Abstract
Aims
Pharmacokinetic (PK) studies suggest that there is a room for improvement in clinical use of rituximab through more individualized treatment. The objective of this study was to characterize rituximab PK in 29 newly diagnosed patients with diffuse large B‐cell lymphoma treated with rituximab in combination with cyclophosphamide, doxorubicin, vincristine and methylprednisolone every 3 weeks. We also evaluated the association of rituximab PK with clinical outcome.
Methods
Rituximab serum levels were determined by enzyme‐linked immunosorbent assay and evaluated by a population PK analysis applying nonlinear mixed effects modelling.
Results
The data were best described by a two‐compartment model comprising linear nonspecific clearance of 0.252 [95% confidence interval (CI): 0.227–0.279] l day–1 and time‐varying specific clearance of 0.278 (95% CI: 0.181–0.390) l day–1, corresponding to target‐mediated drug disposition of rituximab. Nonspecific clearance was lower in older patients and those with lower body weight. Additionally, volume of the central compartment was higher in males. A clear association of clinical response with rituximab PK has been observed. Rate constant of specific clearance decay was 0.143 day−1 (95% CI: 0.0478–0.418) in patients with no disease progression, while in patients with disease progression it was 82.2% lower (95% CI: 33.4–95.0).
Conclusions
This finding indicates that time‐changes in clearance could serve as a predictive marker of response to rituximab. Our report demonstrates the rationale for studies evaluating higher doses of rituximab in selected patients.
Keywords: clinical outcome, diffuse large B‐cell lymphoma, pharmacokinetics, rituximab
What is Already Known about this Subject
Rituximab dosing is based on evidence from clinical practice rather than from consideration of pharmacokinetics and factors influencing individual exposure.
High serum rituximab levels are associated with favourable outcome in indolent non‐Hodgkin lymphoma (NHL) patients.
Pharmacokinetic data are limited in aggressive NHL and dosing in these patients may be suboptimal.
What this Study Adds
This is the first study in aggressive NHL to demonstrate target‐mediated drug disposition of rituximab.
We are the first to show the association of disease progression with the rate of rituximab clearance decay.
The rate of rituximab clearance decay may serve as one of predictive markers of rituximab response.
Tables of Links
| LIGANDS |
|---|
| Rituximab |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to Pharmacology 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Rituximab (Mabthera, F. Hoffmann–La Roche Ltd, Basel, Switzerland) is a chimeric human/mouse immunoglobulin (Ig)G1 monoclonal antibody that binds specifically to the CD20 antigen present on the surface of normal and neoplastic B lymphocytes 3. Possible mechanisms of action include antibody‐dependent cellular cytotoxicity, complement‐dependent cytotoxicity and induction of apoptosis 4, 5, 6. Rituximab has been proved effective in patients with various lymphoid malignancies, including indolent and aggressive forms of B‐cell non‐Hodgkin lymphoma (NHL) 7, 8.
Overall survival of patients with diffuse large B‐cell lymphoma (DLBCL) has been shown to significantly improve when standard 3‐week regimen of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) was combined with rituximab at a dose of 375 mg m–2 (R‐CHOP) 9, 10, 11. R‐CHOP is now considered the standard of care for DLBCL patients. Rituximab dosing schedules were selected based on evidence from clinical practice rather than from consideration of rituximab pharmacokinetics (PK) and factors influencing individual exposure 12, 13.
In a pivotal clinical trial of low‐grade or follicular NHL patients, where rituximab was administered at a dose of 375 mg m–2 once weekly for 4 weeks, median peak concentrations increased from 239.1 mg l–1 after the first infusion to 460.7 mg l–1 after the fourth infusion 14. Rituximab PK was best described by a two‐compartment model, with mean half‐lives of about 1.3 and 19 days for distribution and elimination phase, respectively. The majority of PK data with rituximab are from studies in patients with indolent lymphomas. Despite the widespread use of rituximab in aggressive B‐cell lymphoma, the data on its PK are limited and dosing in these patients may be suboptimal 12, 15.
Therefore, the aim of this trial was to investigate if there is room for improvement in clinical use of rituximab in patients with newly diagnosed DLBCL receiving R‐CHOP through a more individualized treatment. Objectives were to perform population PK (PopPK) analysis of rituximab and to evaluate the association of rituximab PK with clinical outcome.
Methods
Patient selection and study design
Patients older than 18 years with newly diagnosed DLBCL were eligible to participate in this study. All patients received eight cycles of R‐CHOP regimen every 3 weeks according to the national guidelines for the treatment of NHL 16. To minimize adverse drug reactions associated with the treatment regimen, patients were premedicated with clemastine, acetaminophen and methylprednisolone, followed by rituximab at a dose of 375 mg m–2. Subsequently, patients received granisetron and chemotherapy regimen consisting of cyclophosphamide, doxorubicin and vincristine. In the first cycle rituximab was administered as slow infusion started at 50 mg h–1 during the first 30 min. If no signs of toxicity were observed, infusion rate was gradually increased by 50 mg h–1 every 30 min to a maximum of 400 mg h–1. If the first cycle was well tolerated, subsequent rituximab doses were administered as fast infusions. In this scenario, 20% of the rituximab dose was infused over 30 min, followed by the remaining 80% over 60 min. To exclude treatment‐related factors on response to therapy, relative dose intensity (RDI) was calculated for R‐CHOP protocol, which represents the ratio of the amount of a drug combination actually administered to the amount planned for a fixed time period 17.
Patient exclusion criteria were history of central nervous system lymphomatous disease, other malignancies, infections or any other medical condition that would preclude treatment with eight cycles of R‐CHOP regimen. Clinical response was evaluated according to the revised response criteria for malignant lymphoma proposed by International Harmonization Project 18. The study was approved by the Institutional Review Board at Institute of Oncology Ljubljana (Approval number 03‐Z/KSOPKR‐22) and National Medical Ethics Committee of Republic of Slovenia (Approval number 38/10/09). Informed consent was obtained from all patients before inclusion in the study.
Blood sample collection for pharmacokinetic analysis
Overall, 18 blood samples (10 mL) per patient were collected for pharmacokinetic analysis. In cycles 1–7 of the R‐CHOP treatment, two samples per cycle were obtained to determine rituximab peak and trough serum levels. Peak serum levels samples were collected in the time‐frame from 15 min up to 3 h after rituximab infusion and trough samples were collected immediately before rituximab infusion in the subsequent cycle. In the last cycle (cycle 8) four additional samples were collected (peak and 1, 3, and 6 months after rituximab infusion) to determine rituximab disposition. Blood was drawn into Z Serum Clot Activator Vacuette Tube (Greiner Bio‐One GmbH, Kremsmunster, Austria). Samples were centrifuged at 1000 × g for 10 min at room temperature and stored at –80°C until analysis.
Determination of rituximab serum concentration
Rituximab serum levels were determined by enzyme‐linked immunosorbent assay (ELISA) according to the previously published method 19. Microtitre 96‐well plates were coated with rat anti‐rituximab IgG2a antibody at a concentration of 1 μg ml–1 diluted in 0.05 mol l–1 carbonate–bicarbonate buffer at pH 9.6. Following incubation at 4°C for 24 h, the plates were washed three times with 0.05% Tween‐20 in phosphate buffered saline (PBS). The remaining protein‐binding sites were saturated with 1% bovine serum albumin (BSA) in PBS at room temperature for 2 h and subsequently washed three times as described above. Diluted standards, quality control (QC) samples, and patient samples were added to the wells and incubated for 1 h at room temperature. After five washings, goat peroxidase‐conjugated anti‐human IgG antibody diluted 1/60 000 in 1% BSA in PBS was added to each well. Plates were incubated at room temperature for 90 min. Following five washings, O‐phenylenediamine was added and the plates were incubated in the dark at room temperature for 30 min. The colour reaction was stopped by adding 3 mol l–1 H2SO4 per well. The plate was shaken for 30 seconds and read at 490 nm with ELISA plate reader (Epoch Microplate Spectrophotometer, BioTek, Bad Friedrichshall, Germany). Rituximab serum concentration in patient samples and QCs was calculated from a standard curve fitted with a five‐parameter logistic equation (ReaderFit, Hitachi Solutions, Irvine, California, USA).
The rat anti‐rituximab IgG2a monoclonal antibody MB2A4 and goat anti‐human IgG polyclonal antibody AHP1323P were purchased from AbD Serotec (Oxford, UK). Microtiter 96‐well solid plates (Nunc‐Immuno MicroWell 96 well solid plates), carbonate–bicarbonate buffer capsules, PBS, BSA, PBS containing Tween‐20, and O‐phenylenediamine tablets were supplied by Sigma–Aldrich (St Louis, MO, USA). Mabthera (rituximab) 100 mg, supplied as a solution for infusion, was obtained from Roche Pharmaceuticals (Basel, Switzerland).
Rituximab calibration standards at nominal concentrations of 10, 30, 50, 100, 160, 230, 350, 600, 900, 1400 and 2000 μg ml–1 were prepared by dilution in 1% BSA and 0.05% Tween‐20 in PBS. QC samples at 20, 200 and 1000 μg ml–1 were prepared by spiking blank serum with rituximab. Samples, calibration standards and QC samples were diluted 1/20 000 with 1% BSA and 0.05% Tween‐20 in PBS immediately before assay.
Samples, calibration standards and QC samples were analysed in duplicate and the mean value was reported. For study samples, the criterion for an acceptable run was a coefficient of variation (CV) of the duplicate analysis ≤20%. Between‐run and within‐run precision and accuracy were determined for the three QC samples in six replicates run on 3 separate days. Accuracy, determined as deviation of the calculated from the nominal QC sample concentration was ≤13.7%, within‐run and between‐run precision expressed as CV were ≤9.8% and ≤13.8%, respectively.
Pharmacokinetic analysis
Nonlinear mixed‐effects modelling using NONMEM software version 7.3 (Icon plc, Dublin, Ireland) was used for the PK analysis. Model‐building steps were managed by PsN (version 3.5.3, http://psn.sourceforge.net) and Xpose (version 4.4.0, http://xpose.sourceforge.net) software. Fortran subroutines were compiled with the Intel Visual Fortran Compiler (version 11.0, Intel; Santa Clara, CA, USA).
Structural model development
The base model of rituximab PK was developed in the first step. The structural models investigated were one‐ and two‐compartment models. Initially, rituximab elimination was modelled as constant clearance (CL1), assuming linear PK. Subsequently, target‐mediated disposition of rituximab was modelled as nonlinear clearance (CL2), approximated by time‐dependent (Equation (1)) or concentration‐dependent (Michaelis–Menten type) clearance (Equation (2))
| (1) |
| (2) |
where KD is the rate constant of clearance decay with time (t); C(t) is rituximab serum concentration at time t; and C50 is rituximab concentration when clearance is 50% of its maximum value (CL2,0) at time 0, when rituximab concentration is 0. Additionally, combinations of linear and nonlinear elimination pathways were evaluated.
A log‐normal distribution of individual subjects' parameter values was assumed. Interindividual variability (IIV) was therefore described by exponential random effect model, while additive, proportional and combination (additive + proportional) error models were evaluated to describe residual IIV of rituximab serum concentration. The model was coded by differential equations employing NONMEM's ADVAN 6 subroutine. The first‐order conditional estimation method with interaction was used for parameter estimation.
Model development was guided by the minimum value of objective function value (OFV), which is approximately equal to –2 times log‐likelihood of the parameter values given the data. The difference in OFV (ΔOFV) between two nested models is approximately χ2 distributed and a decrease of 3.84 for an extra parameter was considered significant at the 5% significance level. Non‐nested models were compared by Akaike information criteria value (AIC) computed as OFV + 2 × Npar, where Npar is the number of all estimated parameters in the model 20. Additional guidance in model development was convergence of minimization, number of significant digits >3, successful covariance step, gradients in the final iteration between 10−3 and 102 and absence of substantial η‐ and ε‐shrinkage.
Covariate model
Initially, the base model without covariates was used to describe rituximab serum concentration–time data and to obtain empirical Bayesian estimates of individual parameters. The association between various covariates and individual parameters was evaluated by graphical exploration followed by testing within NONMEM with a stepwise covariate modelling procedure. Among covariate effects considered for inclusion were patients' age, sex, weight, international prognostic index (IPI), irradiation following R‐CHOP in first line treatment (yes/no), treatment response (complete response/partial response/stable disease/progressive disease), and disease progression during follow‐up (yes/no). For inclusion of continuous covariates, linear and power models were investigated. Categorical covariates were modelled to estimate proportional change in the value of the PK parameter. Likelihood ratio tests were used to evaluate the significance of covariate relationships (p < 0.05 during forward inclusion and p < 0.01 during backward elimination step). Reduction in unexplained IIV and biologically plausible parameter estimates were additional criteria for retention of a covariate in the model.
Model evaluation
The final model was evaluated by standard diagnostic plots and visual predictive check (VPC) applying prediction and variability correction. With VPC, rituximab concentration profiles were simulated with 1000 replications of the original dataset and the 95% confidence intervals (95% CIs) of the simulated 5th, 50th (median) and 95th percentiles were compared with the 5th, 50th and 95th percentiles of rituximab concentration observed in the data. Precision of parameter estimates was derived through bootstrap of 1000 samples and nonparametric 95% CIs were calculated.
Statistical analysis
Statistical analyses of patients' characteristics were performed using the SPSS Statistics 22.0 software package (SPSS Inc., Chicago, IL, USA). The group of patients with disease progression was compared to the group of patients without disease progression for the difference in RDI, age, sex and IPI using independent samples t test and χ2‐test. Significance level was set at p < 0.05.
Results
Patients' characteristics
The study population consisted of 29 DLBCL patients, 13 women and 16 men, of Caucasian ethnic origin. At the start of treatment, the median age was 62 (range: 48–84) years and body weight 74 (range: 54–100) kg. Baseline characteristics of participants, including Ann Arbour clinical stage, IPI and presence of bulky disease are presented in Table 1. Sixteen patients with residual disease following R‐CHOP were additionally irradiated as a part of the frontline treatment.
Table 1.
Patients' characteristics
| Patients' characteristics | Number of patients (n = 29) | % | |
|---|---|---|---|
| Sex | Male | 16 | 55.2 |
| Female | 13 | 44.8 | |
| Age | > 60 years | 17 | 58.6 |
| ≤ 60 years | 12 | 41.4 | |
| Ann Arbour clinical stage | I‐II | 11 | 37.9 |
| III‐IV | 18 | 62.1 | |
| IPI | 0–2 | 19 | 65.5 |
| 3–5 | 10 | 34.5 | |
| Bulky disease | Yes | 10 | 65.5 |
| No | 19 | 34.5 | |
| Irradiation first line | Yes | 16 | 55.2 |
| No | 13 | 44.8 | |
| Response to frontline R‐CHOP ± radiotherapy | Complete response | 25 | 86.2 |
| Partial response | 2 | 6.9 | |
| Progressive disease/stable disease | 2 | 6.9 | |
| Overall response rate (complete + partial response) | 27 | 93.1 | |
IPI, International prognostic index.
Comorbidities and concomitant medications were not expected to affect rituximab pharmacokinetics. Most common comorbidities in our study were arterial hypertension, benign prostatic hyperplasia and hypercholesterolaemia and most common concomitant medications were angiotensin‐converting enzyme inhibitors/angiotensin II receptor antagonists, proton‐pump inhibitors and nonsteroidal anti‐inflammatory drugs.
Response to frontline therapy
Response to R‐CHOP therapy was as follows: 12 patients achieved complete response; 15 patients partial response; and two patients progressive disease. After additional radiotherapy as a part of frontline therapy, 25 patients achieved complete response, two patients partial response and two patients progressive disease. Therefore, after sole R‐CHOP and after frontline R‐CHOP ± radiotherapy both overall response rates were 93%. During follow‐up, six patients experienced disease progression, three with IPI = 4 and three with IPI = 1. Four progressions occurred 3 months after completion of R‐CHOP ± radiotherapy, the other two at 4 and 5 months after completion of therapy. At a median observation period of 52.9 (range: 9.7–66.3) months, the projected 5‐year progression free survival and overall survival were 79%.
Mean RDI in our study was 92.2% for rituximab, cyclophosphamide, doxorubicin and vincristine combined. The dose intensity was 92.5% in the group of patients who did not progress and 91.1% in the group of patients who progressed. The dose intensities did not differ between the two groups (p = 0.757). Likewise, the two groups of patients did not differ in terms of sex, age, or IPI.
Rituximab serum levels
A total of 512 samples (16–18 per patient) were available for analysis. Patients received median dose of 700 (range: 500–800) mg of rituximab per cycle. There was significant rituximab accumulation resulting in increasing trough serum levels over the entire eight R‐CHOP cycles, from 23.2 (range: 17.9–31.5) mg l–1 before the second infusion to 80.7 (range: 50.0–129.4) mg l–1 before infusion in the last cycle. After completion of therapy, serum levels decreased continuously and remained detectable 6 months after the last cycle in most of the patients (22 out of 23 available patients).
Rituximab pharmacokinetic analysis
Rituximab displayed biexponential decay in serum concentration. Consequently, PK were more adequately described with a two‐compartment (AIC = 3544) than a one‐compartment (AIC = 4060) linear model. The model was further improved with the addition of time‐dependent clearance (AIC = 3528). To describe nonlinearity in PK, time‐dependent was better than concentration‐dependent clearance mechanism. The base model was parameterized in terms of linear system‐nonspecific clearance (CL1), system‐specific time‐varying clearance (CL2), rate constant of clearance decay (KD), volumes of the central and peripheral compartments (V1 and V2, respectively), and distribution clearance between the central and peripheral compartments (Q). CL1 was estimated at 0.223 l day–1, CL2 at 0.241 l day–1, KD at 0.0279 day−1, V1 at 4.15 L, V2 at 8.31 L, and Q at 1.04 l day–1. We were able to estimate IIV (CV%) in CL1 (19.7%), KD (1050%), and V1 (16.6%). A combination error model composed of additive (2.49 mg l–1) and proportional (15.8%) component was used for residual IIV of rituximab concentration.
Of all covariate relationships tested, we observed a significant association of CL1 with body weight and age, V1 with sex, and KD with disease progression (Figure 1). Parameters of the final model are summarized in Table 2.
Figure 1.
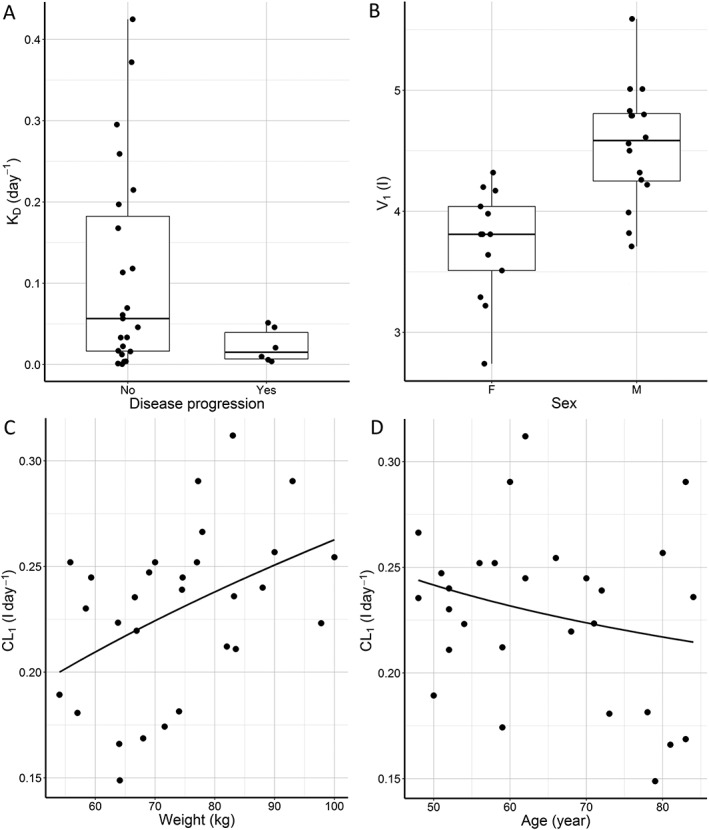
Posthoc estimates of individual patient's pharmacokinetic parameters of rituximab obtained by the base population pharmacokinetic model according to (A) disease progression, (B) sex, (C) weight and (D) age
Table 2.
Pharmacokinetic parameters of rituximab in patients with diffuse large B‐cell lymphoma
| Parameter | Estimate | Bootstrap | ||
|---|---|---|---|---|
| Median | Nonparametric 95% confidence interval | |||
| CL1, l day–1 | 0.252 | 0.251 | 0.227 | 0.279 |
| Covariate effects on CL1 | ||||
| Age | –0.00820 | –0.00799 | –0.01449 | –0.00102 |
| Weight | 1.23 | 1.21 | 0.70 | 1.73 |
| CL2,0, l day–1 | 0.278 | 0.281 | 0.181 | 0.390 |
| KD, day−1 | 0.143 | 0.141 | 0.0478 | 0.418 |
| Covariate effects on KD | ||||
| Disease progression | –0.822 | –0.813 | –0.950 | –0.334 |
| V1, L | 4.62 | 4.62 | 4.34 | 4.93 |
| Covariate effects on V1 | ||||
| Sex | –0.214 | –0.212 | –0.296 | –0.135 |
| V2, L | 8.61 | 8.58 | 7.45 | 9.81 |
| Q, l day–1 | 1.02 | 1.01 | 0.664 | 1.95 |
| Interindividual variability | ||||
| IIVCL1 (CV% [shrinkage]) | 18.5 [5.3] | 17.2 | 11.5 | 21.8 |
| IIVKD (CV% [shrinkage]) | 161 [22.7] | 155 | 67.4 | 311 |
| IIVV1 (CV% [shrinkage]) | 11.6 [14.2] | 11.0 | 5.65 | 15.0 |
| Residual variability [shrinkage] | [6.0] | |||
| Additive, mg l–1 | 2.46 | 2.32 | 1.05 | 4.36 |
| Proportional, % | 15.9 | 15.7 | 14.3 | 17.5 |
CL1, nonspecific linear clearance; CL2,0, time‐varying specific clearance at time zero; KD, rate constant of specific clearance decay; V1, volume of the central compartment; V2, volume of the peripheral compartment; Q, distribution clearance.
Rituximab elimination is a sum of linear (CL1) and time varying (CL2) clearance. CL1 is reduced in older patients and patients with lower body weight. The association with age is linear, while the relationship with weight is more adequately described with the power model (Equation (3)).
| (3) |
Our model predicts a decrease of CL1 by 0.82% for every year above 60 years. The exponent for the effect of weight is 1.23 and the 95% CI (0.70, 1.73) includes the theoretical allometric value of 0.75. At the beginning of treatment, CL2 (0.278 l day–1) is comparable to CL1 and then exponentially decreases with time according to Equation (1). KD was estimated at 0.143 day−1 in patients with no disease progression, while in patients with disease progression it was 82.2% (95% CI: 33.4, 95.0) lower (0.0254 day−1). As a result, half‐life of CL2 decay is 27.2 days in patients with disease progression compared to 4.85 days in patients without disease progression. Collectively, our model predicts slower decrease of time‐varying clearance and therefore higher total clearance of rituximab in patients with disease progression. Additionally, we observed that volume of the central compartment in women is 21.4% lower compared to men.
The final PopPK model of rituximab was evaluated with VPC (Figure 2). The observed median, 5th and 95th percentiles were in good agreement with the simulated median, 5th and 95th percentiles, and generally within 95% CI of the simulations. Thus, the model adequately described the typical rituximab concentration profile and its variability.
Figure 2.
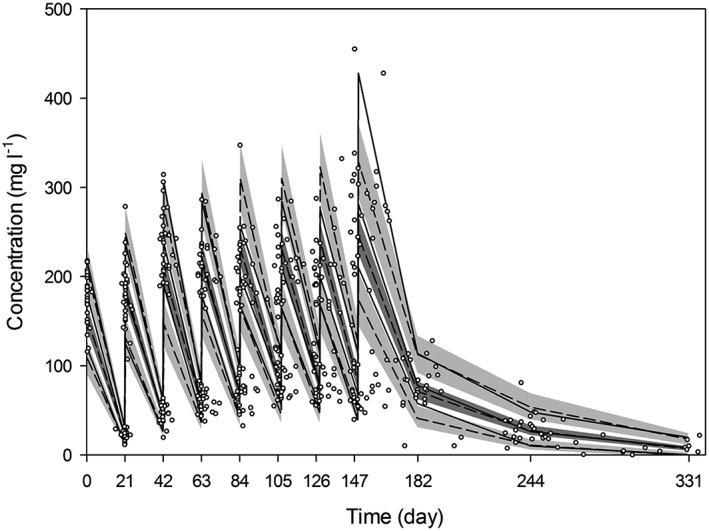
Visual predictive check of the final pharmacokinetic model applying prediction and variability correction. Circles depict the observed concentrations, and solid lines depict the observed median, 5th and 95th percentiles. Dashed lines indicate the predicted median, 5th and 95th percentiles, and shaded areas represent the 95% confidence interval of the predictions
To visualize the association of clinical response with rituximab PK, the final model was used for simulation of rituximab serum concentration profile in a typical male patient with and without disease progression (Figure 3).
Figure 3.
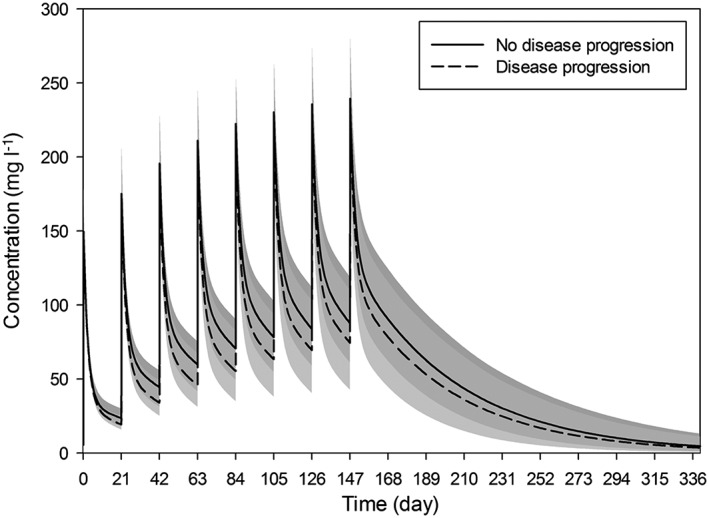
Association of time course of serum rituximab concentration with disease progression. Simulation was performed for a typical male patient (70 kg body weight, age 60 years) receiving standard therapy with rituximab (700 mg per cycle). Median predicted concentration (lines) and 90% prediction intervals (shaded areas)
Discussion
In an effort to elucidate the PK properties of rituximab in DLBCL patients we performed a prospective observational clinical trial and monitored rituximab serum levels in 29 patients during their treatment period. Our findings demonstrate that rituximab PK is well described by a two‐compartment model corresponding to target‐mediated drug disposition, and that rituximab clearance is related to clinical response.
The observed two‐compartment model is in concordance with the current literature 12, 13, 21, 22. In contrast, the nonlinear model with constant and time‐varying clearance detected in our study was previously shown only by Li et al. 13, 21. This might be due to our larger sample size. Additionally, studies differ in terms of design, patient inclusion criteria and treatment protocol. Muller et al. 12 evaluated PK properties of rituximab at a dose of 375 mg m–2 in 20 elderly patients aged 61–80 years in a dose‐dense 2‐week R‐CHOP regimen, whereas Li et al. 13 in 21 patients with relapsed or refractory chronic lymphocytic leukaemia (CLL) receiving rituximab at a dose of 375 mg m–2 in cycle 1, followed by 500 mg m–2 in cycles 2–6 in combination with fludarabine and cyclophosphamide. Another study by Li et al. 21 was a large retrospective analysis of follicular lymphoma and DLBCL patients, who received rituximab as a single agent or in combination with CHOP regimen. Nevertheless, nonlinearity in rituximab clearance is consistent with the target‐mediated drug disposition phenomenon of monoclonal antibodies 23, 24. The constant clearance component is believed to reflect the endogenous catabolic processes of IgG degradation, hence its linearity. The time‐varying clearance component corresponds to the binding of rituximab to its respective target CD20. After initial infusions, the target‐mediated elimination is decreased because of a reduction in the available target antigen.
Initial PK studies suggested that high serum rituximab levels are associated with favourable outcome in NHL patients. Berinstein et al. 14 reported higher median serum concentrations in responders with low‐grade or follicular lymphoma, whereas Tobinai et al. 25 detected higher mean values of trough levels and area under the curve (AUC) of rituximab in responders with aggressive B‐cell lymphoma. Accordingly, Li et al. 13 found higher median trough levels of rituximab and AUC in CLL responders, while Jager et al. 26 observed correlation between trough levels of rituximab and remission quality in patients with follicular lymphoma. In line with these findings, our study likewise reports a clear association between rituximab PK properties and clinical response. Our study is the first to show the association of disease progression status with time‐varying clearance decay (KD). Patients with disease progression had slower time‐varying clearance decay and thus higher total rituximab clearance. Merely correlating rituximab serum concentration or AUC with clinical response might be oversimplified. Additionally, it carries the risk of incorrect assumptions as rituximab infusions might be postponed due to adverse effects or other unpredictable events, affecting both, the serum concentration and AUC. The PK modelling approach includes these deviations into consideration and indeed verifies the association of clinical response with rituximab PK. The time‐varying clearance decay might therefore serve as one of the predictive markers of rituximab response. Nevertheless, further studies in larger patient cohort are needed to validate this association.
Our young patients and males had lower serum rituximab levels, indicated by higher rituximab constant clearance and higher volume of the central compartment, respectively. Similarly, several other studies have shown that young patients and males benefit less from addition of rituximab to the treatment protocols 15, 26, 27, 28, 29, 30. Whether this is due to lower serum rituximab levels in this patient subpopulation is still not known. Herein, we were able to identify another subpopulation of patients with lower serum rituximab levels, those with low KD, and inferior response to treatment. Based on our data, trials with higher doses and/or prolonged exposure times of rituximab are warranted in males, young patients and patients with low KD. There is already literature on the approaches that might overcome the differences in rituximab treatment efficiency 31, 32. Additionally, as patients with low KD are more likely to experience disease progression, closer follow‐up could be conducted for this patient subpopulation.
The major limitation of our study is the modest sample size and scarce sampling design. Moreover, determination of rituximab pharmacokinetics including KD is not performed routinely. The study population was homogenous, which might preclude applying results globally. Furthermore, we did not include histological subtyping (germinal centre B‐cell‐like or activated B‐cell‐like) in our analysis, which might impact treatment outcomes. Nevertheless, the PK parameters of rituximab were estimated with reasonable precision. Our model could serve as a starting point to design further PK studies of rituximab in DLBCL to optimize dosing in subpopulations of patients and to pinpoint the differences in PK between responders and nonresponders.
To our knowledge, this is the first prospective study evaluating PopPK of rituximab in newly diagnosed DLBCL patients treated with standard 3‐week R‐CHOP regimen. To improve efficacy in the treatment of DLBCL it is necessary to understand the relationship between rituximab PK and clinical outcome. In our study, age, weight and sex were shown to affect rituximab PK. A clear association of clinical response with rituximab PK has also been observed. The report demonstrates the rationale for studies evaluating higher doses of rituximab in selected patients.
Competing Interests
There are no competing interests to declare.
The authors express gratitude to all colleagues and patients for participating in the study. The authors also thank Dr J.Z. Rozman for her critical review of the manuscript. This research was partially supported by Slovenian Research Agency (Program P3‐0321).
Contributors
S.R., A.M. and B.J.N. designed the study; B.J.N. provided the clinical data; S.R. collected the PK samples and clinical data; S.R. and S.N. analysed PK data; S.R. and I.G. performed the PK modelling; S.R. and I.G. performed the statistical analysis; all authors participated in writing the manuscript and approved the final version of it.
Rozman, S. , Grabnar, I. , Novaković, S. , Mrhar, A. , and Jezeršek Novaković, B. (2017) Population pharmacokinetics of rituximab in patients with diffuse large B‐cell lymphoma and association with clinical outcome. Br J Clin Pharmacol, 83: 1782–1790. doi: 10.1111/bcp.13271.
Principal investigator: Barbara Jezeršek Novaković, MD, PhD.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, et al. The concise guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 2015; 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cartron G, Watier H, Golay J, Solal‐Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood 2004; 104: 2635–2642. [DOI] [PubMed] [Google Scholar]
- 4. Johnson P, Glennie M. The mechanisms of action of rituximab in the elimination of tumor cells. Semin Oncol 2003; 30: 3–8. [DOI] [PubMed] [Google Scholar]
- 5. Jazirehi AR, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti‐CD20 mAb) in non‐Hodgkin's lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene 2005; 24: 2121–2143. [DOI] [PubMed] [Google Scholar]
- 6. Jezeršek Novaković B, Kotnik V, Južnič Šetina T, Vovk M, Novaković S. Testing of mechanisms of action of rituximab and clinical results in high‐risk patients with aggressive CD20+ lymphoma. Radiol Oncol 2007; 41: 23–32. [Google Scholar]
- 7. Plosker GL, Figgitt DP. Rituximab: a review of its use in non‐Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs 2003; 63: 803–843. [DOI] [PubMed] [Google Scholar]
- 8. Molina A. A decade of rituximab: improving survival outcomes in non‐Hodgkin's lymphoma. Annu Rev Med 2008; 59: 237–250. [DOI] [PubMed] [Google Scholar]
- 9. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B‐cell lymphoma. N Engl J Med 2002; 346: 235–242. [DOI] [PubMed] [Google Scholar]
- 10. Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. J Clin Oncol 2006; 24: 3121–3127. [DOI] [PubMed] [Google Scholar]
- 11. Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP‐like chemotherapy plus rituximab versus CHOP‐like chemotherapy alone in young patients with good‐prognosis diffuse large B‐cell lymphoma: a randomized controlled trial by the Mabthera international trial (MInT) group. Lancet Oncol 2006; 7: 379–391. [DOI] [PubMed] [Google Scholar]
- 12. Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, et al. The role of sex and weight on rituximab clearance and serum elimination half‐life in elderly patients with DLBCL. Blood 2012; 119: 3276–3284. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Zhi J, Wenger M, Valente N, Dmoszynska A, Robak T, et al. Population pharmacokinetics of rituximab in patients with chronic lymphocytic leukemia. J Clin Pharmacol 2012; 52: 1918–1926. [DOI] [PubMed] [Google Scholar]
- 14. Berinstein NL, Grillo‐Lopez AJ, White CA, Bence‐Bruckler I, Maloney D, Czuczman, et al. Association of serum rituximab (IDEC‐C2B8) concentration and anti‐tumor response in the treatment of recurrent low‐grade or follicular non‐Hodgkin's lymphoma. Ann Oncol 1998; 9: 995–1001. [DOI] [PubMed] [Google Scholar]
- 15. Pfreundschuh M, Muller C, Zeynalova S, Kuhnt E, Wiesen MH, Held G, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood 2014; 123: 640–646. [DOI] [PubMed] [Google Scholar]
- 16. Institute of Oncology Ljubljana, Slovenia . National guidelines for the treatment of NHL. Available at http://www.onko‐i.si/fileadmin/user_upload/Doktrina_maligni_limfomi_2016.pdf. (last accessed 6 Jun 2016).
- 17. Gutierrez A, Bento L, Bautista‐Gili AM, Garcia F, Martinez‐Serra J, Sanchez B, et al. Differential impact of relative dose‐intensity reductions in diffuse large B‐cell lymphoma treated with R‐CHOP21 or R‐CHO14. PLoS One 2015; 10: e0123978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 19. Hampson G, Ward TH, Cummings J, Bayne M, Tutt AL, Cragg MS, et al. Validation of an ELISA for the determination of rituximab pharmacokinetics in clinical trial subjects. J Immunol Methods 2010; 360: 30–38. [DOI] [PubMed] [Google Scholar]
- 20. Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike information criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm 1994; 22: 431–445. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Levi M, Charoin JE, Frey N, Kheoh T, Ren S, et al. Rituximab exhibits a long half‐life based on a population pharmacokinetic analysis in non‐Hodgkin's lymphoma (NHL) patients [abstract 2371]. Blood 2007; 110: 700a. [Google Scholar]
- 22. Blasco H, Chatelut E, de Bretagne IB, Congy‐Jolivet N, Le Guellec C. Pharmacokinetics of rituximab associated with CHOP chemotherapy in B‐cell non‐Hodgkin's lymphoma. Fundam Clin Pharmacol 2009; 23: 601–608. [DOI] [PubMed] [Google Scholar]
- 23. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008; 84: 548–558. [DOI] [PubMed] [Google Scholar]
- 24. Golay J, Semenzato G, Rambaldi A, Foa R, Gaidano G, Gamba E, et al. Lessons for the clinic from rituximab pharmacokinetics and pharmacodynamics. MAbs 2013; 5: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tobinai K, Igarashi T, Itoh K, Kobayashi Y, Taniwaki M, Ogura M, et al. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B‐cell lymphoma. Ann Oncol 2004; 15: 821–830. [DOI] [PubMed] [Google Scholar]
- 26. Jager U, Fridrik M, Zeitlinger M, Heintel D, Hopfinger G, Burgstaller S, et al. Rituximab serum concentrations during immuno‐chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica 2012; 97: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi‐weekly CHOP‐14 with or without rituximab in elderly patients with aggressive CD20+ B‐cell lymphomas: a randomized controlled trial (RICOVER‐60). Lancet Oncol 2008; 9: 105–116. [DOI] [PubMed] [Google Scholar]
- 28. Riihijarvi S, Taskinen M, Jerkman M, Leppa S. Male gender is an adverse prognostic factor in B‐cell lymphoma patients treated with immunochemotherapy. Eur J Haematol 2011; 86: 124–128. [DOI] [PubMed] [Google Scholar]
- 29. Habermann TM. Is rituximab one for all ages and each sex? Blood 2014; 123: 602–603. [DOI] [PubMed] [Google Scholar]
- 30. Ngo L, Hee SW, Lim LC, Tao M, Quek R, Yap SP, et al. Prognostic factors in patients with diffuse large B cell lymphoma: before and after the introduction of rituximab. Leuk Lymphoma 2008; 49: 462–469. [DOI] [PubMed] [Google Scholar]
- 31. Pfreundschuh M, Held G, Zeynalova S, Zwick C, Haenel M, Truemper L, et al. Increased rituximab (R) doses and effect on risk of elderly male patients with aggressive CD20+ B cell lymphomas: results from the SEXIE‐R‐CHOP‐14 trial of the DSHNHL [abstract 8501]. J Clin Oncol 2014; 32: 5s. [Google Scholar]
- 32. Pfreundschuh M, Poeschel V, Zeynalova S, Hänel M, Held G, Schmitz N, et al. Optimization of rituximab for the treatment of diffuse large B‐cell lymphoma (II): extended rituximab exposure time in the SMARTE‐R‐CHOP‐14 trial of the German high‐grade non‐Hodgkin lymphoma study group. J Clin Oncol 2014; 32: 4127–4133. [DOI] [PubMed] [Google Scholar]


