Abstract
Aim
Tranexamic acid (TXA) continues to be one of the antifibrinolytics of choice during paediatric cardiac surgery. However, in infants less than 1 year of age, the optimal dosing based on pharmacokinetic (PK) considerations is still under discussion.
Methods
Forty‐three children less than 1 year of age were enrolled, of whom 37 required the use of cardiopulmonary bypass (CPB) and six were operated on without CPB. Administration of 50 mg kg–1 TXA intravenously at the induction of anaesthesia was followed by 50 mg kg–1 into the CPB prime in the CPB group. Plasma concentrations of TXA were analysed by gas chromatography–mass spectrometry. PK data were investigated using nonlinear mixed‐effect models.
Results
A two‐compartment model was fitted, with the main covariates being allometrically scaled bodyweight, CPB, postmenstrual age (PMA). Intercompartmental clearance (Q), peripheral volume (V2), systemic clearance, (CL) and the central volume (V1) were calculated. Typical values of the PK parameter estimates were as follows: CL = 3.78 [95 % confidence interval (CI) 2.52, 5.05] l h–1; central volume of distribution = 13.6 (CI 11.7, 15.5) l; Q = 16.3 (CI 13.5, 19.2) l h–1; V2 = 18.0 (CI 16.1, 19.9) l. Independently of age, 10 mg kg–1 TXA as a bolus, a subsequent infusion of 10 mg kg–1 h–1, then a 4 mg kg−1 bolus into the prime and a reduced infusion of 4 mg kg–1 h–1 after the start of CPB are required to maintain TXA concentrations continuously above 20 μg ml–1, the threshold value for an effective inhibition of fibrinolysis and far lower than the usual peak concentrations (the ‘10‐10‐4‐4 rule’).
Conclusions
The introduction of a modified dosing regimen using a starting bolus followed by an infusion and a CPB prime bolus would prohibit the potential risk of seizures caused by high peak concentrations and also maintain therapeutic plasma concentration above 20 μg ml–1.
Keywords: tranexamic acid, antifibrinolytic agents, pharmacokinetics, cardiac surgical procedures, congenital heart defects
What is Already Known about this Subject
Tranexamic acid remains the cornerstone of antifibrinolytic therapy during paediatric cardiac surgery.
Side effects are rare, but could include seizures and thrombosis.
Better dosing regimens are warranted to avoid unnecessary high concentrations and subsequent complications.
What this Study Adds
Paediatric doses are different to those for adults.
The main covariates are allometrically scaled bodyweight, on or off cardiopulmonary bypass (CPB), and postmenstrual age.
Concentrations within the therapeutic range can be achieved by using a dosing schedule including a starting bolus followed by an infusion and a CPB prime bolus.
Table of Links
| LIGANDS |
|---|
| Tranexamic acid |
This Table lists key ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1.
Introduction
Tranexamic acid (TXA) has been used to reduce blood loss in cardiac surgery following its first proof of effectiveness in 1990 2. As aprotinin has been withdrawn from the market in many countries, only two lysine analogues remain as the main antifibrinolytics – namely, TXA and ɛ‐aminocaproic acid. TXA is considered to be the most effective 3 but significant side effects have been reported, including dose‐dependent seizures after cardiac surgery 4. Therefore, in order to optimize its use, new studies have examined the pharmacokinetic (PK) profile in different patient populations 5, 6. As a quality improvement exercise, we examined TXA PK with our standard dosing regimen. We expected the TXA PK in neonates and infants to be mostly influenced by dilutional effects during cardiopulmonary bypass (CPB), age, weight and renal function. The goal of our study was to quantify these covariates with a population approach in these age groups and to determine the effects of CPB. Based on these findings, we suggest a dosing recommendation for patients less than 1 year of age. As our study differs, in terms of patient population and bypass management, from the two studies published so far in this age group 7, 8, it provides valuable new information on this topic.
Methods
Clinical protocol
The study was approved by the ethics committee of the Medical Faculty of the Technical University Munich (ref. 5600/12) and registered with the World Health Organization International Clinical Trials Registry Platform/German Clinical Trials Register No. DRKS‐4538. Written parental consent was obtained in all cases. Patients were children less than 1 year of age with a congenital heart defect undergoing cardiac surgery with (n = 37) or without (n = 6) CPB between January and June 2013. Exclusion criteria were a known allergy to TXA; any coagulopathy, as defined by laboratory levels outside the norm, adjusted for age; or preoperative renal insufficiency. Per standard protocol, 50 mg kg–1 TXA was given over 5 s after induction and arterial line placement. If CPB was used, another 50 mg kg–1 was added to the prime volume. Anaesthesia was induced and maintained with midazolam, sufentanil and pancuronium. Volume replacement before CPB was accomplished with albumin 5% or Ringer's acetate. The CPB priming fluid included Ringer's acetate, 5000 IU of heparin, 100–150 ml washed packed red blood cells, 100–150 ml fresh frozen plasma and sodium bicarbonate. The final prime volumes achieved were approximately 370–380 ml. A noncoated bypass circuit was used. Depending on the patient's size, either a Dideco D100 for neonates <5 kg (Sorin Group, Milan, Italy) or a Terumo Baby RX oxygenator (Terumo, Ann Arbor, MI, USA) was used. Mild hypothermia at 32–34°C was applied on CPB. After discontinuation of CPB and routine modified ultrafiltration (MUF), protamine was administered in a heparin : protamine ratio of 1:1.3. Additional protamine was based on residual heparin levels. Our institutional transfusion management was applied and haemoglobin levels were maintained above 10 g dl–1.
Sample acquisition, handling, and processing
Blood samples (1.2 ml) were drawn into ethylene diamine tetra‐acetic acid‐coated tubes (Sarstedt, Nuembrecht, Germany). After induction of anaesthesia, a radial or femoral artery catheter was inserted, TXA was injected and samples were drawn after 5, 15, 30, 60, 90, 120, 150, 180, 240 and 360 min or until CPB was initiated as dictated by clinical circumstances. Additional samples were drawn before the initiation of CPB and 5, 15, 30, 60, 90, 120, 150, 180, 240 and 360 min after the start of CPB. Plasma was separated by centrifugation (4000 g, 10 min) at room temperature and stored at −80°C.
Tranexamic acid assay
Plasma concentrations were analysed by gas chromatography–mass spectrometry using a published analytical amino‐acid quantification method 9 adapted to TXA (see Appendix). A relative mean intra‐assay recovery of 92.6±3.7 % (n = 49) was measured at a concentration level of 122 μg ml–1, with an interassay coefficient of variation of 12.9 %. The calibration curve was linear (r 2 = 0.99995) and the lower limit of quantification (LLOQ) was 4.6 μg ml–1.
Data analysis
Descriptive analysis was performed using PASW Statistics v21.0 (IBM Corporation, Somer, NY, USA).
PK modelling
Structural model
PK data were analysed using the nonlinear mixed‐effects modelling software Phoenix 64 NLME 7.0 (Certara Inc., Princeton, NJ, USA) 10. The fixed parameters are elimination (CL) and intercompartmental (Q) clearances, and the volume of the central (V1) and peripheral (V2) compartments. A two‐compartment open model without covariates was first fitted to our TXA data.
Covariate analysis
Using a forward addition procedure, the following covariate effects were tested, based on physiological and developmental principles during the first year of life: weight, postmenstrual age (PMA), CPB prime volumes, CPB as a categorical variable (on/off) and modified ultrafiltration (MUF) volumes. All covariates were centred to the adult values and their effects was estimated as θcov. We evaluated three approaches for body size, one with no normalization, one in which parameters were normalized for weight, and an allometric approach in which the PK parameters were allometrically scaled for bodyweight (BW). Individual values of PK parameters (Pi) were described for BW as follows:
where i denotes the i th individual and tvP the typical value for the population. The power exponents θ were tested with and without fixation at 0.75 for the clearance and 1 for the volume parameters (allometric scaling) 11.
θCPB was introduced as a modifying factor and estimated using NLME during the period of CPB:
where Pi denotes a clearance or volume term for the ith individual, tvP is the typical parameter value, CPB is a binary variable (θCPB 1 = θCPB when on CPB, θCPB 0 = 0 otherwise), with θCPB as the influential parameter. For the impact of age on clearance, a sigmoidal maturation function (Fmat) based on the postmenstrual age was chosen:
with PMA being postmenstrual age in years, θPMA the Hill coefficient and PMA50 the maturation half‐life 11.
Model evaluation and selection
The likelihood ratio test (‐2LL), Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used to test different hypotheses regarding the final model. The final residual variability (εPROP) was described using a proportional error model. From the final model, 1000 simulations were performed to generate a visual predictive check (VPC) 12. The goodness‐of‐fit plots were examined, with particular emphasis on the observed vs. the population‐predicted concentrations for the best predictive model. The results were visualized as a scatterplot for the population conditional weighted residuals (CWRES) vs. individual predicted concentrations or time after dose and a prediction‐corrected VCP (pcVPC) stratified for BW (n = 1000 simulations). A nonparametric bootstrap analysis (n = 1000 samples) was used to assess parameter precision.
Simulation of TXA concentrations
For the simulation, patient BWs of 2.5, 5, 7.5 and 10 kg were chosen. Using the final model, concentration courses were simulated for the first 8 h, assuming a 60‐min interval between the first dose and initiation of CPB with an additional duration of CPB of 90 min. We considered 20 μg ml–1 a reasonable lower border of the therapeutic window during the time of surgery as previously proposed 13, 14. This target concentration was selected to minimize any possible proconvulsive effects while maintaining clinical efficacy.
Results
Population characteristics and TXA concentrations
The demographic and CPB characteristics are listed in Table 1.
Table 1.
Demographics and cardiopulmonary bypass (CPB) characteristics
| Demographics | n = 43 |
|---|---|
| Age, days (min–max) | 123 (6–348) |
| Weight, kg (min–max) | 4.95 (2.3–9.5) |
| Length, cm | 58 ± 9 |
| Male gender (%) | 22 (51) |
| Cyanosis (%) | 15 (35) |
| Aristotle basic score, median (25–75% percentile) | 8 (6–9) |
| CLCREA, mean ± standard deviation (ml min–1 1.73m–2) (min–max) | 75 ± 29 (26–149) |
| CPB characteristics | n = 37 |
|---|---|
| Priming volume (ml) | 382 ± 65 |
| Packed red blood cell volume in the prime (ml) | 113 ± 17 |
| Fresh frozen plasma volume in the prime (ml) | 100 ± 0 |
| CPB time (min) | 82 ± 34 |
| Cross‐clamping n (%) | 27 (73) |
| Cross‐clamp time (min) | 49 ± 29 |
CLCREA, estimated preoperative creatinine clearance using the Schwartz formula
One terminal sample was missing because the child required cardiopulmonary resuscitation shortly before the final sampling point. A total of 637 samples were obtained, with three outliers excluded and no sample below the LLOQ. Notable side effects related to TXA were a series of three seizures on the day of the operation in one infant 15. Individual PK courses of TXA are shown in Figure 1. Measured peak concentrations were 213 ± 54 μg ml–1 (mean ± standard deviation) 5 min after the first bolus, and the TXA concentration fell to 75 ± 19 μg ml–1 shortly before CPB. The second dose, on CPB, achieved a peak level of 236 ± 67 μg ml–1. 5.6 ± 0.43 h after the start of CPB, measured concentrations were 54 ± 15 μg ml–1. The six patients without CPB had residual TXA concentrations of 26 ± 10 μg ml–1 6 h after their first dose.
Figure 1.
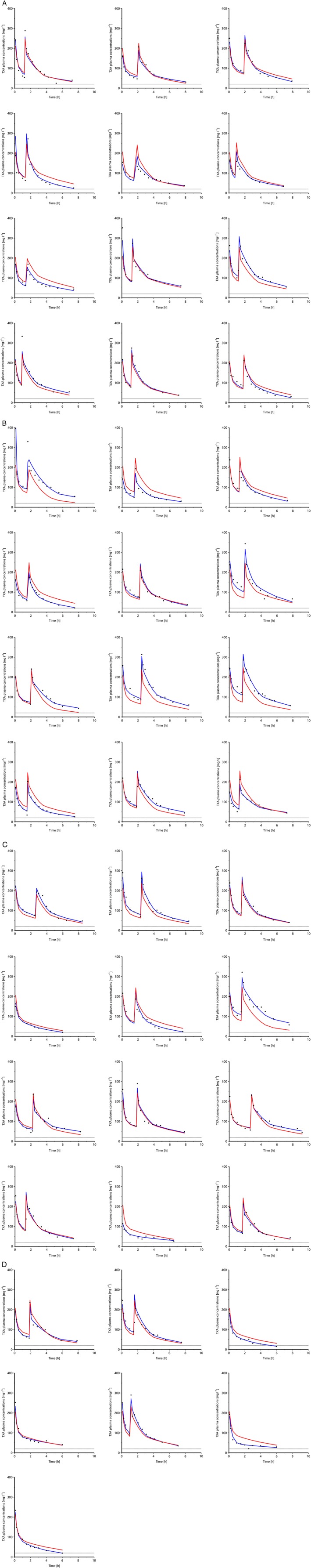
Latticed scatter plot by indi vidual, containing all population observations, population predictions (red lines) and individual predictions (blue lines) vs. time. The first dose was applied at the time zero and the second dose was given upon initiation of cardiopulmonary bypass (if used), 5 min before the second peak. TXA, tranexamic acid
Population PK modelling
The model building steps are summarized in Table 2. Significant covariates of the final model were allometrically scaled BW, CPB as a categorical covariate and PMA, but not MUF or prime volume. First, we evaluated three approaches to normalizing the PK parameters to BW. Compared with a covariate‐free model, the weight‐standardized model improved the objective function. The allometric model yielded a further improvement and was retained. Thereafter, adding the effect of PMA on CL and CPB again improved the model. The addition of prime or MUF volumes did not improve the model beyond the categorical effects of CPB (not shown).
Table 2.
Model building summary, base scenario with allometric weight on (V1, V2, CL, Q), sorted by BIC
| Additional covariates | –2LL | AIC | BIC | Number of parameters |
|---|---|---|---|---|
| None | 5616 | 5636 | 5680 | 10 |
| CPB (V2, V1) | 5609 | 5633 | 5686 | 12 |
| CPB (V2) | 5613 | 5635 | 5684 | 11 |
| CBP (V1) | 5611 | 5633 | 5682 | 11 |
| CPB (V2, V1), PMA (CL) | 5595 | 5621 | 5679 | 13 |
| CPB (V2), PMA (CL) | 5600 | 5624 | 5677 | 12 |
| CPB (V1), PMA (CL) | 5597 | 5621 | 5675 | 12 |
| PMA (CL) | 5602 | 5624 | 5673 | 11 |
| CPB (Q, V2) | 5593 | 5617 | 5671 | 12 |
| CPC (Q) | 5595 | 5617 | 5666 | 11 |
| CPB (Q, V2, V1) | 5582 | 5608 | 5666 | 13 |
| CPB (Q, V2), PMA (CL) | 5579 | 5605 | 5663 | 13 |
| CPB (Q, V1) | 5584 | 5608 | 5662 | 12 |
| CPB (Q), PMA (CL) | 5580 | 5604 | 5658 | 12 |
| CPB (Q, V2, V1), PMA (CL) | 5568 | 5596 | 5658 | 14 |
| CPB (Q, V1), PMA (CL) | 5570 | 5596 | 5653 | 13 |
| CPB (CL) | 5489 | 5511 | 5560 | 11 |
| CPB (Cl), PMA (CL) | 5478 | 5502 | 5556 | 12 |
| CPB (V1, CL) | 5467 | 5491 | 5544 | 12 |
| CPB (V1, CL), PMA (CL) | 5456 | 5482 | 5540 | 13 |
| CPB (V2, CL) | 5448 | 5472 | 5526 | 12 |
| CPB (V2, CL), PMA (CL) | 5439 | 5465 | 5522 | 13 |
| CPB (V2, V1, CL) | 5391 | 5417 | 5475 | 13 |
| CPB (V1, V1, CL), PMA (CL) | 5383 | 5411 | 5473 | 14 |
| CBP (Q, V1, CL) | 5378 | 5404 | 5462 | 13 |
| CPB (Q, V1, CL), PMA (CL) | 5368 | 5396 | 5459 | 14 |
| CPB (Q, CL) | 5379 | 5403 | 5456 | 12 |
| CPB (Q, CL), PMA (CL) | 5369 | 5395 | 5453 | 13 |
| CPB (Q, V2, V1, CL) | 5348 | 5376 | 5438 | 14 |
| CPB (Q, V2, V1, CL), PMA (CL) | 5339 | 5369 | 5436 | 15 |
| CPB (Q, V2, CL) | 5350 | 5376 | 5434 | 13 |
| CPB (Q, V2, CL), PMA (CL ) | 5341 | 5369 | 5432 | 14 |
−2LL, minus two times the log likelihood; weight allometric= bodyweight with theta values fixed to 0.75 (CL, Q) and 1 (V1, V2)
AIC, Akaike information criterion; BIC, Bayesian information criterion; CL, systemic clearance; CPB, cardiopulmonary bypass; PMA, postmenstrual age; Q, intercompartmental clearance; V1, central volume of distribution; V2, peripheral volume of distribution. Bold number, the lowest values for the model quality parameters
The final covariates used in the model with the lowest BIC values therefore were: allometrically fixed BW using theory‐based exponents of 0.75 on (CL, Q) and 1 on (V1, V2); CPB on (Q, V2, CL) and PMA on CL.
Table 3 shows the final population PK estimates, with the typical values for BW groups presented in Table 4. Figures 2 and 3 compare the covariate‐free to the final model. In Figure 2, the initial asymmetrical distribution observed for the covariate‐free model is corrected to a slightly more homogenous distribution when the covariates are taken into account. Figure 3 depicts the pcVPC for the final model.
Table 3.
Final population parameters
| Individual parameters | All 43 patients | |||
|---|---|---|---|---|
| Typical value (tv) estimate (%RSE) | IIV (%RSE) | Bootstrap median (%RSE) | IIV (%RSE) | |
| V1 (l) = tvV1 * (weight/70) θweight * exp (ηV1) | 13.58 (7.0) | 0.101 (45.8) | 13.77 (7.3) | 0.105 (66) |
| θ weight | 1 (0) | 1 (0) | ||
| V2 (l) = tvV2 * (weight/70) θweight * exp (θCPB*(CPB==1)) * exp (ηV2) | 17.99 (5.4) | 0.090 (32.9) | 17.92 (5.5) | 0.092 (34.3) |
| θ CPB | ‐0.153 (30.8) | –0.157 (31.8) | ||
| θ weight | 1 (0) | 1 (0) | ||
| CL (l h −1 ) = tvCL * (weight/70) θweight *1/(1 + (PMA50/PMA) θPMA ) * exp (θCPB*(CPB==1)) *exp (ηCl) | 3.78 (12.6) | 0.067 (18.3) | 3.66 (25.3) | 0.063 (19.3) |
| θ CPB | 0.842 (7.7) | 0.843 (8.0) | ||
| θ weight | 0.75 (0) | 0.75 (0) | ||
| θ PMA | 2.95 (46.0) | 3.02 (619) | ||
| PMA50 | 0.632 (14.5) | 0.679 (42.0) | ||
| Q (l h −1 ) = tvQ * (weight/70) θweight * exp (θCPB*(CPB = 1)) * exp (ηQ) | 16.32 (8.7) | 9.92 E‐06 (6.3) | 16.14 (8.8) | 1.53E‐05 (541) |
| θ CPB | 1.87 (13.0) | 1.88 (14.9) | ||
| θ weight | 0.75 (0) | 0.75 (0) | ||
| Standard deviation of the multiplicative error mode | 0.128 | 0.127 | ||
CL, elimination clearance; CPB, cardiopulmonary bypass; IIV, interindividual variability; %RSE, percent relative standard error, PMA50, PMA at which normalized clearance is equal to 50% of the maximum value (in years); Q, intercompartmental clearance; V1, central volume of distribution; V2, peripheral volume of distribution
Table 4.
Dosing proposal and mean pharmacokinetic parameters for tranexamic acid in children undergoing cardiac surgery with cardiopulmonary bypass (CPB) for a 20 μg ml–1 minimum target concentration
| Weight (kg) | CPB | CL (l h–1) | V1 (l) | Q (l h–1) | V2 (l) | Fast t½ (h) | Slow t½ (h) | First dose (mg kg–1) | Rate (mg kg–1 h–1) |
CPB dose (mg kg–1) |
Rate post‐CPB (mg kg–1 h–1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 0 | 0.932 | 2.101 | 3.79 | 2.75 | 0.191 | 4.11 | 10 | 10 | 4 | 4 |
| 1 | 2.16 | 2.101 | 24.7 | 2.36 | 0.028 | 1.54 | |||||
| 7.5 | 0 | 0.728 | 1.511 | 3.05 | 2.01 | 0.171 | 3.83 | 10 | 10 | 4 | 4 |
| 1 | 1.691 | 1.511 | 19.9 | 1.72 | 0.025 | 1.43 | |||||
| 5 | 0 | 0.506 | 0.997 | 2.25 | 1.35 | 0.155 | 3.64 | 10 | 10 | 4 | 4 |
| 1 | 1.177 | 0.997 | 14.7 | 1.16 | 0.023 | 1.36 | |||||
| 2.5 | 0 | 0.213 | 0.517 | 1.34 | 0.70 | 0.139 | 4.51 | 10 | 10 | 4 | 4 |
| 1 | 0.494 | 0.517 | 8.74 | 0.60 | 0.020 | 1.72 |
CL, elimination clearance; fast t½, primary elimination half‐life; Q, intercompartmental clearance; slow t½, terminal elimination half‐life; V1, central volume of distribution; V2, peripheral volume of distribution
Figure 2.
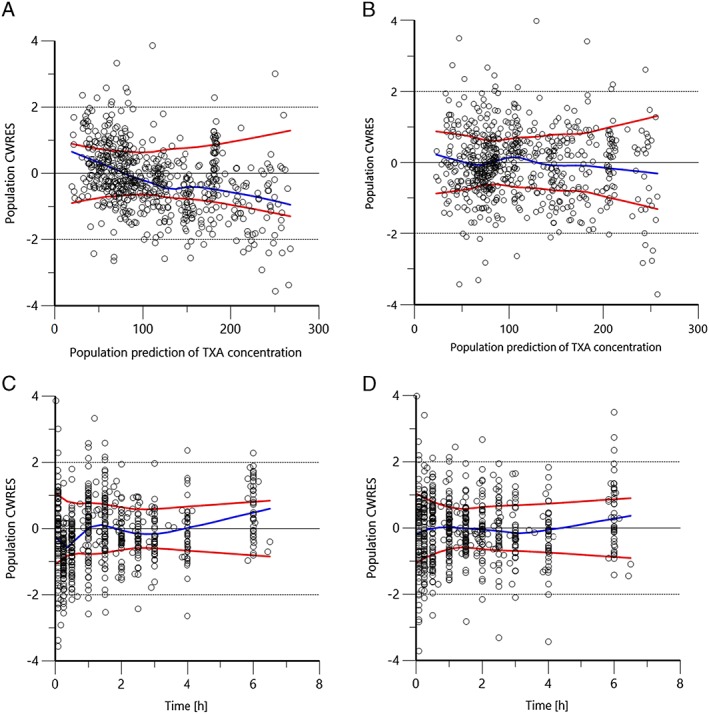
Development of the predictive performance of the population CWRES against concentration or time after dose. (A), (C): covariate free model. (B), (D): final model. Values should be between y=–2 and y=+2. Blue line= Normal Loess smooth line, Red lines= Absolute Loess regression lines. CWRES, conditional weighted residuals
Figure 3.
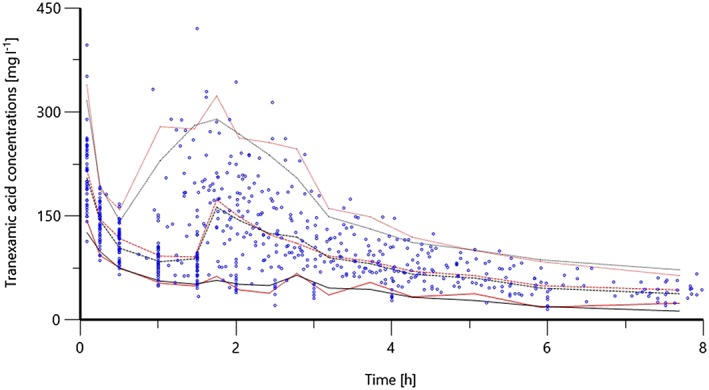
Visual predictive check from a Monte Carlo simulation (n = 1000) of the final model. Raw data points as well as predicted (black) and observed (red) 5th, 50th and 95th percentiles are shown
Simulation of TXA concentrations
As allometric BW, CPB and PMA were the main covariates, we proposed a weight‐adjusted scheme (Table 4). Despite the benefits conferred by the introduction of allometric scaling into our final model, we retained a weight‐adjusted dosing regimen based on the allometric PK parameters for adult BW values as dosing based on BW satisfies most clinical situations in the operating room 16. Therefore, concentration curves for children of less than 10 kg BW were simulated after receiving a loading dose of 10 mg kg–1 TXA, followed by a continuous infusion of 10 mg kg–1 h–1 until initiation of CPB, then a 4 mg kg–1 bolus, followed by a 4 mg kg–1 h–1 infusion to maintain a target blood concentration of 20 mg l–1. This is known as the ‘10‐10‐4‐4’ rule (Figure 4).
Figure 4.
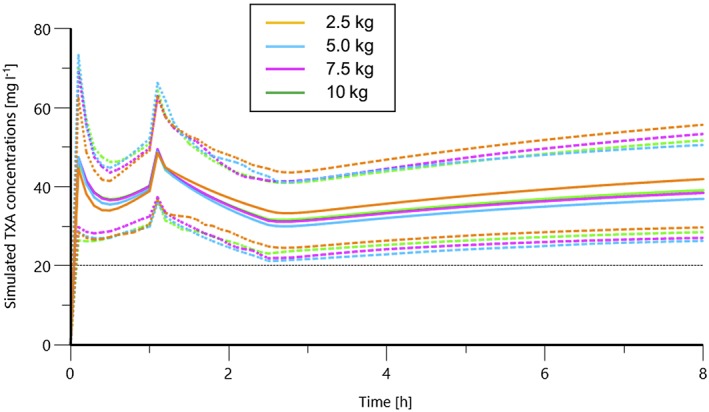
Simulation of concentration curves using the final model with the ‘10‐10‐4‐4’ dosing recommendations for tranexamic acid (TXA) [a loading dose of 10 mg kg–1 followed by a continuous infusion of 10 mg kg–1 h–1 until initiation of cardiopulmonary bypass (CPB), then a 4 mg kg–1 bolus, followed by 4 mg kg–1 h–1 after CPB] for maintaining a target concentration of 20 mg l–1. Means and 2.5th and 97.5th percentiles are given
Discussion
We have described TXA PK in infants less than 1 year of age undergoing cardiac surgery with and without CPB but omitting those with deep hypothermic circulatory arrest. Using a two‐compartmental model, model improvements from baseline were seen by adding allometric scaling of BW, CPB as a categorical factor and PMA. Finally, a dose recommendation was developed to ensure a threshold concentration of 20 mg l–1, which can be described as the ‘ 10‐10‐4‐4 rule’.
The effect of covariates on PK
The two main aspects of PK disturbances on bypass are distribution and elimination. For TXA, Dowd et al. 13 found that, in adults, CPB induced an increase in Vc (V1). Haemodilution has been described to be responsible for an increase in the apparent Vc 17. The impact of haemodilution is mostly apparent upon initiation of CPB and is more pronounced in neonates 18, where prime volumes often exceed 100% of the patient's blood volume. Therefore redosing on CPB seems justified to compensate for this drop in concentration. Previous studies in adults reported either concentrations only 19 or a full set of PK parameters 7, 13, 20. Recently, paediatric data in children undergoing cardiac surgery 7, 8 and craniofacial surgery 21 have been presented.
Our study was an extension of the previous work by Grassin‐Delyle et al. 7 that investigated children with a mean age of 5 years and only showed an effect of CPB on Q. By contrast, Wesley et al. 8, in children less than 4 years of age, found an effect of CPB on volume and clearance parameters. Our results did not reveal an effect of CPB on V1. However, using a model including V1 was only marginally worse and actually provided the best AIC and ‐2LL values. Therefore, we were able to confirm the findings by Wesley et al. that favour an unrestricted model centred on the median of the respective population parameter. During our model‐building process, the clearance maturation was clearly dependent on PMA. This is explained primarily by the renal excretion of TXA and the fast maturation of the glomerular filtration rate: at birth, it is approximately 35% of adult values, reaching 50% at 48 weeks PMA and 90 % at 1 year postnatal age 22. All children had a normal preoperative creatinine clearance according to the Schwartz formula and values were found to be within the range for ‘noncardiac’ children 21. Taken together, our estimates are in the range of previously published ones in paediatric patients 7, 8, 21, as well as adult values (Table S1) 13, 19, 20, 23, 24.
Dosing and range of effective concentrations
The primary aim of the present study was to reduce peak levels and the total amount of TXA administered. We propose a dosing regimen based on our PK model (Table 4). This involves slightly higher doses than those proposed by Wesley et al. 8, (with a loading dose of 9–15 mg kg–1, an infusion rate of 2.0–2.5 mg kg–1 h–1 and a CPB prime dose of 20 mg l–1) and that reported by Grassin‐Delyle et al. 7 (with a loading dose of 6.4 mg kg–1 followed by an infusion rate of 2.0–3.1 mg kg–1 h–1 without a prime bolus) based on data from older children with a mean BW of 18 kg. The main reasons for the higher doses indicated in the present study and a different approach to PK analysis. We created a simulation with only 90 min of CPB time, whereas Wesley et al. presented their analysis using an ongoing effect of CPB from its initiation onwards. In comparison, the combination of these two probably increases the clearance (CL) and our model may be more realistic during regular bypass with mild hypothermia. An additional bolus into the CPB prime avoids exaggerated dilutional effects on CPB 25. Also, the TXA boulus into the prime fluid was higher since our prime volumes were also slightly higher. In contrast to Wesley et al. 8, we did not find a different TXA dose requirement in the neonatal group.
Any dosing recommendation in our investigation is aiming at target plasma TXA concentration of 20 mg l–1. This target is a topic of great discrepancy; the main target concentrations in the literature range from 10 μg ml–1 to 126 μg ml–1 13, 19. In vivo, higher doses may be clinically more effective in reducing the perioperative blood loss 13, even though this did not translate into differences in mortality 26. We used one of the lowest effective target concentrations reported, based on the work by Yee et al. 14. These authors explored the maximal inhibitory effects of TXA on fibrinolysis in vitro. Using pooled cord blood from term neonates, they induced fibrinolysis with 1000 IU ml‐1 tissue plasminogen activator (t‐PA), and titrated TXA to complete inhibition. Effective inhibitory concentrations to abolish fibrinolysis were found at TXA plasma concentrations of 6.54 μg ml–1 , 17.5 μg ml–1 and 9.83 μg ml–1 for neonatal plasma, adult plasma and a mixture of both, respectively, during routine paediatric CPB. One limitation of their study was the fact that normal plasma levels of t‐PA in children on CPB are only between 2.5 IU ml–1 and 4 IU ml–1 27, 28, so the applied t‐PA concentration was more than 250 times higher. Despite this, the study underlined the concept of maintaining TXA concentrations around 20 μg ml–1. This is particularly important in light of the increasing risk of seizures to 2.5 –3.5% in adult cardiac surgery patients at doses greater than 100 mg kg–1 of TXA 29. In fact, one of our patients suffered a series of seizures 6 h after the second dose of TXA 15, possibly related to the recently discovered competitive antagonism of TXA at glycine and GABAA receptors 30, 31 and the fact that peak cerebrospinal fluid (CSF) levels are delayed by approximately 5–6 h 30. In addition, changes in the blood–brain barrier after CPB 32 might be responsible for unexpectedly high CSF levels, which are even higher in neonates and infants with a naturally more fragile endothelium.
Limitations
All of the children in our study underwent continuous ultrafiltration and MUF. As we did not take separate samples before and after MUF, or standardize the filtration process, we cannot comment on its effect on TXA plasma concentrations. However, introducing MUF volumes as a covariate did not improve the model. In addition, the effect of moderate and deep hypothermia on TXA levels is unclear and we limited our population to cases using mild hypothermia between 32°C and 36°C 33. This is in agreement with the study by Wesley et al. 8 where neither MUF nor hypothermia were found to be influential factors.
Conclusions
We investigated TXA PK in children of less than 1 year of age undergoing cardiac surgery. PK parameters are significantly influenced by BW, maturation and CPB. A simple dosing regimen of 50 mg kg–1 twice at the beginning and repeated on CPB delivers effective TXA concentrations above 20 μg ml–1 until 6 h after the last dose but at the risk of high peak concentrations, which eventually lead to seizures. Alternatively, a reduced dosing regimen according to desired target concentrations of e.g. 20 μg ml–1 throughout surgery would comprise the ‘10‐10‐4‐4 rule’: an initial bolus of 10 mg kg–1 is followed by an infusion of 10 mg kg–1 h–1 until initiation of CPB and then a 4 mg kg–1 h–1 infusion after the start of CPB, with a bolus of 4 mg kg–1 into the CPB prime. Further studies should aim to relate these results to pharmacodynamics – in this case, the associated blood loss and clinical outcome – as demonstrated in adults by Sigaut et al. 26.
Competing Interests
There are no competing interests to declare.
We would like to gratefully acknowledge the work of the medical technicians at our hospital laboratory for separating the blood samples, and the University Hospital Regensburg for their expert analysis. Special thanks also to Magdalena Waldhier, Institute of Functional Genomics, University Regensburg, Germany, for providing background knowledge on chromatography–mass spectrometry. Financial support was provided from departmental sources and the Kommission für klinische Forschung (KKF), Medizinische Fakultät der Technischen Universität München.
Contributors
R.G. helped to design the study, conduct the study, recruit the patients, collect the data, analyse the data and write the manuscript. M.G. helped to design the study, analyse the data and write the manuscript. S.G.‐D. helped to analyse the data and write the manuscript. S.U. helped to analyse the data and write the manuscript. K.M. helped to design the study, conduct the study, recruit the patients, collect the data, analyse the data and write the manuscript. P.T.‐P. helped to design the study, analyse the data and write the manuscript. S.B. helped to conduct the study, collect the data, analyse the data and write the manuscript. S.B. helped to analyse the data and write the manuscript. G.W. helped to design the study, conduct the study, analyse the data and write the manuscript. All authors approved the final manuscript.
Supporting information
Table S1 Kinetic parameters for comparative pharmacokinetic studies separated into paediatric and adult data, as available
An Agilent GCMS (5890 plus and 5972 MSD) system in electron impact (EI) mode was used. Plasma preparation started by adding 1 μg (10 μl) internal standard solution 13C,15N‐tranexamic acid (Sigma, Steinheim, Germany) and 1 μg 6‐aminohexanoic acid (AHA;10 μl, Sigma) to a 25 μl sample. Deproteination was achieved with an additional volume of 150 μl of cold methanol (–20°C) (Baker, Deventer, the Netherlands). The mixture was centrifuged at 3200 g for 4 min, the pellet was washed twice with 25 μl methanol/H2O [4/1 (vol/vol)] and the combined supernatants were dried in an N2 stream at 40°C. The residual was resolved in 50 μl water, 25 μl methanol and 6 μl pyridine (Acros, Fisher scientific, Nidderau, Germany). Methylchloroformate (Sigma) was added in two portions of 5 μl each with intercepted mixing. The derivatives were shifted into an organic phase by adding 50 μl of CHCl3 (Sigma). The lower CHCl3 phase was transferred into micro‐inserts (CZT, Kriftel, Germany). The chromatographical setup was: injection volume 0.3 μl (splitless for 0.5 min at 285°C), a Phenomenex ZB‐1 ms capillary column with dimensions of 30 m × 0.25 mm × 0.25 μm (Phenomenex, Aschaffenburg, Germany). Mass spurs (m/z) and retention times (RT) of the substances: internal standards isotopic enhanced TXA (m/z = 201.1; RT = 6.654 min) and AHA (m/z = 172.1; RT = 5.737 min) and target TXA (m/z = 198.1; RT = 6.654 min). The used temperature characteristic as follows: starting with 70°C for 1 min, a subsequent gradient of 30 K min–1 up to 280°C was used. Calibration was found to be linear over the whole concentration range.
Gertler, R. , Gruber, M. , Grassin‐Delyle, S. , Urien, S. , Martin, K. , Tassani‐Prell, P. , Braun, S. , Burg, S. , and Wiesner, G. (2017) Pharmacokinetics of tranexamic acid in neonates and infants undergoing cardiac surgery. Br J Clin Pharmacol, 83: 1745–1757. doi: 10.1111/bcp.13274.
Trial registration:The study was approved by the ethics committee of the Medical Faculty of the Technical University Munich (ref. 5600/12) and registered with the World Health Organization International Clinical Trials Registry Platform/German Clinical Trials Register No. DRKS‐4538.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horrow JC, Hlavacek J, Strong MD, Collier W, Brodsky I, Goldman SM, et al. Prophylactic tranexamic acid decreases bleeding after cardiac operations. J Thorac Cardiovasc Surg 1990; 99: 70–74. [PubMed] [Google Scholar]
- 3. Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, Ker K. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2011: CD001886. [DOI] [PubMed] [Google Scholar]
- 4. Martin K, Knorr J, Breuer T, Gertler R, Macguill M, Lange R, et al. Seizures after open heart surgery: comparison of epsilon‐aminocaproic acid and tranexamic acid. J Cardiothorac Vasc Anesth 2011; 25: 20–25. [DOI] [PubMed] [Google Scholar]
- 5. Faraoni D, Goobie SM. New insights about the use of tranexamic acid in children undergoing cardiac surgery: from pharmacokinetics to pharmacodynamics. Anesth Analg 2013; 117: 760–762. [DOI] [PubMed] [Google Scholar]
- 6. Chauhan S. Comparison of tranexamic acid with aprotinin in pediatric cardiac surgery. Ann Card Anaesth 2015; 18: 27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grassin‐Delyle S, Couturier R, Abe E, Alvarez JC, Devillier P, Urien S. A practical tranexamic acid dosing scheme based on population pharmacokinetics in children undergoing cardiac surgery. Anesthesiology 2013; 118: 853–862. [DOI] [PubMed] [Google Scholar]
- 8. Wesley MC, Pereira LM, Scharp LA, Emani SM, McGowan FX Jr, DiNardo JA. Pharmacokinetics of tranexamic acid in neonates, infants, and children undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology 2015; 122: 746–758. [DOI] [PubMed] [Google Scholar]
- 9. Waldhier MC, Dettmer K, Gruber MA, Oefner PJ. Comparison of derivatization and chromatographic methods for GC‐MS analysis of amino acid enantiomers in physiological samples. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 1103–1112. [DOI] [PubMed] [Google Scholar]
- 10. Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 2005; 49: 1020–1038. [Google Scholar]
- 11. Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci 2013; 102: 2941–2952. [DOI] [PubMed] [Google Scholar]
- 12. Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed‐effect models: the npde add‐on package for R. Comput Methods Programs Biomed 2008; 90: 154–166. [DOI] [PubMed] [Google Scholar]
- 13. Dowd NP, Karski JM, Cheng DC, Carroll JA, Lin Y, James RL, et al. Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology 2002; 97: 390–399. [DOI] [PubMed] [Google Scholar]
- 14. Yee BE, Wissler RN, Zanghi CN, Feng C, Eaton MP. The effective concentration of tranexamic acid for inhibition of fibrinolysis in neonatal plasma in vitro . Anesth Analg 2013; 117: 767–772. [DOI] [PubMed] [Google Scholar]
- 15. Gertler R, Wiesner G, Tassani‐Prell P, Martin K, Gruber M. Measurement of tranexamic acid serum concentrations in a 7‐month‐old infant with clinical seizures after open heart surgery. Pediatr Neurol 2014; 51: e1–e2. [DOI] [PubMed] [Google Scholar]
- 16. Anderson BJ, Meakin GH. Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth 2002; 12: 205–219. [DOI] [PubMed] [Google Scholar]
- 17. Rosen DA, Rosen KR. Elimination of drugs and toxins during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997; 11: 337–340. [DOI] [PubMed] [Google Scholar]
- 18. Kern FH, Morana NJ, Sears JJ, Hickey PR. Coagulation defects in neonates during cardiopulmonary bypass. Ann Thorac Surg 1992; 54: 541–546. [DOI] [PubMed] [Google Scholar]
- 19. Fiechtner BK, Nuttall GA, Johnson ME, Dong Y, Sujirattanawimol N, Oliver WC Jr, et al. Plasma tranexamic acid concentrations during cardiopulmonary bypass. Anesth Analg 2001; 92: 1131–1136. [DOI] [PubMed] [Google Scholar]
- 20. Sharma V, Fan J, Jerath A, Pang KS, Bojko B, Pawliszyn J, et al. Pharmacokinetics of tranexamic acid in patients undergoing cardiac surgery with use of cardiopulmonary bypass. Anaesthesia 2012; 67: 1242–1250. [DOI] [PubMed] [Google Scholar]
- 21. Goobie SM, Meier PM, Sethna NF, Soriano SG, Zurakowski D, Samant S, et al. Population pharmacokinetics of tranexamic acid in paediatric patients undergoing craniosynostosis surgery. Clin Pharmacokinet 2013; 52: 267–276. [DOI] [PubMed] [Google Scholar]
- 22. Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 2009; 24: 67–76. [DOI] [PubMed] [Google Scholar]
- 23. Grassin‐Delyle S, Tremey B, Abe E, Fischler M, Alvarez JC, Devillier P, et al. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth 2013; 111: 916–924. [DOI] [PubMed] [Google Scholar]
- 24. Yang QJ, Jerath A, Bies RR, Wasowicz M, Pang KS. Pharmacokinetic modeling of tranexamic acid for patients undergoing cardiac surgery with normal renal function and model simulations for patients with renal impairment. Biopharm Drug Dispos 2015; 36: 294–307. [DOI] [PubMed] [Google Scholar]
- 25. Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child 2013; 98: 737–744. [DOI] [PubMed] [Google Scholar]
- 26. Sigaut S, Tremey B, Ouattara A, Couturier R, Taberlet C, Grassin‐Delyle S, et al. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology 2014; 120: 590–600. [DOI] [PubMed] [Google Scholar]
- 27. Eisses MJ, Chandler WL. Cardiopulmonary bypass parameters and hemostatic response to cardiopulmonary bypass in infants versus children. J Cardiothorac Vasc Anesth 2008; 22: 53–59. [DOI] [PubMed] [Google Scholar]
- 28. Couturier R, Rubatti M, Credico C, Louvain‐Quintard V, Anerkian V, Doubine S, et al. Continuous or discontinuous tranexamic acid effectively inhibits fibrinolysis in children undergoing cardiac surgery with cardiopulmonary bypass. Blood Coagul Fibrinolysis 2014; 25: 259–265. [DOI] [PubMed] [Google Scholar]
- 29. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High‐dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg 2010; 110: 350–353. [DOI] [PubMed] [Google Scholar]
- 30. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest 2012; 122: 4654–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kratzer S, Irl H, Mattusch C, Burge M, Kurz J, Kochs E, et al. Tranexamic acid impairs gamma‐aminobutyric acid receptor type A‐mediated synaptic transmission in the murine amygdala: a potential mechanism for drug‐induced seizures? Anesthesiology 2014; 120: 639–649. [DOI] [PubMed] [Google Scholar]
- 32. Cavaglia M, Seshadri SG, Marchand JE, Ochocki CL, Mee RB, Bokesch PM. Increased transcription factor expression and permeability of the blood brain barrier associated with cardiopulmonary bypass in lambs. Ann Thorac Surg 2004; 78: 1418–1425. [DOI] [PubMed] [Google Scholar]
- 33. van Saet A, de Wildt SN, Knibbe CA, Bogers AD, Stolker RJ, Tibboel D. The effect of adult and pediatric cardiopulmonary bypass on pharmacokinetic and pharmacodynamic parameters. Curr Clin Pharmacol 2013; 8: 297–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Kinetic parameters for comparative pharmacokinetic studies separated into paediatric and adult data, as available


