Abstract
Aims
Nonvitamin K antagonist oral anticoagulants (NOACs) are now available for the prevention of stroke in patients with atrial fibrillation (AF) as an alternative to vitamin K antagonists (VKA) and aspirin. The comparative effectiveness and safety in daily practice of these different drug classes is still unclear. The objective of this study was to evaluate the risk of major bleeding and stroke in AF patients using NOACs, VKAs or aspirin.
Methods
A retrospective cohort study was conducted among AF patients using the UK Clinical Practice Research Datalink (March 2008–October 2014). New users of VKAs, NOACs and low dose aspirin were followed from the date of first prescription of an antithrombotic drug until the occurrence of stroke or major bleeding. Analyses were adjusted for a history of comorbidities and drug use with Cox regression analysis.
Results
A total of 31 497 patients were eligible for the study. The hazard ratio (HR) of major bleeding was 2.07 [95% confidence interval (CI) 1.27–3.38] for NOACs compared with VKAs, which was mainly attributed by the increased risk of gastrointestinal bleeding (HR 2.63, 95% CI 1.50–4.62). This increased bleeding risk was restricted to women (HR 3.14, 95% CI 1.76–5.60). Aspirin showed a similar bleeding risk as VKAs. NOACs showed equal effectiveness as VKA in preventing ischaemic stroke (HR 1.22, 95% CI 0.67–2.19). VKAs were more effective than aspirin (HR 2.18, 95% CI 1.83–2.59).
Conclusions
NOACs were associated with a higher risk on gastrointestinal bleeding, particularly in women. The use of NOACs in patients who are vulnerable for this type of bleeding should be carefully considered. NOACs and VKAs are equally effective in preventing stroke. Aspirin was not effective in the prevention of stroke in AF.
Keywords: anticoagulants, aspirin, atrial fibrillation, gastrointestinal haemorrhage, intracranial haemorrhage, stroke
What is Already Known about this Subject
Randomized clinical trials show that nonvitamin K antagonist oral anticoagulants (NOACs) are at least as effective in the prevention of ischaemic stroke in atrial fibrillation as vitamin K antagonists (VKAs).
There is no sound evidence for a preference starting either VKAs or NOACs.
Asprin has no place in the prevention of ischaemic stroke in patients with atrial fibrillation.
What this Study Adds
In UK general practice, it is confirmed that VKAs and NOACs are equally effective in the prevention of ischaemic stroke.
Women have a higher risk on gastrointestinal bleeding when using NOACs compared to VKAs.
Although aspirin is still commonly used in patient with atrial fibrillation in UK general practice, it is confirmed that is less effective and carries an equal bleeding risk compared to VKAs.
Tables of Links
| TARGETS | |
|---|---|
| Enzymes 2 | COX‐1 |
| VKORC1 | Thrombin |
| Coagulation factor X |
| LIGANDS | |
|---|---|
| aspirin | rivaroxaban |
| dabigatran | warfarin |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Atrial fibrillation (AF) has a prevalence of 1–2% and is associated with a doubled rate of death and a 5‐fold increased rate of stroke 3, 4. Antithrombotic therapy such as vitamin K antagonists (VKAs), nonvitamin K antagonist oral anticoagulants (NOACs) and low dose aspirin are used treatment options for AF and can reduce stroke rates by up to 20%–60% 5, 6, 7. The CHA2DS2‐VASC risk score guides the choice of antithrombotic treatment using known risk factors for stroke: congestive heart failure, hypertension, age, diabetes, prior stroke or thromboembolism, vascular disease and female sex.
Studies have shown that NOACs may significantly reduce the risk of stroke and intracranial bleeding, when compared with warfarin 8, 9, 10, 11. In line with these findings the European guidelines now recommend using NOACs over VKAs for most patients with AF (2). The use of aspirin was used only in the treatment of patients at low risk for stroke, however, more recently it is advised that aspirin should be confined to those that refuse NOAC or VKA therapy.
While NOACs are effective in reducing stroke risk, the evidence remains inconclusive with respect to its risks of major and gastrointestinal bleeding 8, 9, 11, 12, 13, 14, 15, 16. This complicates the choice in antithrombotic therapy in daily practice as the harm–benefit ratio is uncertain in patients with higher baseline risks for bleeding. Furthermore, the risk of antithrombotic therapy in real world patients may differ from those in the randomized controlled trials (RCTs). Patients in RCTs using warfarin spent more time in the therapeutic range compared with patients monitored by community physicians 17. Secondly, patients who are seen in everyday clinical practice have a different risk profile, as the patients in the trials were obligated to meet specific inclusion and exclusion criteria 18.
To investigate the safety and efficacy of NOACs compared with VKA in real‐world patients, several observational studies have been conducted, but these were limited to the evaluation of dabigatran, and they were unable to statistically adjust for life style factors such as body mass index and smoking status 12, 13, 14, 15, 16.
The aim of this study was to evaluate the risk of major bleeding and stroke in AF patients using VKAs, NOACs and low dose aspirin in a UK general practice population.
Methods
Data source
We conducted a retrospective cohort study within the Clinical Practice Research Datalink (CPRD). This database contains computerized medical records of around 674 primary care practises in the UK, covering 11.3 million patients, representing 6.9% of the total UK population 19. Data recorded in the CPRD include demographic information, laboratory tests, specialist referrals, hospital admissions, prescription details, and lifestyle variables such as body mass index (BMI), smoking, and alcohol consumption. Previous studies have shown a high validity of registration and high degrees of accuracy and completeness of these data have been shown for various diagnoses (including 85.3% for diagnoses related to the circulatory system and 87.4% for diagnoses related to the digestive system) and for smoking status 19, 20, 21, 22, 23.
Study population
The study population consisted of all patients aged ≥18 years with a first ever recorded diagnosis of AF during a patient's period of valid data collection. Only patients with follow‐up time between 18th March 2008 (the date of market introduction of the NOACs) and 1 October 2014 were included. Within this cohort of AF patients, we identified new users of antithrombotic drugs: VKAs, NOACs and low dose (≤325 mg) aspirin. New users were defined as patients who had never been exposed previously to any one of the drugs of interest.
Exposure
Patients were followed from the start of antithrombotic treatment until the end of follow‐up, death, or an outcome of interest, whichever date came first. The period of follow‐up was divided into 30‐day periods, starting with the index date. At the start date of each period, exposure to antithrombotic agents in the 30 days before was defined as current users and past users were defined as those who had discontinued their antithrombotic agents >30 days before the start of the interval. An example of exposure definition for a hypothetical patient is given in Figure 1. During follow‐up, patients were able to move between current and past exposure groups. Patients were defined as current users of VKA only (warfarin, acenocoumarol and phenindione), NOAC only (dabigatran, rivaroxaban and apixaban), aspirin only, or mixed use of more than one of the three main study drugs. These groups were identified regardless of past use as it is expected that medications taken >30 days from an exposure period would no longer impact a patient's likelihood for the outcome. Patients could only contribute to one current user group during an interval. Among patients who were not considered current users, past use was defined as past VKA, NOAC, or aspirin use, and patients could contribute to more than one past user group in an interval.
Figure 1.
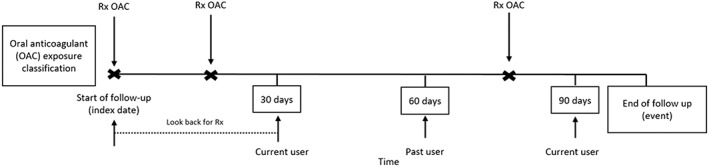
Diagram of exposure definition demonstrating a hypothetical case example of a patient classified as oral anticoagulants user at time of a stroke or bleeding event
Outcomes
The primary outcome of interest was major bleeding. Secondary outcomes were gastrointestinal bleeding, intracranial bleeding, stroke, ischaemic stroke and haemorrhagic stroke. The UK Read code system was used to define outcomes. Major bleeding was defined as a bleeding at a critical site or organ and the selected Read‐codes were reviewed by a clinician for relevancy. The codes used for defining the primary outcome can be found in Appendix 1.
Potential confounders
Potential confounders considered in this study were based on literature review. The presence of a covariate was assessed by reviewing the computerized medical records for any record of a covariate. For each outcome, sex, BMI, smoking status and alcohol status were considered at baseline and age at the start of each interval. The following covariates were evaluated prior to the start of each interval for bleeding outcomes: oesophagitis, gastritis, cerebrovascular disease and malignancies. The use of the following prescription drugs in the 6 months before an interval were considered: statins, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II (ATII) blockers, diuretics, β‐blockers, antiplatelet drugs (excluding aspirin), anticoagulant drugs (excluding VKAs and NOACs), antiarrhythmic drugs, nitrates, antidiabetic drugs, nonsteroidal anti‐inflammatory drugs (NSAIDs), systemic glucocorticoids, selective serotonin reuptake inhibitors (SSRIs). Proton‐pump inhibitors and histamine 2 receptor antagonists were assessed in the 3 months before an interval. For stroke, covariates included history of congestive heart failure, hypertension, cerebrovascular disease, ischaemic heart disease, peripheral artery disease, acute or chronic renal failure. Prescriptions in the 6 months prior were also considered for stroke: statins, calcium channel blockers, ACE‐inhibitors, ATII‐blockers, diuretics, β‐blockers, clonidine, monoxide, doxazosin, antipsychotics, SSRIs, NSAIDs, antiplatelet drugs, anticoagulant drugs, antiarrhythmic drugs, nitrates, antidiabetic drugs and insulin.
Statistical analysis
The outcomes of interest were incident first‐ever events; patients with a history of the outcome were excluded. Baseline characteristics were summarized as means and standard deviations or proportions where appropriate. Crude incidence rates of outcomes within 1 year per 1000 person‐years were calculated. Cox proportional hazard regression analysis estimated the adjusted hazard ratios (HR) using the SAS 9.2 PHREG procedure. Potential confounders were included in the final model if they independently changed the β‐coefficient for current use with the outcome of interest by at least 5%, or when a consensus about inclusion existed within the team of researchers, supported by clinical evidence from the literature. Current use of VKAs served as the reference group and was used to compare to the other exposure groups (current use of NOAC only, aspirin only, mixed use and past use). Analyses were stratified by sex and the CHA2DS2‐VASC risk score. Missing data were dealt with by including an indicator for missingness in the model.
Patient involvement
For this study, we did not actively involve patients.
Results
We identified 31 497 patients with an AF diagnosis and a first‐ever prescription of antithrombotic therapy. Figure 2 shows the study flowchart.
Figure 2.
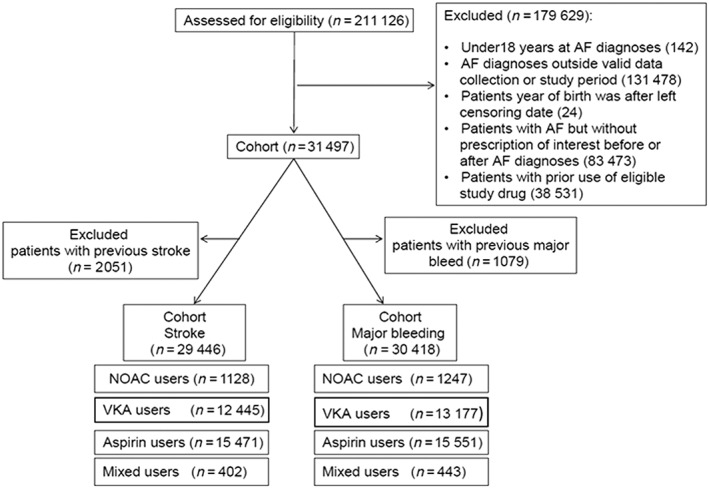
Flow diagram of cohort assembly
Baseline characteristics are presented in Table 1. At the index date, 16 094 (51.1%) patients were prescribed aspirin, 13 643 (43.3%) VKAs, 1306 (4.1%) NOACs, and 453 (1.4%) a mix of these agents. In the NOACs group 28.5% of patients were using dabigatran and 71.5% rivaroxaban. None were using apixaban. The mean duration of follow‐up was shorter for users of NOACs (1.0 years) than for users of VKAs (2.7 years) or aspirin (2.8 years). Age, BMI, smoking status and alcohol use did not differ much between exposure groups at baseline. Users of NOACs (18.9%) had more often a history of cerebrovascular disease as compared with users of VKAs (13.4%) or low dose aspirin (6.1%). Appendix 2 shows baseline characteristics of the two cohorts excluding history of the respective outcomes, stroke or major bleed.
Table 1.
Baseline characteristics for users of NOACs, VKAs, aspirin or mixed users at the index date
| Characteristic |
NOAC‐users
(n = 1306) |
VKA‐users (n = 13 643) |
Aspirin‐users
(n = 16 094) |
Mixed users (n = 454) |
|---|---|---|---|---|
| Follow up, years (SD) | 0.95 (0.63) | 2.71 (1.86) | 2.84 (1.87) | 2.94 (1.97) |
| Number of women | 589 (45.1%) | 6283 (46.1%) | 8008 (49.8%) | 163 (35.9%) |
| Age | ||||
| Mean (years, SD) | 72.6 (12.6) | 72.1 (11.9) | 73.6 (12.7) | 72.2 (10.6) |
| 18–49 | 59 (4.5%) | 673 (4.9%) | 665 (4.1%) | 14 (3.1%) |
| 50–59 | 159 (12.2%) | 1288 (9.4%) | 1454 (9.0%) | 39 (8.6%) |
| 60–69 | 258 (19.8%) | 3017 (22.1%) | 3662 (22.8%) | 119 (26.2%) |
| 70–79 | 419 (32.1%) | 4663 (34.2%) | 4408 (27.4%) | 163 (35.9%) |
| 80+ | 411 (31.5%) | 4002 (29.3%) | 5905 (36.7%) | 119 (26.2%) |
| CHA2DS2‐VASc | ||||
| Mean | 2.6 | 2.6 | 2.5 | 2.6 |
| 0–1 | 25.0% | 24.6% | 27.2% | 22.7% |
| 2 | 20.7% | 21.0% | 21.1% | 23.4% |
| 3–10 | 54.3% | 54.5% | 51.8% | 54.0% |
| BMI (kg/m 2 ) | ||||
| Mean (SD) | 27.9 (6.2) | 28.7 (6.3) | 27.8 (6.2) | 28.9 (6.6) |
| < 20 | 82 (6.3%) | 543 (4.0%) | 963 (6.0%) | 21 (4.6%) |
| 20–25 | 334 (25.6%) | 3165 (23.2%) | 4134 (25.7%) | 96 (21.2%) |
| 25–30 | 420 (32.2%) | 4629 (33.9%) | 5318 (33.0%) | 155 (34.1%) |
| 30–35 | 248 (19.0%) | 2672 (19.6%) | 2754 (17.1%) | 91 (20.0%) |
| >35 | 142 (10.9%) | 1800 (13.2%) | 1659 (10.3%) | 60 (13.2%) |
| Missing | 80 (6.1%) | 834 (6.1%) | 1266 (7.9%) | 31 (6.8%) |
| Smoking status | ||||
| Never | 566 (43.3%) | 5659 (41.5%) | 7074 (44.0%) | 173 (38.1%) |
| Current | 105 (8.0%) | 1230 (9.0%) | 1548 (9.6%) | 54 (11.9%) |
| Ex | 628 (48.1%) | 6691 (49.0%) | 7391 (45.9%) | 225 (49.6%) |
| Missing | 7 (0.5%) | 63 (0.5%) | 81 (0.5%) | <5 |
| Alcohol status | ||||
| Yes | 905 (69.3%) | 9513 (69.7%) | 11 002 (68.4%) | 313 (68.9%) |
| No | 288 (22.1) | 3158 (23.2%) | 3794 (23.6%) | 100 (22.0%) |
| Missing | 113 (8.7) | 972 (7.1%) | 1298 (8.1%) | 41 (9.0%) |
| History of comorbidities | ||||
| Acute renal failure | 7 (0.5%) | 65 (0.5%) | 120 (0.8%) | <5 |
| Cerebrovascular disease | 247 (18.9%) | 1822 (13.4%) | 988 (6.1%) | 73 (16.1%) |
| Chronic renal failure | 7 (0.5%) | 157 (1.2%) | 158 (1.0%) | <5 |
| Congestive heart failure | 98 (7.5%) | 1396 (10.2%) | 961 (6.0%) | 68 (15.0%) |
| Gastritis | 82 (6.3%) | 849 (6.2%) | 933 (5.8%) | 18 (4.0%) |
| GI bleeding | 42 (3.2%) | 374 (4.7%) | 410 (2.6%) | 7 (1.5%) |
| Hypertension | 713 (54.6%) | 7323 (53.7%) | 8048 (50.0%) | 233 (51.3%) |
| Ischaemic heart disease | 111 (8.5%) | 1461 (10.7%) | 1499 (9.3%) | 115 (25.3%) |
| Liver disease | <5 | 15 (0.1%) | 35 (0.2%) | <5 |
| Oesophagitis | 126 (9.6%) | 1179 (8.6%) | 1298 (8.1%) | 34 (7.5%) |
| Cancer | 15 (1.2%) | 125 (0.9%) | 122 (0.8%) | <5 |
| Peripheral artery disease | 72 (5.5%) | 712 (5.2%) | 661 (4.1%) | 26 (5.7%) |
| History of medication use (6 months before index date) | ||||
| Antiarrhythmic drugs | 81 (6.2%) | 907 (6.7%) | 679 (4.2%) | 13 (2.9%) |
| Anticoagulant drugs | 17 (1.3%) | 209 (1.5%) | 65 (0.4%) | 0 (0.0%) |
| Antidiabetic drugs (including insulin) | 102 (7.8%) | 1058 (7.8%) | 911 (5.7%) | 38 (8.4%) |
| Antihypertensive drugs | 342 (26.2%) | 3849 (28.2%) | 3574 (22.2%) | 104 (22.9%) |
| Antiplatelet drugs | 9 (0.7%) | 207 (1.5%) | 95 (0.6%) | <5 |
| NSAIDs | 143 (11.0%) | 1597 (11.7%) | 2107 (13.1%) | 60 (13.2%) |
| SSRIs | 91 (7.0%) | 800 (5.9%) | 1073 (6.7%) | 17 (3.7%) |
| Statins | 394 (30.2%) | 4083 (29.9%) | 3385 (21.0%) | 113 (24.9%) |
| Glucocorticoids | 127 (9.7%) | 1342 (9.8%) | 1278 (7.9%) | 32 (7.1%) |
| History of medication use (3 months before index date) | ||||
| H2 receptor‐antagonists | 32 (2.5%) | 324 (2.4%) | 306 (1.9%) | 13 (2.9%) |
| PPIs | 360 (27.6%) | 3334 (24.4%) | 3542 (22.0%) | 84 (18.5%) |
NOAC, nonvitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; SD, standard deviation; BMI, body mass index; NSAIDs, nonsteroidal anti‐inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors; H2, histamine 2; PPIs, proton pump inhibitors, GI, gastrointestinal.
The incidence rate for major bleeding per 1000 person‐years was 10.6 for current NOAC use, 5.8 for current VKA use, 7.5 for current aspirin use and 8.2 for current mixed use (Table 2). A 2‐fold increased risk of major bleeding was found with current use of NOACs [adjusted hazard ratio (HR) 2.08; 95% confidence interval (CI) 1.28–3.40], which dropped after discontinuation (HR 1.13; 95% CI 0.42–3.05). Current use of aspirin did not have an increased risk of major bleeding (HR 1.05; 95% CI 0.84–1.32), as compared with current use of VKAs. The doubled risk of major bleeding with current users of NOACS was largely explained by an increased risk for gastrointestinal bleeding (HR 2.63; 95% CI 1.50–4.60) for current NOAC users as compared with current VKA users. No difference was found for the occurrence of intracranial bleeding in current users of NOACs as compared with current use of VKAs (HR 1.39; 95% CI 0.55–3.52).
Table 2.
Risk of bleeding outcomes in NOAC, aspirin, and mixed users compared with VKA users
| Outcome | Number of events | Incidence rate per 1000 person‐years | Age/sex adjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) * |
|---|---|---|---|---|
| Major bleeding | ||||
| Current (≤30 days before index date) | ||||
| VKA only use | 167 | 5.78 | Reference | Reference |
| NOAC only use | 19 | 10.63 | 2.07 (1.27–3.38) | 2.08 (1.28–3.40) |
| Aspirin only use | 140 | 7.51 | 1.05 (0.84–1.32) | 1.05 (0.84–1.32) |
| Mixed use | 9 | 8.16 | 1.37 (0.70–2.68) | 1.37 (0.70–2.68) |
| Past (>30 days before index date) | ||||
| VKA use | 123 | 6.47 | 1.23 (0.98–1.54) | 1.23 (0.98–1.54) |
| NOAC use | <5 | ** | 1.13 (0.42–3.06) | 1.13 (0.42–3.05) |
| Aspirin use | 130 | 5.91 | 0.93 (0.74–1.16) | 0.94 (0.75–1.17) |
| GI bleeding | ||||
| Current (≤30 days before index date) | ||||
| VKA only use | 107 | 3.68 | Reference | Reference |
| NOAC only use | 15 | 7.73 | 2.63 (1.50–4.62) | 2.63 (1.50–4.60) |
| Aspirin only use | 103 | 5.29 | 1.18 (0.89–1.55) | 1.17 (0.89–1.54) |
| Mixed use | 7 | 6.31 | 1.67 (0.77–3.59) | 1.63 (0.76–3.50) |
| Past (>30 days before index date) | ||||
| VKA use | 73 | 3.61 | 1.11 (0.83–1.48) | 1.11 (0.83–1.48) |
| NOAC use | <5 | ** | 0.91 (0.22–3.71) | 0.90 (0.22–3.67) |
| Aspirin use | 85 | 3.65 | 1.00 (0.76–1.33) | 1.01 (0.77–1.34) |
| Intracranial bleeding | ||||
| Current (≤30 days before index date) | ||||
| VKA only use | 62 | 2.09 | Reference | Reference |
| NOAC only use | 5 | 2.53 | 1.39 (0.55–3.53) | 1.42 (0.56–3.61) |
| Aspirin only use | 38 | 1.91 | 0.80 (0.53–1.20) | 0.80 (0.53–1.20) |
| Mixed use | <5 | *** | 0.85 (0.21–3.47) | 0.87 (0.21–3.56) |
| Past (>30 days before index date) | ||||
| VKA use | 53 | 2.56 | 1.41 (0.99–2.00) | 1.41 (0.99–2.00) |
| NOAC use | <5 | *** | 1.37 (0.33–5.62) | 1.37 (0.33–5.64) |
| Aspirin use | 50 | 2.10 | 0.87 (0.61–1.24) | 0.87 (0.61–1.24) |
CI, confidence interval; GI, gastrointestinal; VKA, vitamin K antagonist; NOAC, non‐vitamin K antagonist oral anticoagulant; ATII, angiotensin II; NSAID, nonsteroidal anti‐inflammatory drug; H2, histamine 2; PPI, proton pump inhibitor; SSRI, selective serotonin receptor inhibitor.
Adjusted for age, sex, body mass index, alcohol status, smoking status, anticoagulants, antiplatelets, cerebrovascular disease, PPIs.
Suppressed due to fewer than five patients (ISAC regulations).
Table 3 shows that there was no difference in the risks of ischaemic and haemorrhagic stroke between current use of NOAC and VKA (HR 1.22, 95% CI 0.67–2.19 and HR 1.56, 95% CI 0.61–3.99, respectively). The risk of ischaemic stroke was doubled with current use of low dose aspirin compared with current use of VKAs (HR 2.18; 95% CI 1.72–2.39). A higher risk was also found for past use of low dose aspirin compared with current use of VKA (HR 1.65; 95% CI 1.38–1.97).
Table 3.
Risk of stroke outcomes in NOAC, aspirin and mixed users compared with VKA users
| Outcome | Number of events | Incidence rate per 1000 person‐years | Age/sex adjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) * |
|---|---|---|---|---|
| Stroke | ||||
| Current (≤30 days before index date) | ||||
| VKA only use | 181 | 6.72 | Reference | Reference |
| NOAC only use | 15 | 8.81 | 1.37 (0.81–2.33) | 1.38 (0.81–2.35) |
| Aspirin only use | 342 | 17.85 | 1.99 (1.69–2.36) | 2.03 (1.72–2.39) |
| Mixed use | 16 | 15.62 | 1.98 (1.19–3.29) | 2.04 (1.23–3.39) |
| Past (>30 days) | ||||
| VKA use | 195 | 10.15 | 1.11 (0.93–1.32) | 1.10 (0.92–1.31) |
| NOAC use | 8 | 10.36 | 1.37 (0.68–2.78) | 1.37 (0.68–2.78) |
| Aspirin use | 276 | 12.19 | 1.53 (1.29–1.81) | 1.54 (1.30–1.82) |
| Haemorrhagic stroke | ||||
| Current (≤30 days before index date) | ||||
| VKA only use | 57 | 1.92 | Reference | Reference |
| NOAC only use | 5 | 2.53 | 1.54 (0.60–3.93) | 1.56 (0.61–3.99) |
| Aspirin only use | 38 | 1.91 | 0.87 (0.58–1.31) | 0.87 (0.58–1.31) |
| Mixed use | <5 | ** | 0.94 (0.23–3.84) | 0.95 (0.23–3.89) |
| Past (>30 days before index date) | ||||
| VKA use | 49 | 2.37 | 1.37 (0.95–1.97) | 1.37 (0.95–1.97) |
| NOAC use | <5 | ** | 1.50 (0.36–6.18) | 1.50 (0.36–6.18) |
| Aspirin use | 47 | 1.97 | 0.89 (0.62–1.30) | 0.89 (0.62–1.29) |
| Ischaemic stroke | ||||
| Current (≤30 days before index date) | ||||
| VKA only use | 156 | 5.76 | Reference | Reference |
| NOAC only use | 12 | 7.00 | 1.20 (0.67–2.17) | 1.22 (0.67–2.19) |
| Aspirin only use | 327 | 16.95 | 2.13 (1.79–2.54) | 2.18 (1.83–2.59) |
| Mixed use | 15 | 14.58 | 2.09 (1.24–3.53) | 2.16 (1.28–3.64) |
| Past (>30 days befor index date) | ||||
| VKA use | 170 | 8.79 | 1.04 (0.86–1.26) | 1.03 (0.86–1.25) |
| NOAC use | 6 | 7.68 | 1.10 (0.49–2.49) | 1.10 (0.49–2.48) |
| Aspirin use | 256 | 11.22 | 1.63 (1.37–1.95) | 1.65 (1.38–1.97) |
CI, confidence interval VKA, vitamin K antagonist; NOAC, nonvitamin K antagonist oral anticoagulant; ACE, angiotensin converting enzyme; ATII, angiotensin II; NSAIDs, nonsteroidal anti‐inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
*Adjusted for age, sex, body mass index, alcohol status, smoking status, antidiabetics, ACE‐inhibitors, antiplatelets, ATII antagonists, calcium channel blockers, cerebrovascular disease, hypertension, peripheral artery disease, statins.
Suppressed due to fewer than five patients (ISAC regulations).
Results stratified by sex (Table 4) showed that the risk of major bleed in NOAC users was elevated in women (HR 3.14, 95% CI 1.76–5.60) but not in men (HR 0.94, 95% CI 0.34–2.59). Table 5 shows the results stratified by CHA2DS2‐VASc risk score. Current NOAC users with a high stroke risk (CHA2DS2‐VASc >3) had a higher risk of major bleeding compared with current VKA users with a high stroke risk (HR 2.62, 95% CI 1.41–4.87). Across all risk categories, current low dose aspirin use showed an increased risk for ischaemic stroke compared with current VKA use.
Table 4.
Risk of major bleeding and ischaemic stroke in current NOAC and aspirin users compared with current VKA users stratified by sex
| Major bleeding | Stroke | |||||
|---|---|---|---|---|---|---|
| Exposure | Number of events | Incidence rate per 1000 person years | Adjusted HR (95% CI) * | Number of events | Incidence rate per 1000 person years | Adjusted HR (95% CI) ** |
| Women | ||||||
| Current (≤30 days before index date) | ||||||
| VKA only use | 82 | 6.08 | Reference | 87 | 6.94 | Reference |
| NOAC only use | 15 | 16.32 | 3.14 (1.76–5.60) | 8 | 10.04 | 1.40 (0.77–2.56) |
| Aspirin only use | 76 | 7.64 | 1.02 (0.74–1.40) | 197 | 20.01 | 1.74 (1.42–2.14) |
| Mixed use | <5 | *** | 1.14 (0.36–3.61) | 11 | 28.16 | 2.38 (1.29–4.38) |
| Males | ||||||
| Current (≤30 days before index date) | ||||||
| VKA only use | 85 | 5.54 | Reference | 94 | 6.52 | Reference |
| NOAC only use | <5 | *** | 0.94 (0.34–2.59) | 7 | 7.73 | 1.45 (0.75–2.78) |
| Aspirin only use | 64 | 6.89 | 1.08 (0.78–1.49) | 145 | 15.57 | 1.91 (1.53–2.38) |
| Mixed use | 6 | 8.84 | 1.52 (0.66–3.48) | 5 | 7.89 | 1.00 (0.44–2.26) |
CI, confidence interval VKA, vitamin K antagonist; NOAC, nonvitamin K antagonist oral anticoagulant; PPIs, proton pump inhibitors; ATII, angiotensin II ; NSAIDs, nonsteroidal anti‐inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
Adjusted for age, sex, body mass index, alcohol status, smoking status, anticoagulants, antiplatelets, cerebrovascular disease, PPIs
Adjusted for age, sex, body mass index, alcohol status, smoking status, antidiabetics ACE‐inhibitors, antiplatelets, ATII antagonists, calcium channel blockers, cerebrovascular disease, hypertension, peripheral artery disease, statins.
Suppressed due to <5 patients (ISAC regulations).
Table 5.
Risk of major bleeding and ischaemic stroke in current NOAC and aspirin users compared with current VKA users stratified by CHA2DS2‐VASC‐score
| Major bleeding | Stroke | |||||
|---|---|---|---|---|---|---|
| Exposure | Number of events | Incidence rate per 1000 person‐years | Adjusted HR (95% CI) * | Number of events | Incidence rate per 1000 person‐years | Adjusted HR (95% CI) ** |
| High CHA 2 DS 2 ‐VASC (>3) | ||||||
| Current (≤30 days before index date) | ||||||
| VKA only use | 71 | 8.98 | Reference | 69 | 9.96 | Reference |
| NOAC only use | 13 | 24.10 | 2.62 (1.41–4.87) | 9 | 21.22 | 1.86 (0.93–3.76) |
| Aspirin only use | 58 | 10.87 | 0.96 (0.67–1.38) | 132 | 25.88 | 1.79 (1.36–2.35) |
| Mixed use | <5 | *** | 1.01 (0.32–3.23) | 7 | 26.47 | 2.16 (1.00–4.66) |
| Medium CHA 2 DS 2 ‐VASC (2–3) | ||||||
| Current (≤30 days before index date) | ||||||
| VKA only use | 75 | 5.34 | Reference | 82 | 6.16 | Reference |
| NOAC only use | 6 | 6.82 | 1.75 (0.75–4.09) | 5 | 6.04 | 1.19 (0.48–2.95) |
| Aspirin only use | 68 | 7.79 | 1.26 (0.90–1.75) | 162 | 18.35 | 2.29 (1.78–2.93) |
| Mixed use | 5 | 9.21 | 1.71 (0.69–4.23) | 8 | 15.91 | 2.34 (1.14–4.81) |
| Low CHA 2 DS 2 ‐VASC (0–1) | ||||||
| Current (≤30 days before index date) | ||||||
| VKA only use | 21 | 3.05 | Reference | 30 | 4.48 | Reference |
| NOAC only use | 0 | 0.00 | ‐ | <5 | *** | 0.47 (0.06–3.42) |
| Aspirin only use | 14 | 2.71 | 0.90 (0.46–1.77) | 48 | 9.18 | 2.02 (1.34–3.07) |
| Mixed use | <5 | *** | 1.02 (0.14–7.62) | <5 | *** | 0.74 (0.10–5.37) |
Abbreviations: CI, confidence interval VKA, vitamin K antagonist; NOAC, nonvitamin K antagonist oral anticoagulant; PPIs, proton pump inhibitors; ATII, angiotensin II inhibitors; NSAIDs, nonsteroidal anti‐inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
NB: 0 counts for current NOAC users in the Low CHA2DS2‐VASC stratum for the major bleed outcome. Current NOAC not included in this model.
Adjusted for age, sex, body mass index, alcohol status, smoking status, anticoagulants, antiplatelets, cerebrovascular disease, PPIs
Adjusted for age, sex, body mass index, alcohol status, smoking status, antidiabetics ACE‐inhibitors, antiplatelets, ATII antagonists, calcium channel blockers, cerebrovascular disease, hypertension, peripheral artery disease, statin.
Supressed due to <5 patients (ISAC regulations).
Discussion
This study showed a tw2o‐fold increase in the risk of major bleeding with current NOAC use compared with current VKA use. This was largely explained by the increase in gastrointestinal bleeding risk; there was no difference in intracranial haemorrhage risk. The increased risk of gastrointestinal bleeding diminished after NOAC discontinuation, as expected. NOACs were equally effective as VKA in the prevention of ischaemic stroke, whereas aspirin was less effective. Our results further suggest that the increased risk for bleeding for NOAC users was restricted to women.
Our main finding of an increased risk of major bleed is not in line with a large meta‐analysis from four phase III randomized trials of four different NOACs (dabigatran, rivaroxaban, apixaban, edoxaban) 10. This study showed that the risk of major bleeding was lower compared with warfarin. A 52% decreased risk for intracranial haemorrhage was found with the usage of NOACs compared with warfarin, although the risk of gastrointestinal bleeding was found to be increased with 25%, which is in line with the results from the current study. Patients who were prone to bleeding were excluded from the clinical trials. Although we have excluded patients with a history of a major bleeding event, we did not exclude patients with other comorbidities (e.g. renal failure, malignancies, gastritis) or concomitant medication (NSAIDs, SSRIs) that increases the risk of bleeding, which might have selected patients with a different baseline risk. In contrast to the trials, we excluded patients with prior events of interest and therefore our results are not directly comparable with the results from the trials.
Several observational studies have been carried out that assessed the bleeding risk of NOACs compared with warfarin. A study using US Medicare data compared dabigatran with warfarin and found results that were partially in line with our findings. In this study, an increase in gastrointestinal bleeding was found as well (HR 1.28, 95% CI 1.14–1.44), but a decrease in intracranial bleeding (HR 0.34, 95% CI 0.26–0.46) was shown 16. No difference for gastrointestinal bleeding was found in a study using Optum Labs Data Warehouse data (a different claims database) in the USA 14. Similar to the current study, the two observational studies mentioned above used a new user design. By excluding all previous users, the effects of switching and long‐term use are reduced. However, in this study we not only identified new users of NOACs and VKAs, but additionally we excluded patients who had used aspirin before. Also the in‐ or exclusion of prior events of the outcome might be a reason for differences in bleeding risks.
The results on major bleeding may strongly depend on the definition of this outcome, and this may explain some of the discrepancies in the current body of literature on this outcome. The most common definition of major bleeding is that of the International Society of Thrombosis and Haemostasis including bleeding at critical sites, need for transfusion of more than two units of blood and a fall in haemoglobin level of >20 g l−1 24. This definition, or a derivate of this definition, is used in the different trials comparing VKAs and NOACs 25, 26, 27. In the current study, we used the Read coding system as opposed to the other observational studies that use ICD‐9‐CM (international classification of diseases, 9th revision, clinical modification) codes 14, 16, which might explain variation in results.
This study underlines that NOACs are equally effective in reducing ischaemic stroke as VKAs as is found in meta‐analyses of RCTs 10, 28. Similar results were found in a study undertaken in new users of warfarin from the Danish registry 13. The >2‐fold increased risk of ischaemic stroke with aspirin use is in line with a Cochrane review that shows less frequent ischaemic stroke for oral anticoagulants (all VKAs) when compared with antiplatelet therapy in patients with nonvalvular AF without a history of stroke and transient ischaemic attack 29.
The higher risk of gastrointestinal bleeding might be explained by the pharmacokinetic in the case of dabigatran. Dabigatran exilate is a prodrug that is hydrolysed to the active drug by esterase. This leads to progressively high concentrations of the active drug during transit in the gastrointestinal tract. This local effect might aggravate bleeding in (pre‐)existing diseased mucosa 28. However, this explanation is not applicable for rivaroxaban, which accounts for most prescriptions in the NOAC group for this study.
The study by Graham et al. 16 identified a trend for a higher risk of gastrointestinal bleeding with dabigatran compared with warfarin in women aged 75–84 years and ≥85 years. This is confirmed in our study where we also found an increased risk in major bleed for women, but not in men. These results are not in line with the differences found for sex in RCTs 10, 30. It has been shown that dabigatran concentrations were dependent on several demographic characteristics including, among others, female sex 31. In women, concentrations of dabigatran were 30% higher compared with men and higher plasma concentrations were found to be related with a higher probability of major bleed. Further study should give more insight about the difference in benefit risk balance of antithrombotic agents for men and women.
In addition to those already identified, this study has several limitations. Benefit risk balance might be different for dabigatran and rivaroxaban and also across different dosages. This study lacked power to compare different NOACs or different dosages. Although we have adjusted the results for various risk factors, there might still be (unobserved) confounding. Despite the fact that the UK guidelines do not give a preference for either starting a NOAC or a VKA, we expect that these agents are prescribed to a selected group of people, which complicates comparison. Some misclassification of exposure might occur. If a patient starts a NOAC at day 1 of a specific month, it will take 29 days until this patient is classified as exposed in the next period. If they suffer a bleed on day 15, this will be wrongly attributed to nonexposure.
Despite the limitations, this study has several strengths, including inclusion of a diverse real‐world population. We included all antithrombotic therapies used for AF in our investigation to provide a complete overview of efficacy and safety of these therapies. We have classified exposure in a time‐dependent manner as well as confounders to minimize misclassification.
This study adds to the information that is already available on real life use of the different antithrombotic agents. The usage of NOACs poses a greater risk on major bleeding, especially on gastrointestinal bleeding, compared with the usage of VKAs. The use of NOACs in patients who are vulnerable for this type of bleeding should be carefully considered. Next to that, women might have a greater risk for bleeding events, which makes this group less suitable for the treatment with NOACs. Further studies should be performed that are designed to study effectiveness and safety in subgroups.
Competing Interests
The Division of Pharmacoepidemiology & Clinical Pharmacology employing authors Frank de Vries, Anthonius de Boer, Tjeerd van Staa, Hendrika van den Ham and Andrea Burden has received unrestricted funding from the Netherlands Organization for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), the Royal Dutch Pharmacists Association (KNMP), the private‐public funded Top Institute Pharma (www.tipharma.nl), includes cofunding from universities, government, and industry), the EU Innovative Medicines Initiative (IMI), the EU 7th Framework Program (FP7), the Dutch Ministry of Health and industry (including GlaxoSmithKline, Pfizer, and others). Andrea Burden is supported by a Canadian Institutes of Health Research (CIHR) Post Doctoral Fellowship.
Author contributions
E.G., H.H., H.O., J.B., C.K., A.B., F.V. and A.B. conceived and designed the study. E.G., H.H. and A.B. collected the data, carried out the statistical analysis and drafted the manuscript. All authors analysed and interpreted the data and critically revised the manuscript for important intellectual content.
Appendix 1.
Read codes for major bleed
| Read code | Medical code | Clinical event | Read term |
| J680.00 | 1188 | Bleeding | Haematemesis |
| J681.00 | 397 | Bleeding | Melaena |
| J68z.11 | 1642 | Bleeding | Gastrointestinal bleeding |
| J68.00 | 3097 | Bleeding | Gastrointestinal haemorrhage |
| J680.11 | 2712 | Bleeding | Vomiting of blood |
| J68z200 | 4354 | Bleeding | Upper gastrointestinal haemorrhage |
| G850.00 | 24 989 | Bleeding | Oesophageal varices with bleeding |
| J12y100 | 2814 | Bleeding | Unspecified duodenal ulcer with haemorrhage |
| J68zz00 | 4636 | Bleeding | Gastrointestinal tract haemorrhage NOS |
| J68z000 | 15 517 | Bleeding | Gastric haemorrhage NOS |
| J120100 | 18 001 | Bleeding | Acute duodenal ulcer with haemorrhage |
| J68z.00 | 12 471 | Bleeding | Gastrointestinal haemorrhage unspecified |
| J68z100 | 2150 | Bleeding | Intestinal haemorrhage NOS |
| J121111 | 18 625 | Bleeding | Bleeding chronic duodenal ulcer |
| 19E4.12 | 18 313 | Bleeding | Complains of melaena |
| J110111 | 11 124 | Bleeding | Bleeding acute gastric ulcer |
| 1994.00 | 44 489 | Bleeding | Vomiting blood – fresh |
| J110100 | 30 054 | Bleeding | Acute gastric ulcer with haemorrhage |
| J121100 | 48 951 | Bleeding | Chronic duodenal ulcer with haemorrhage |
| J10y000 | 16 114 | Bleeding | Haemorrhage of oesophagus |
| 4737.11 | 37 299 | Bleeding | Melaena – on examination of faeces |
| J130100 | 44 637 | Bleeding | Acute peptic ulcer with haemorrhage |
| J111100 | 63 582 | Bleeding | Chronic gastric ulcer with haemorrhage |
| J111111 | 36 583 | Bleeding | Bleeding chronic gastric ulcer |
| J11y100 | 57 958 | Bleeding | Unspecified gastric ulcer with haemorrhage |
| J120300 | 48 730 | Bleeding | Acute duodenal ulcer with haemorrhage and perforation |
| J131100 | 53 126 | Bleeding | Chronic peptic ulcer with haemorrhage |
| J11yy00 | 94 397 | Bleeding | Unspecified gastric ulcer; unspecified haemorrhage and/or perforation |
| J13y100 | 70 456 | Bleeding | Unspecified peptic ulcer with haemorrhage |
| J110300 | 71 403 | Bleeding | Acute gastric ulcer with haemorrhage and perforation |
| J111300 | 71 897 | Bleeding | Chronic gastric ulcer with haemorrhage and perforation |
| J121300 | 71 881 | Bleeding | Chronic duodenal ulcer with haemorrhage and perforation |
| J140100 | 96 628 | Bleeding | Acute gastrojejunal ulcer with haemorrhage |
| J12y300 | 93 436 | Bleeding | Unspecified duodenal ulcer with haemorrhage and perforation |
| J13y300 | 96 622 | Bleeding | Unspecified peptic ulcer with haemorrhage and perforation |
| J140300 | 106 330 | Bleeding | Acute gastrojejunal ulcer with haemorrhage and perforation |
| G60.00 | 1786 | Bleeding | Subarachnoid haemorrhage |
| G61.00 | 5051 | Bleeding | Intracerebral haemorrhage |
| G61.11 | 6960 | Bleeding | Cerebrovascular accident due to intracerebral haemorrhage |
| G622.00 | 17 734 | Bleeding | Subdural haematoma ‐ nontraumatic |
| G623.00 | 18 912 | Bleeding | Subdural haemorrhage NOS |
| G621.00 | 4273 | Bleeding | Subdural haemorrhage ‐ nontraumatic |
| G61z.00 | 3535 | Bleeding | Intracerebral haemorrhage NOS |
| G61.12 | 18 604 | Bleeding | Stroke due to intracerebral haemorrhage |
| G613.00 | 13 564 | Bleeding | Cerebellar haemorrhage |
| S62.13 | 6569 | Bleeding | Subdural haemorrhage following injury |
| S62A.00 | 18 411 | Bleeding | Traumatic extradural haematoma |
| S622.00 | 2883 | Bleeding | Closed traumatic subdural haemorrhage |
| G60z.00 | 23 580 | Bleeding | Subarachnoid haemorrhage NOS |
| G617.00 | 30 202 | Bleeding | Intracerebral haemorrhage, intraventricular |
| S62.00 | 5682 | Bleeding | Cerebral haemorrhage following injury |
| G62z.00 | 20 284 | Bleeding | Intracranial haemorrhage NOS |
| S62.11 | 27 661 | Bleeding | Extradural haemorrhage following injury |
| G602.00 | 19 412 | Bleeding | Subarachnoid haemorrhage from middle cerebral artery |
| G614.00 | 7912 | Bleeding | Pontine haemorrhage |
| G61X000 | 28 314 | Bleeding | Left sided intracerebral haemorrhage, unspecified |
| G611.00 | 40 338 | Bleeding | Internal capsule haemorrhage |
| G60X.00 | 17 326 | Bleeding | Subarachnoid haemorrhage from intracranial artery, unspecified |
| G620.00 | 36 178 | Bleeding | Extradural haemorrhage ‐ nontraumatic |
| G61X100 | 19 201 | Bleeding | Right sided intracerebral haemorrhage, unspecified |
| G62.00 | 31 805 | Bleeding | Other and unspecified intracranial haemorrhage |
| G603.00 | 42 331 | Bleeding | Subarachnoid haemorrhage from anterior communicating artery |
| S62.12 | 28 807 | Bleeding | Subarachnoid haemorrhage following injury |
| G610.00 | 31 595 | Bleeding | Cortical haemorrhage |
| G612.00 | 46 316 | Bleeding | Basal nucleus haemorrhage |
| S630.12 | 35 867 | Bleeding | Intracranial haematoma following injury |
| G61X.00 | 31 060 | Bleeding | Intracerebral haemorrhage in hemisphere, unspecified |
| S628.00 | 8181 | Bleeding | Traumatic subdural haemorrhage |
| G604.00 | 9696 | Bleeding | Subarachnoid haemorrhage from posterior communicating artery |
| G605.00 | 41 910 | Bleeding | Subarachnoid haemorrhage from basilar artery |
| S62z.00 | 46 545 | Bleeding | Cerebral haemorrhage following injury NOS |
| S62.14 | 28 077 | Bleeding | Traumatic cerebral haemorrhage |
| S620.00 | 38 304 | Bleeding | Closed traumatic subarachnoid haemorrhage |
| S624.00 | 45 421 | Bleeding | Closed traumatic extradural haemorrhage |
| S627.00 | 58 545 | Bleeding | Traumatic subarachnoid haemorrhage |
| G616.00 | 30 045 | Bleeding | External capsule haemorrhage |
| G618.00 | 57 315 | Bleeding | Intracerebral haemorrhage, multiple localized |
| Gyu6200 | 53 810 | Bleeding | Other intracerebral haemorrhage |
| S629000 | 53 980 | Bleeding | Traumatic subdural haematoma without open intracranial wound |
| G615.00 | 62 342 | Bleeding | Bulbar haemorrhage |
| Gyu6100 | 65 745 | Bleeding | Other subarachnoid haemorrhage |
| G606.00 | 60 692 | Bleeding | Subarachnoid haemorrhage from vertebral artery |
| S625.00 | 73 471 | Bleeding | Open traumatic extradural haemorrhage |
| S63z.00 | 42 283 | Bleeding | Other cerebral haemorrhage following injury NOS |
| S629100 | 96 677 | Bleeding | Traumatic subdural haematoma with open intracranial wound |
Appendix 2.
Baseline characteristics for users of NOACs, VKAs or aspirin at index date for the cohort with history of major bleed excluded and cohort with history of stroke excluded
| Characteristic | Cohort outcome bleed | Cohort outcome stroke | ||||||
|---|---|---|---|---|---|---|---|---|
| NOAC‐users (n = 1247) | VKA‐users (n = 13 177) | Aspirin‐users (n = 15 551) | Mixed‐users (n = 443) | NOAC (n = 1128) | VKA (n = 12 445) | Aspirin (n = 15 471) | Mixed use (n = 402) | |
| Follow‐up (years, SD) | 1.0 (0.6) | 2.7 (1.9) | 2.9 (1.9) | 2.9 (2.0) | 0.9 (0.6) | 2.7 (1.9) | 2.9 (1.9) | 3.0 (2.0) |
| Number of women | 566 (45.4%) | 6073 (46.1%) | 7753 (49.9%) | 159 (35.9%) | 501 (44.4%) | 5683 (45.7%) | 7665 (49.5%) | 142 (35.3%) |
| Age | ||||||||
| Mean age at index date (years, SD) | 72.4 (12.6) | 71.9 (11.9) | 73.5 (12.7) | 72.2 (10.6) | 72.0 (12.8) | 71.7 (12.0) | 73.4 (12.7) | 71.8 (10.5) |
| 18–49 years | 59 (4.7%) | 663 (5.0%) | 654 (4.21%) | 13 (2.9%) | 54 (4.8%) | 651 (5.2%) | 661 (4.3%) | 13 (3.2%) |
| 50–59 years | 155 (12.4%) | 1263 (9.6%) | 1421 (9.14%) | 37 (8.4%) | 148 (13.1%) | 1231 (9.9%) | 1428 (9.2%) | 35 (8.7%) |
| 60–69 years | 252 (20.2%) | 22 943 (22.3) | 359 (23.1%) | 117 (26.4%) | 237 (21.0%) | 2788 (22.4%) | 3589 (23.2)% | 110 (27.4%) |
| 70–79 years | 401 (32.2%) | 4498 (34.1%) | 4259 (27.4%) | 160 (36.1%) | 350 (31.0%) | 4221 (33.9%) | 4238 (27.4%) | 143 (35.6%) |
| 80+ years | 380 (30.5%) | 3810 (28.9%) | 5627 (36.2%) | 116 (26.2%) | 339 (30.1%) | 3554 (28.6%) | 5555 (35.9%) | 101 (25.1%) |
| BMI | ||||||||
| Mean BMI at index date (SD) | 28.0 (6.2) | 28.7 (6.3) | 27.8 (6.2) | 28.9 (6.5) | 28.0 (6.2) | 28.8 (6.4) | 27.8 (6.2) | 29.0 (6.6) |
| < 20 kg m–2 | 77 (6.2%) | 522 (4.0%) | 925 (5.95%) | 20 (4.5%) | 70 (6.2%) | 482 (3.9%) | 926 (6.0%) | 17 (4.2%) |
| 20–25 kg m–2 | 312 (25.0%) | 3056 (23.2%) | 3984 (25.6%) | 94 (21.2%) | 285 (25.3%) | 2886 (23.2%) | 3955 (25.6%) | 83 (20.7%) |
| 25–30 kg m–2 | 403 (32.3%) | 4468 (33.9%) | 5149 (33.1%) | 151 (34.1%) | 364 (32.3%) | 4200 (33.8%) | 5134 (33.2%) | 138 (34.3%) |
| 30–35 kg m–2 | 243 (19.5%) | 2570 (19.5%) | 2668 (17.2%) | 90 (20.3%) | 217 (19.2%) | 2463 (19.8%) | 2664 (17.2%) | 81 (20.2%) |
| >35 kg m–2 | 136 (10.9%) | 1747 (13.3%) | 1617 (10.4%) | 59 (13.3%) | 129 (11.4%) | 1680 (13.5%) | 1619 (10.5%) | 56 (13.9%) |
| Missing | 76 (6.1%) | 814 (6.2%) | 1208 (7.8%) | 29 (6.5%) | 63 (5.6%) | 734 (5.9%) | 1173 (7.58%) | 27 (6.7%) |
| Smoking status | ||||||||
| Never | 545 (43.7%) | 5483 (41.6%) | 6856 (44.1%) | 168 (37.9%) | 493 (43.7%) | 5160 (41.5%) | 6797 (43.9%) | 154 (38.3%) |
| Current | 97 (7.8%) | 1191 (9.0%) | 1493 (9.6%) | 51 (11.5%) | 93 (8.2%) | 1124 (9.0%) | 1495 (9.7%) | 44 (11.0%) |
| Ex | 598 (48.0%) | 6440 (48.9%) | 7126 (45.8%) | 222 (50.1%) | 537 (47.6%) | 6106 (49.1%) | 7105 (45.9%) | 202 (50.3%) |
| Missing | 7 (0.6%) | 63 (0.5%) | 76 (0.5%) | <5 | 5 (0.4%) | 55 (0.4%) | 74 (0.5%) | <5 |
| Alcohol | ||||||||
| Yes | 869 (69.7%) | 9196 (69.8%) | 10 650 (68.5%) | 305 (68.8%) | 795 (70.5%) | 8755 (70.4%) | 10 628 (68.7%) | 277 (68.9%) |
| No | 269 (21.6) | 3034 (23.0%) | 3646 (23.4%) | 100 (22.6%) | 240 (21.3%) | 2835 (22.8%) | 3612 (23.4%) | 89 (22.1%) |
| Missing | 109 (8.7%) | 947 (7.2%) | 1255 (8.1%) | 38 (8.6%) | 93 (8.2%) | 855 (6.9%) | 1231 (8.0%) | 36 (9.0%) |
| CHA 2 DS 2 ‐VASc score | ||||||||
| Mean (SD) | 2.6 (1.5) | 2.6 (1.5) | 2.5 (1.5) | 2.6 (1.4) | 2.4 (1.5%) | 2.5 (1.5%) | 2.5 (1.4%) | 2.5 (1.4%) |
| Low | 564 (45.2%) | 6029 (45.8%) | 7062 (45.4%) | 219 (49.4%) | 525 (46.5%) | 5790 (46.5%) | 7135 (46.1%) | 205 (51.0%) |
| Medium | 319 (25.6%) | 3294 (25.0%) | 4312 (27.7%) | 101 (22.8%) | 310 (27.5%) | 3260 (26.2%) | 4341 (28.1%) | 97 (24.1%) |
| High | 364 (29.2%) | 3854 (29.2%) | 4177 (26.9%) | 123 (27.8%) | 293 (26.0%) | 3395 (27.3%) | 3995 (25.8%) | 100 (24.9%) |
| History of disease ever before | ||||||||
| Acute renal failure | 7 (0.6%) | 60 (0.5%) | 110 (0.7%) | <5 | 5 (0.4%) | 58 (0.5%) | 114 (0.7%) | <5 |
| Cerebrovascular disease | 215 (17.2%) | 1665 (12.6%) | 814 (5.2%) | 69 (15.6%) | 69 (6.1%) | 624 (5.0%) | 365 (2.4%) | 21 (5.2%) |
| Chronic renal failure | 6 (0.5%) | 150 (1.1%) | 150 (1.0%) | <5 | 6 (0.5%) | 134 (1.0%) | 147 (1.0%) | <5 |
| Congestive heart failure | 90 (7.2%) | 1333 (10.1%) | 906 (5.8%) | 66 (14.9%) | 84 (7.5%) | 1297 (10.4%) | 890 (5.8%) | 63 (15.7%) |
| Gastritis | 74 (5.9%) | 768 (5.8%) | 866 (5.6%) | 16 (3.6%) | 67 (5.9%) | 780 (6.3%) | 892 (5.8%) | 17 (4.2%) |
| GI‐bleed | <5 | <5 | <5 | <5 | 32 (2.8%) | 328 (2.6%) | 380 (2.5%) | 6 (1.5%) |
| Hypertension | 675 (54.1%) | 7027 (53.3%) | 7718 (49.6%) | 227 (5.2%) | 604 (53.6%) | 6600 (53.0%) | 7642 (49.4%) | 205 (51.0%) |
| Liver disease | 2 (0.2%) | 15 (0.1%) | 29 (0.2%) | 1 (0.2%) | 2 (0.2%) | 14 (0.1%) | 34 (0.2%) | 0 (0.0%) |
| Cancer | 11 (0.9%) | 120 (0.9%) | 114 (0.7%) | 4 (0.9%) | 15 (1.3%) | 118 (1.0%) | 118 (0.8%) | 4 (1.0%) |
| Peripheral artery disease | 64 (5.1%) | 660 (5.0%) | 612 (3.9%) | 26 (5.9%) | 61 (5.4%) | 623 (5.0%) | 613 (4.0%) | 24 (6.0%) |
| Ischaemic heart disease | 103 (8.3%) | 1343 (10.2%) | 1398 (9.0%) | 111 (25.1%) | 87 (7.7%) | 1255 (10.1%) | 1374 (8.9%) | 105 (26.1%) |
| History of medication use (< 6 months before index date) | ||||||||
| Antiarrhythmic drugs | 78 (6.3%) | 866 (6.6%) | 664 (4.3%) | 13 (2.9%) | 75 (6.7%) | 845 (6.8%) | 655 (4.2%) | 12 (3.0%) |
| Anticoagulant drugs | 16 (1.3%) | 202 (1.5%) | 63 (0.4%) | <5 | 16 (1.2%) | 202 (1.5%) | 63 (0.4%) | <5 |
| Antidiabetic drugs | 93 (7.5%) | 1000 (7.6%) | 876 (5.6%) | 36 (8.1%) | 82 (7.3%) | 952 (7.7%) | 852 (5.5%) | 32 (8.0%) |
| Antihypertensive drugs | 329 (26.3%) | 3690 (28.0%) | 3411 (21.9%) | 102 (23.0%) | 292 (25.9%) | 3480 (28.0%) | 3416 (22.1%) | 98 (24.4%) |
| Antiplatelet drugs | 9 (0.7%) | 190 (1.4%) | 90 (0.6%) | <5 | 4 (0.4%) | 125 (1.0%) | 63 (0.4%) | <5 |
| Insulin | 17 (1.4%) | 203 (1.5%) | 160 (1.0%) | 10 (2.3%) | 15 (1.3%) | 187 (1.5%) | 156 (1.0%) | 9 (2.2%) |
| NSAID's | 140 (11/2%) | 1556 (11.8%) | 2066 (13.3%) | 60 (13.5%) | 123 (10.9%) | 1505 (12.1%) | 2067 (13.4%) | 55 (13.7%) |
| SSRI's | 90 (7.2%) | 761 (5.8%) | 1018 (6.5%) | 17 (3.8%) | 80 (7.1%) | 704 (5.7%) | 1005 (6.5%) | 15 (3.7%) |
| Statins | 371 (29.8%) | 3878 (29.4%) | 3229 (20.8%) | 110 (24.8%) | 301 (26.7%) | 3443 (27.7%) | 3152 (20.4%) | 100 (24.9%) |
| Glucocorticoids | 121 (9.7%) | 1297 (9.8%) | 1226 (7.9%) | 32 (7.2%) | 118 (10.5%) | 1245 (10.0%) | 1235 (8.0%) | 29 (7.2%) |
| History of medication use (<3 months before index date) | ||||||||
| H2 receptor‐antagonists | 30 (2.4%) | 291 (2.2%) | 291 (1.9%) | 11 (2.5%) | 22 (2.0%) | 289 (2.3%) | 282 (1.8%) | 12 (3.0%) |
| PPI's | 329 (26.4%) | 3118 (23.7%) | 3327 (21.4%) | 81 (18.3%) | 305 (27.0%) | 3049 (24.5%) | 3401 (22.0%) | 75 (18.7%) |
NOAC, nonvitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; SD, standard deviation; BMI, body mass index; NSAIDs, non‐steroidal anti‐inflammatory drugs; SSRI's, selective serotonin reuptake inhibitors; H2, histamine 2; PPI's, proton pump inhibitors, GI, gastrointestinal.
Gieling, E. M. , van den Ham, H. A. , van Onzenoort, H. , Bos, J. , Kramers, C. , de Boer, A. , de Vries, F. , and Burden, A. M. (2017) Risk of major bleeding and stroke associated with the use of vitamin K antagonists, nonvitamin K antagonist oral anticoagulants and aspirin in patients with atrial fibrillation: a cohort study. Br J Clin Pharmacol, 83: 1844–1859. doi: 10.1111/bcp.13265.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener H, et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German atrial fibrillation competence NETwork and the European heart rhythm association. Europace 2007; 9: 1006–1023. [DOI] [PubMed] [Google Scholar]
- 4. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation an update of the 2010 ESC guidelines for the management of atrial fibrillationDeveloped with the special contribution of the European heart rhythm association. Europace 2012; 14: 1385–1413. [DOI] [PubMed] [Google Scholar]
- 5. Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003; 349: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 6. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 7. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 8. Miller C, Grandi S, Shimony A, Filion K, Eisenberg M. Meta‐analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 2012; 110: 453–460. [DOI] [PubMed] [Google Scholar]
- 9. Ntaios G, Papavasileiou V, Diener H, Makaritsis K, Michel P. Nonvitamin‐K‐antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta‐analysis of randomized controlled trials. Stroke 2012; 43: 3298–3304. [DOI] [PubMed] [Google Scholar]
- 10. Ruff C, Giugliano R, Braunwald E, Hoffman E, Deenadayalu N, Ezekowitz M, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 11. Dentali F, Riva N, Crowther M, Turpie AGG, Lip GYH, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta‐analysis of the literature. Circulation 2012; 126: 2381–2391. [DOI] [PubMed] [Google Scholar]
- 12. Sørensen R, Gislason G, Torp Pedersen C, Olesen J, Fosbøl E, Hvidtfeldt M, et al. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open 2013; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larsen T, Rasmussen L, Skjøth F, Due K, Callréus T, Rosenzweig M, et al. Efficacy and safety of dabigatran etexilate and warfarin in "real‐world" patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol 2013; 61: 2264–2273. [DOI] [PubMed] [Google Scholar]
- 14. Abraham N, Singh S, Alexander GC, Heien H, Haas L, Crown W, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ 2015; 350: h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smythe M, Forman M, Bertran E, Hoffman J, Priziola J, Koerber J. Dabigatran versus warfarin major bleeding in practice: an observational comparison of patient characteristics, management and outcomes in atrial fibrillation patients. J Thromb Thrombolysis 2015; 40: 280–287. [DOI] [PubMed] [Google Scholar]
- 16. Graham D, Reichman M, Wernecke M, Zhang R, Southworth M, Levenson M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015; 131: 157–164. [DOI] [PubMed] [Google Scholar]
- 17. van Walraven C, Jennings A, Oake N, Fergusson D, Forster A. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest 2006; 129: 1155–1166. [DOI] [PubMed] [Google Scholar]
- 18. Lee S, Monz B, Clemens A, Brueckmann M, Lip GYH. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real‐world atrial fibrillation patients in the United Kingdom: a cross‐sectional analysis using the general practice research database. BMJ Open 2012; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the general practice research database: a systematic review. Br J Gen Pract 2010; 60: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis J, Brensinger C. Agreement between GPRD smoking data: a survey of general practitioners and a population‐based survey. Pharmacoepidemiol Drug Saf 2004; 13: 437–441. [DOI] [PubMed] [Google Scholar]
- 22. Van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG. The use of a large pharmacoepidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol Drug Saf 2000; 9: 359–366. [DOI] [PubMed] [Google Scholar]
- 23. Herrett E, Gallagher A, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015; 44: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 25. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 26. Patel M, Mahaffey K, Garg J, Pan G, Singer D, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 27. Granger C, Alexander J, McMurray JJV, Lopes R, Hylek E, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 28. Cameron C, Coyle D, Richter T, Kelly S, Gauthier K, Steiner S, et al. Systematic review and network meta‐analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open 2014; 4: e004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non‐valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2007; 3. [DOI] [PubMed] [Google Scholar]
- 30. Pancholy S, Sharma P, Pancholy D, Patel T, Callans D, Marchlinski F. Meta‐analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol 2014; 113: 485–490. [DOI] [PubMed] [Google Scholar]
- 31. Reilly P, Lehr T, Haertter S, Connolly S, Yusuf S, Eikelboom J, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY trial (randomized evaluation of long‐term anticoagulation therapy). J Am Coll Cardiol 2014; 63: 321–328. [DOI] [PubMed] [Google Scholar]


