabolic changes in either cat. The third cat had grade 3 skin ulceration associated with objective tumor response at the time of posttreatment evaluation (Fig. 2A), and this adverse event was considered definitely drug-related. In this case, less severe changes were noted starting on day 13. There were several other transient, asymptomatic grade 1 metabolic adverse events in one cat each, including increased BUN, increased CPK, increased creatinine, hypocalcemia, and hyperglycemia, and these were considered possibly drug-related.
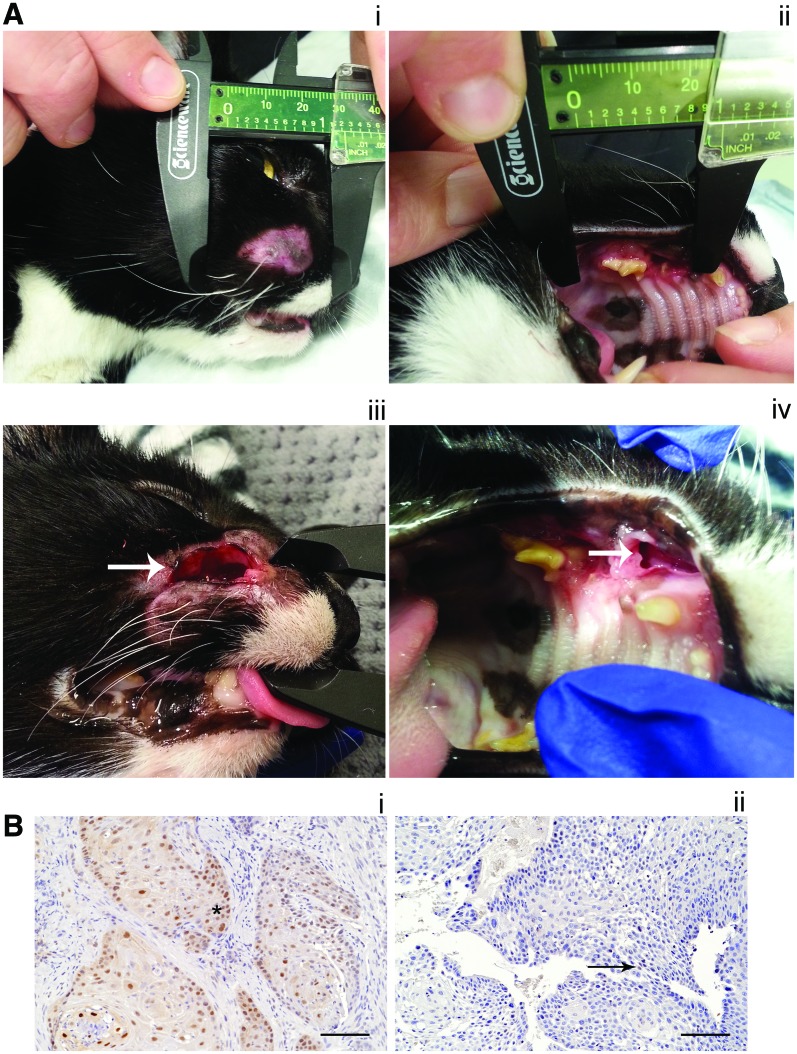
Figure 2.
Clinical and molecular response to treatment in feline oral SCC treated with TBG-RNAi-fCK2αα′. (A) Clinical response in one cat (cat 8; see Table 2). Panels i and ii: Pretreatment, tumor affecting right maxilla and facial soft tissues. Panels iii and iv: Posttreatment. There has been a 40% reduction in longest tumor diameter, which has resulted in an open wound externally on the right lateral muzzle (arrow, iii) and progression of the defect in the maxilla (arrow, iv). (B) CK2α IHC in oral SCC pretreatment (panel i) and posttreatment (panel ii) in one cat (cat 4; see Table 2). There is reduced CK2α labeling in the posttreatment (score 1) sample compared with the pretreatment (score 2) sample. In the pretreatment sample, CK2α labeling is present in both the nucleus and cytoplasm of tumor cells (*). In the posttreatment sample, CK2α labeling is present only in scattered nuclei (arrow shows example) and is of lower intensity overall. Color images available online at www.liebertpub.com/humc
Objective tumor response is seen in cats with oral SCC treated with systemic nanoencapsulated RNAi-CK2
Objective tumor response (using RECIST [Response Evaluation Criteria in Solid Tumors] criteria) and changes in CK2α in tumor and normal tissue are summarized in Table 2, and examples are shown in Fig. 2. One cat had a partial response to treatment (40% decrease in longest diameter), with associated grade 3 skin ulceration and an open wound (Fig. 2A). There was no apparent correlation between tumor response, based on RECIST criteria, and change in tumor CK2α IHC score. An example of decreased CK2α IHC score in tumor posttreatment is shown in Fig. 2B.
Table 2.
Tumor response and change in IHC scores (tumor and normal tissue)
| Cat | Dose level (μg/kg) | Treatments | Tumor response | Percent change in tumor LD | Change in tumor IHC score | Change in normal IHC score |
|---|---|---|---|---|---|---|
| 1 | 2 | 5 | PD | +71 | +1 | 0 |
| 2 | 2 | 6 | PD | +81 | NDa | −1 |
| 3 | 2 | 6 | NDb | NDb | −1 | +1 |
| 4 | 2 | 5 | SD | −24 | −1 | +1 |
| 5 | 2 | 5 | SD | +18 | 0 | −1 |
| 6 | 2 | 6 | PD | +32 | NDa | 0 |
| 7 | 20 | 6 | SD | +5 | NDa | 0 |
| 8 | 20 | 6 | PR | −40 | +1 | −1 |
| 9 | 20 | 5 | PD | +12.9c | 0 | −1 |
Change in tumor IHC was not determined in three cats because of insufficient pretreatment biopsy samples.
Tumor response was not determined in one cat, as the tumor could not be accurately measured to permit RECIST evaluation.
Although the change in tumor diameter did not meet criteria for PD, this cat developed a new lesion (metastatic lymph node).
IHC, immunohistochemistry; LD, longest diameter; ND, not determined; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
Feline oral SCC has been proposed as a general model for human HNC,3,4 and we have demonstrated the relevance of the feline model in the specific case of CK2 as a therapeutic target.7 Here, we extended that study and showed that targeting CK2 with nanoencapsulated RNAi-CK2 was equally effective in feline and human SCC cells; and that in cats with FOSCC the therapy is safe, and an efficacy signal (objective tumor response) is present even in the small number of cats treated and with the short duration of treatment. TBG nanoencapsulated anti-CK2 RNAi has shown promise in preclinical studies of human HNC and other cancers1,33; however, anti-CK2 RNAi has not previously been evaluated in naturally occurring cancer in any species. We have, for the first time, demonstrated that this novel therapeutic strategy can be safely applied in a relevant large-animal model of human head and neck cancer.
Overall, TBG-RNAi-fCK2αα′ was well tolerated in cats with FOSCC, with severity of adverse events in line with other novel systemic therapies assessed for this disease.34–36 Most adverse events seen in our study were considered acceptable. Because anorexia and weight loss are common clinical signs in feline SCC and were noted before treatment in many cats in this study, the effect of tumor is impossible to distinguish from the effect of treatment, as noted by other investigators of FOSCC.34 The grade 3 and grade 4 adverse events are more concerning. The metabolic changes (hypokalemia, and elevated CPK, AST, ALT, and BUN) were not associated with obvious clinical signs. The other grade 3 adverse event (skin ulceration) was associated with tumor response and necrosis, as has been previously shown in nanoencapsulated anti-CK2 RNAi-treated mice.1 The dramatic nature of the partial response causing skin ulceration and necrosis is not desirable. It would be preferable in tumors such as this to aim for a more gradual reduction to allow normal tissue healing and prevent defects such as that seen in this patient, which would require significant management. The authors suggest that if rapid response is noted in future studies, dose and frequency of administration should be modified to try to prevent such an outcome. Hypokalemia has been noted as a DLT of CX-4945, a small-molecule inhibitor currently in early-phase clinical trials in people.28 Diarrhea, the other DLT of CX-4945, was not noted in cats in this study. In future studies of longer duration, close monitoring for adverse events will be necessary to identify new or more severe adverse events that were not seen in the relatively short duration of treatment in this study.
Of eight cats in which tumor response was evaluable, PR was seen in one (12.5%) and stable disease in three (37.5%). Stable disease is often a clinically relevant end point for targeted therapies,37,38 and achieving stable disease or better improves survival in cats with FOSCC.34 Given the aggressive nature of FOSCC,5 any objective tumor response is encouraging. We were unable to demonstrate consistent downregulation of the target in tumors. In vitro, CK2α and CK2α′ expression is reduced by about 90% in feline SCC when using siRNA,7 and CK2α immunostaining in our study was highly variable even within individual samples, so the small differences between pre- and posttreatment IHC scores in tumor and normal oral mucosa in this study could conceivably reflect inherent variability rather than an effect of treatment. We suspect that this is because our selected time point for posttreatment evaluation might be inappropriate. We have documented that in mice with xenograft prostate tumors that responded to TBG-RNAi-CK2, significant downregulation of CK2α and α′ proteins was detectable only 24 hr after intravenous treatment.33 In future, if we can identify the correct time point to detect suppression of CK2α expression in feline SCC tumors, using TBG-RNAi-fCK2αα′, evaluation of CK2α′ expression as well would be ideal to determine whether results in cats with oral SCC recapitulate what has been found in feline SCC in vitro and in rodent xenograft models, that is, that both CK2α and CK2α′ can be effectively suppressed.
In conclusion, we achieved the primary objective of the study, showing that TBG nanoencapsulated RNAi targeting CK2 can be safely administered to cats with FOSCC. Further, we found that there is some evidence of efficacy in cats with advanced disease. Future studies in larger groups of cats may be done to more fully explore the dose, route, and frequency of administration to inform human clinical trials as well as to assess TBG-RNAi-fCK2αα′ as a possible new treatment for oral SCC in domestic cats.
Supplementary Material
Acknowledgments
The authors thank Rachel Isaakson Vogel for statistical assistance, Paula Overn of the Masonic Cancer Center Comparative Pathology Shared resource for assistance with immunohistochemistry, the Rosol laboratory at Ohio State University for SCCF1 cells and Dr. Frank Ondrey of the University of Minnesota Masonic Cancer Center for UM-SCC-11a cells, and the Clinical Investigations Center at the University of Minnesota College of Veterinary Medicine for assistance with conduction of the clinical trial. The authors acknowledge funding from the National Center for Advancing Translational Sciences of the National Institutes of Health (award number UL1TR000114). This work was also supported by a University of Minnesota Clinical and Translational Sciences Award (1UL1 RR033183-01), by merit review research funds (BX001731 and BX003282) awarded by the Department of Veterans Affairs (K. Ahmed), funds from the Animal Cancer Care and Research Program at the University of Minnesota, and by research grants HHSN261201300030C (G.M.U.) and CA150182 (K.A.) awarded by the NCI, NIH, Department of Health and Human Services, and by CA158730 and DK067436-05 awarded by the NCI, NIH, Department of Health and Human Services and the NIDDK, respectively (B.T.K.). The views expressed in this paper are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs or the U.S. government.
Author Disclosure
G.M.U. is the founder and CSO of GeneSegues, Inc., which owns the patent on the nanocapsule used in this study. The authors have no other disclosures.
References
- 1.Unger GM, Kren BT, Korman VL, et al. Mechanism and efficacy of sub-50-nm tenfibgen nanocapsules for cancer cell-directed delivery of anti-CK2 RNAi to primary and metastatic squamous cell carcinoma. Mol Cancer Ther 2014;13:2018–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MS, Diallo OT, Hu M, et al. CK2 modulation of NF-κB, TP53, and the malignant phenotype in head and neck cancer by anti-CK2 oligonucleotides in vitro or in vivo via sub-50-nm nanocapsules. Clin Cancer Res 2010;16:2295–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon C. Cats, cancer and comparative oncology. Vet Sci 2015;2:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wypij JM. A naturally occurring feline model of head and neck squamous cell carcinoma. Pathol Res Int 2013;2013:502197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liptak J, Withrow SJ. Oral tumors. In: Withrow & MacEwen's Small Animal Clinical Oncology. Withrow SJ, Vail DM, Page RL, eds. Elsevier Saunders, St. Louis, MO: 2013; pp. 381–398 [Google Scholar]
- 6.Khanna C, London C, Vail D, et al. Guiding the optimal translation of new cancer treatments from canine to human cancer patients. Clin Cancer Res 2009;15:5671–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon CM, Trembley JH, Kren BT, et al. Evaluation of protein kinase CK2 as a therapeutic target for squamous cell carcinoma of cats. Am J Vet Res 2017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trembley JH, Chen Z, Unger G, et al. Emergence of protein kinase CK2 as a key target in cancer therapy. BioFactors 2010;36:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trembley JH, Unger GM, Tobolt DK, et al. Systemic administration of antisense oligonucleotides simultaneously targeting CK2α and α′ subunits reduces orthotopic xenograft prostate tumors in mice. Mol Cell Biochem 2011;356:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra B, Issinger O. Protein kinase CK2 in human diseases. Curr Med Chem 2008;15:1870–1886 [DOI] [PubMed] [Google Scholar]
- 11.Ahmad KA, Wang G, Slaton J, et al. Targeting CK2 for cancer therapy. Anticancer Drugs 2005;16:1037–1043 [DOI] [PubMed] [Google Scholar]
- 12.Seldin DC, Landesman-Bollag E. The oncogenic potential of CK2. In: Protein Kinase CK2. John Wiley & Sons, Oxford: 2013; pp. 292–304 [Google Scholar]
- 13.Ruzzene M, Pinna LA. Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta 2010;1804:499–504 [DOI] [PubMed] [Google Scholar]
- 14.Trembley JH, Wu J, Unger GM, et al. CK2 Suppression of apoptosis and its implication in cancer biology and therapy. In: Protein Kinase CK2. John Wiley & Sons, Oxford: 2013; pp. 319–343 [Google Scholar]
- 15.Daya-Makin M, Sanghera JS, Mogentale TL, et al. Activation of a tumor-associated protein kinase and casein kinase 2 in human squamous cell carcinomas and adenocarcinomas of the lung. Cancer Res 1994;54:2262–2268 [PubMed] [Google Scholar]
- 16.Kendall JJ, Chaney KE, Patel AV, et al. CK2 blockade causes MPNST cell apoptosis and promotes degradation of β-catenin. Oncotarget 2016;7:53191–53203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giusiano S, Cochet C, Filhol O, et al. Protein kinase CK2α subunit over-expression correlates with metastatic risk in breast carcinomas: quantitative immunohistochemistry in tissue microarrays. Eur J Cancer 2011;47:792–801 [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Eom JI, Cheong J, et al. Protein kinase CK2α as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res 2007;13:1019–1028 [DOI] [PubMed] [Google Scholar]
- 19.Landesman-Bollag E, Song DH, Romieu-Mourez R, et al. Protein kinase CK2: signaling and tumorigenesis in the mammary gland. Mol Cell Biochem 2001;227:153–165 [PubMed] [Google Scholar]
- 20.Lin K, Tai C, Hsu J, et al. Overexpression of nuclear protein kinase CK2 α catalytic subunit (CK2α) as a poor prognosticator in human colorectal cancer. PLoS One 2011;6:e17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laramas M, Pasquier D, Filhol O, et al. Nuclear localization of protein kinase CK2 catalytic subunit (CK2α) is associated with poor prognostic factors in human prostate cancer 5. Eur J Cancer 2007;43:928–934 [DOI] [PubMed] [Google Scholar]
- 22.Piazza FA, Ruzzene M, Gurrieri C, et al. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood 2006;108:1698–1707 [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui-Jain A, Bliesath J, Macalino D, et al. CK2 inhibitor CX-4945 suppresses DNA repair response triggered by DNA-targeted anticancer drugs and augments efficacy: mechanistic rationale for drug combination therapy. Am Assoc Cancer Res 2012;11:193–204 [DOI] [PubMed] [Google Scholar]
- 24.Faust RA, Gapany M, Tristani P, et al. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: association with malignant transformation. Cancer Lett 1996;101:31–35 [DOI] [PubMed] [Google Scholar]
- 25.Williams M, Nguyen T, Carriere P, et al. Protein kinase CK2 expression predicts relapse survival in ERα dependent breast cancer, and modulates ERα expression in vitro. Int J Environ Res Public Health 2015;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gapany M, Faust RA, Tawfic S, et al. Association of elevated protein kinase CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Mol Med 1995;1:659–666 [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqui-Jain A, Drygin D, Streiner N, et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res 2010;70:10288–10298 [DOI] [PubMed] [Google Scholar]
- 28.Marschke RF, Borad MJ, McFarland RW, et al. Findings from the phase I clinical trials of CX-4945, an orally available inhibitor of CK2. J Clin Oncol 2011;29(15 suppl):3087 [Google Scholar]
- 29.Sarduy MR, García I, Coca MA, et al. Optimizing CIGB-300 intralesional delivery in locally advanced cervical cancer. Br J Cancer 2015;112:1636–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trembley JH, Unger GM, Korman VL, et al. Nanoencapsulated anti-CK2 small molecule drug or siRNA specifically targets malignant cancer but not benign cells. Cancer Lett 2012;315:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trembley JH, Unger GM, Korman VL, et al. Tenfibgen ligand nanoencapsulation delivers bi-functional anti-CK2 RNAi oligomer to key sites for prostate cancer targeting using human xenograft tumors in mice. PLoS One 2014;9:e109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trembley JH, Unger GM, Cespedes Gomez O, et al. Tenfibgen-DMAT nanocapsule delivers CK2 inhibitor DMAT to prostate cancer xenograft tumors causing inhibition of cell proliferation. Mol Cell Pharmacol 2014;6:15–25 [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed K, Kren BT, Abedin MJ, et al. CK2 targeted RNAi therapeutic delivered via malignant cell-directed tenfibgen nanocapsule: dose and molecular mechanisms of response in xenograft prostate tumors. Oncotarget 2016;7:61789–61805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiles V, Hohenhaus A, Lamb K, et al. Retrospective evaluation of toceranib phosphate (Palladia) in cats with oral squamous cell carcinoma. J Feline Med Surg 2017;19:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skorupski KA, O'Brien TG, Guerrero T, et al. Phase I/II clinical trial of 2-difluoromethyl-ornithine (DFMO) and a novel polyamine transport inhibitor (MQT 1426) for feline oral squamous cell carcinoma. Vet Comp Oncol 2011;9:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox LE, Rosenthal RC, King RR, et al. Use of cis-bis-neodecanoato-trans-R,R,1,2-diaminocyclohexane platinum(II), a liposomal cisplatin analgoue, in cats with oral squamous cell carcinoma. Am J Vet Res 2000;61:791–795 [DOI] [PubMed] [Google Scholar]
- 37.Tolcher AW. Stable disease is a valid end point in clinical trials. Cancer J 2009;15:374–378 [DOI] [PubMed] [Google Scholar]
- 38.Bernabe LF, Portela R, Nguyen S, et al. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet Res 2013;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.