Abstract
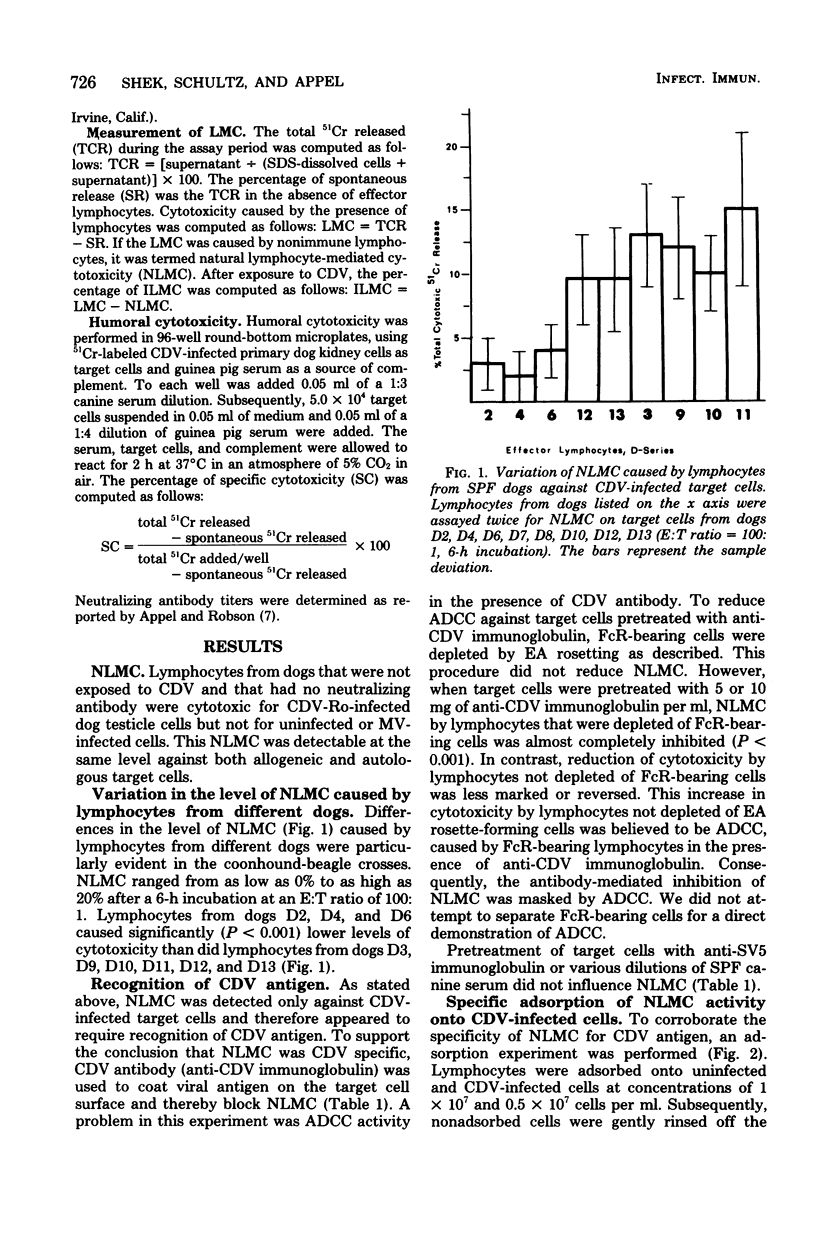
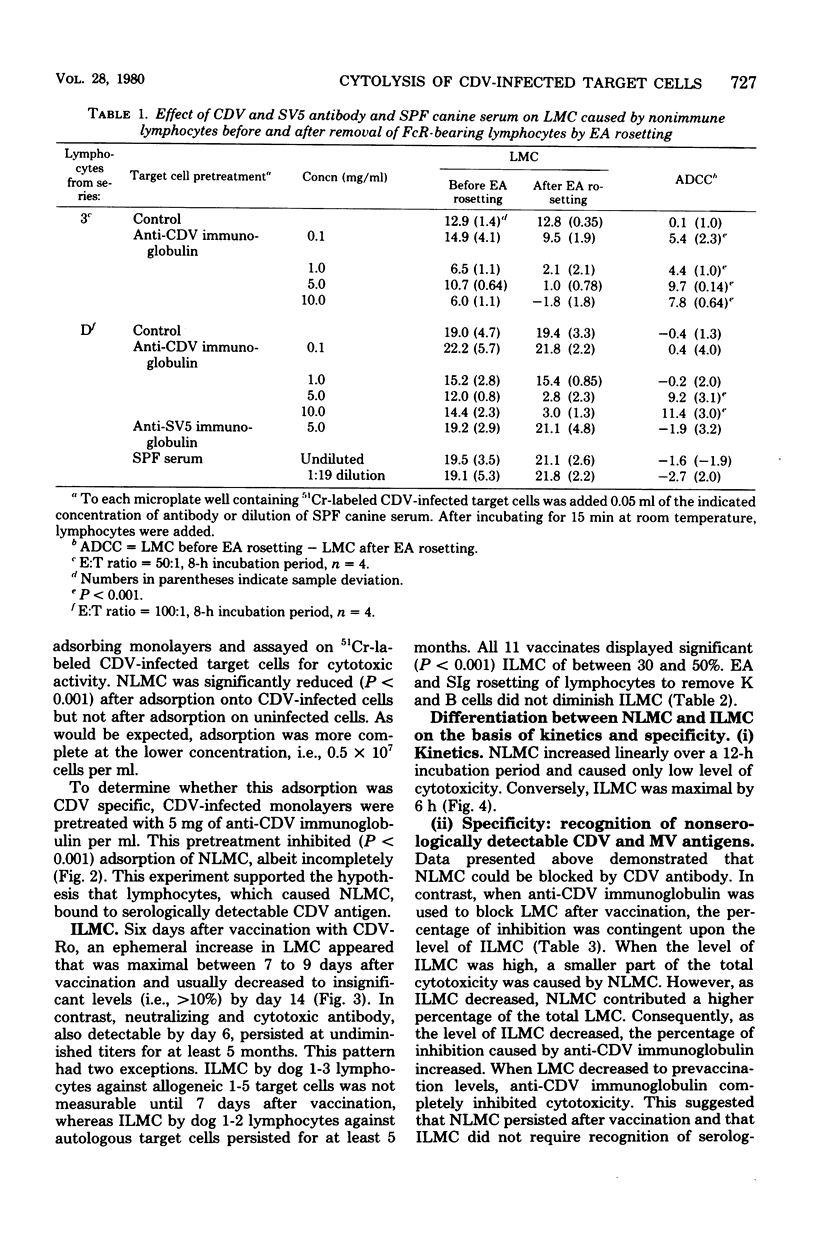
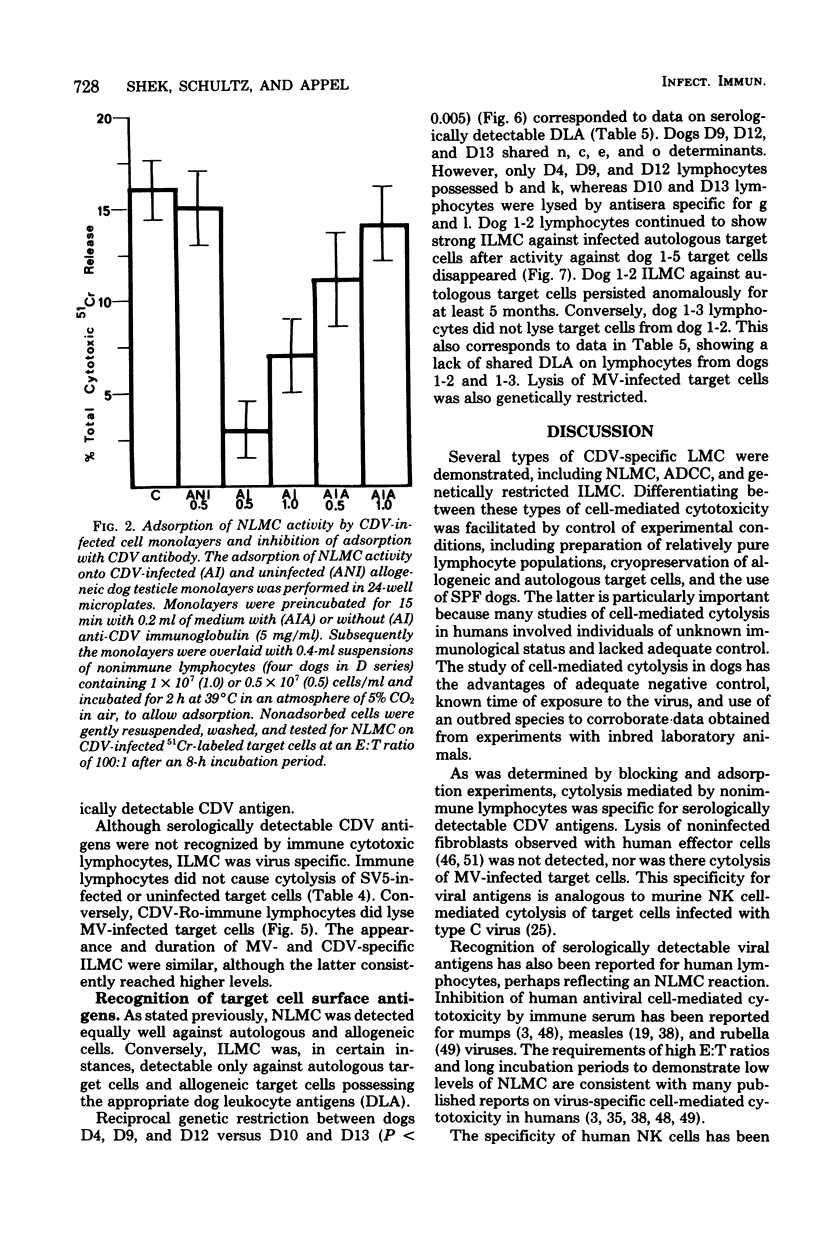
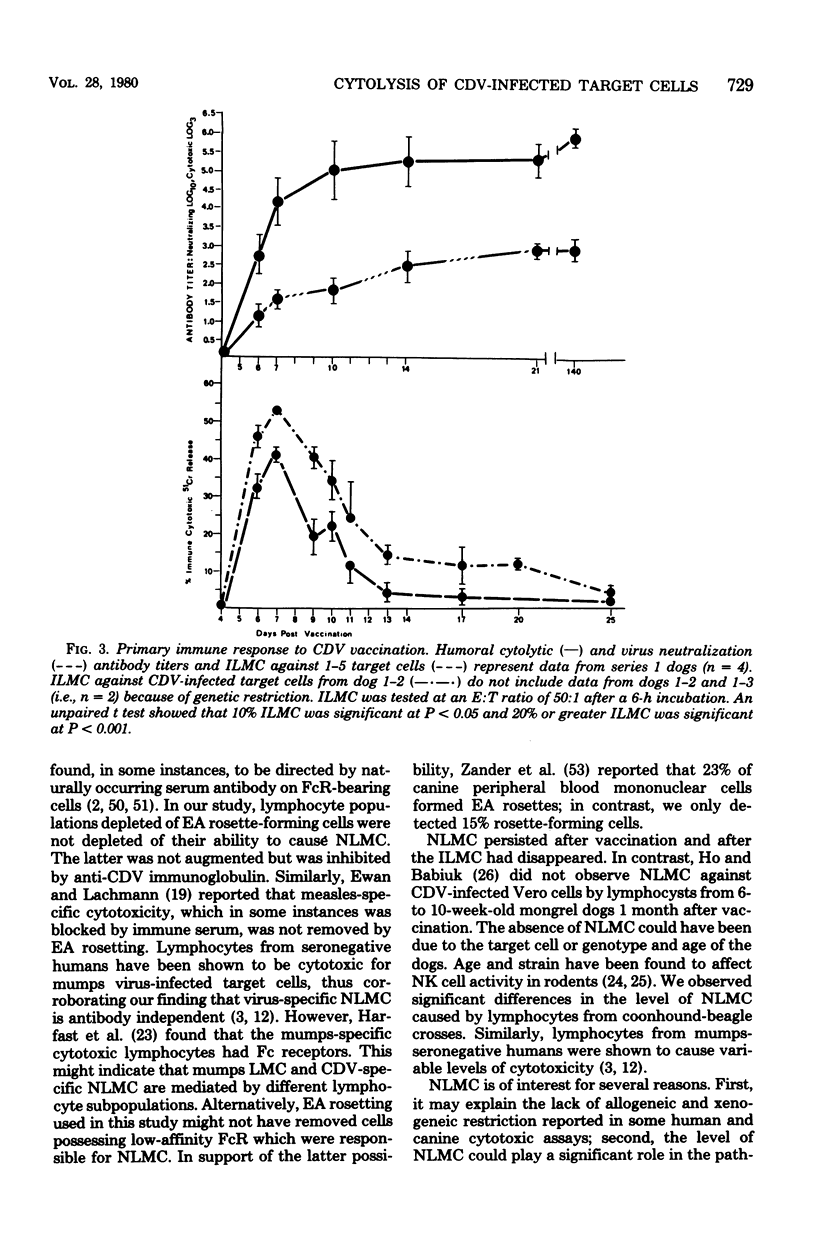
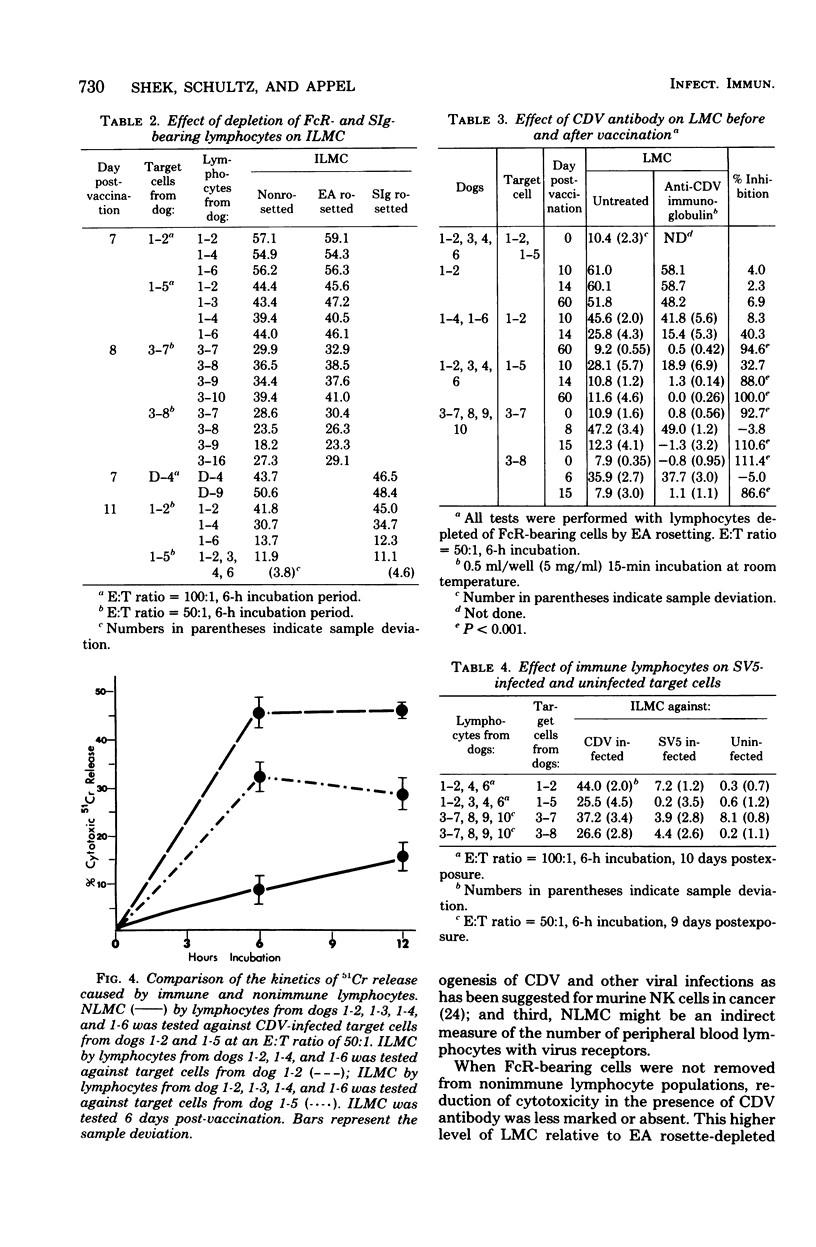
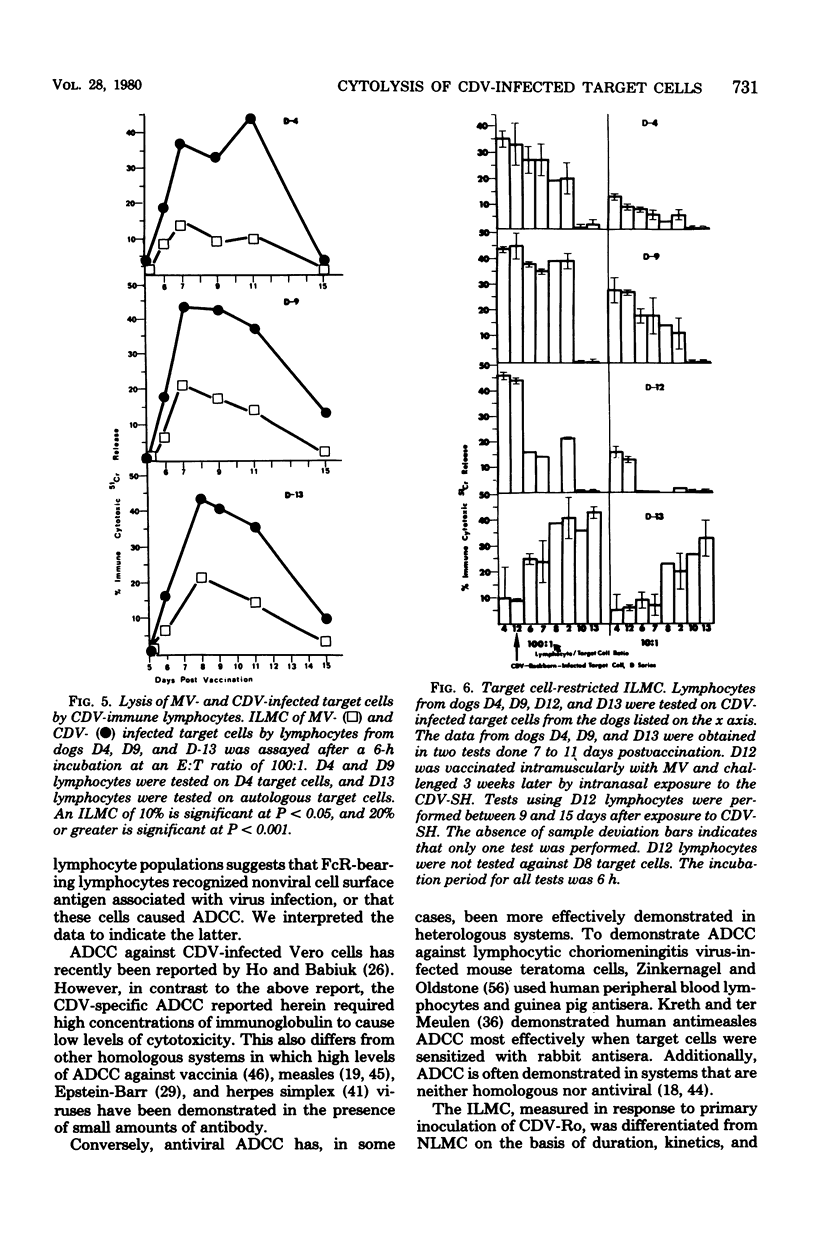
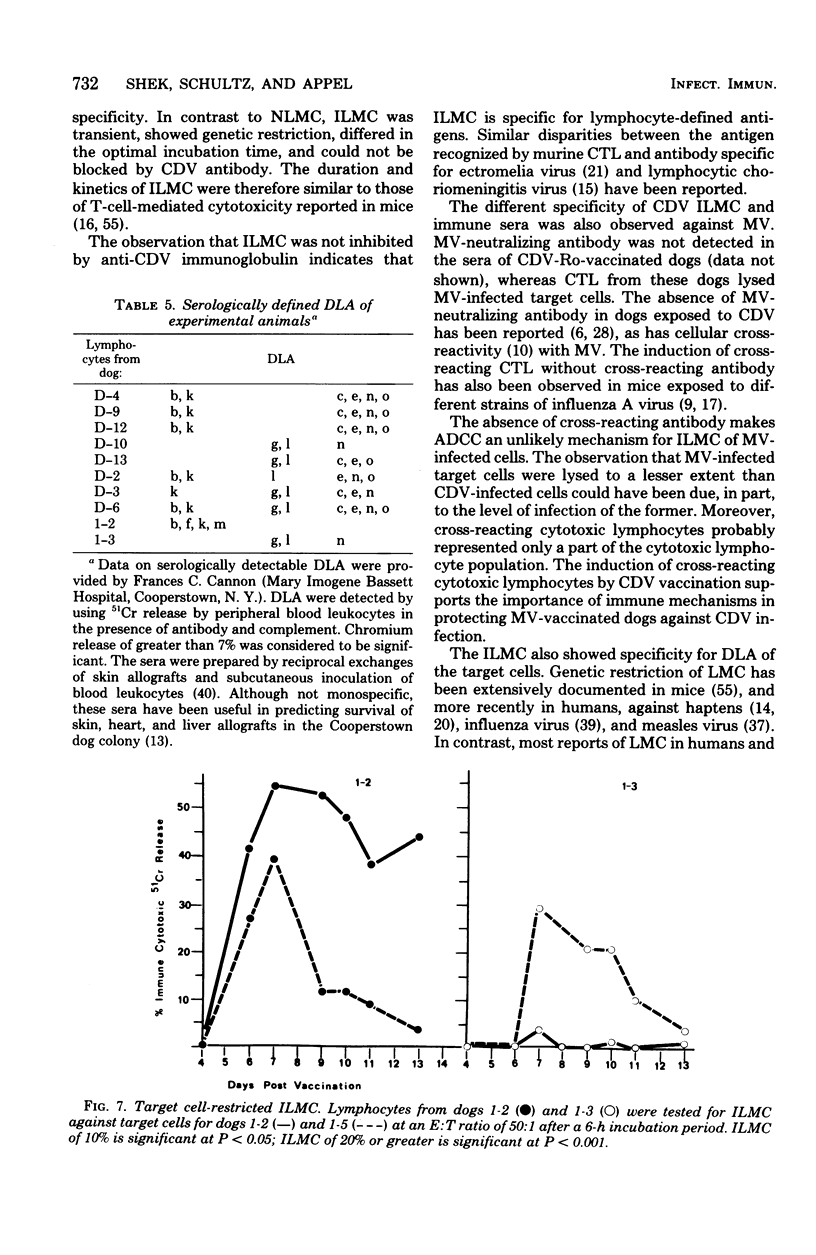
Natural and immune cytolysis of canine distemper virus (CDV)-infected target cells in vitro is described. Lymphocytes expressing natural cytotoxicity were found in specific-pathogen-free beagle dogs and in beagle-coonhound crosses before vaccination with CDV and indefinitely after vaccination, when the ephemeral immune lymphocyte-mediated cytotoxicity (ILMC) had declined. In contrast to the natural lymphocyte-mediated cytotoxicity, the ILMC was genetically restricted, could not be blocked by CDV-specific antibody, and was effective against measles virus-infected as well as CDV-infectd target cells. Lymphocyte populations were depleted of Fc receptor and surface immunoglobulin-bearing cells by rosetting techniques and tested in comparison. An antibody-dependent cell-mediated cytotoxicity was demostrated against CDV-infected target cells that were preincubated with CDV antibody when Fc receptor-bearing lymphocytes were not removed. The ILMC was measurable for approximately 10 days beginning at 6 days post-vaccination. In contrast, CDV antibody measured by virus neutralization and humoral cytotoxicity was detectable by 6 days postvaccination and persisted at peak levels for at least 5 months.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. I., Jackson D. C., Blanden R. V., Hla R. T., Bowern N. A. Changes in the surface of virus-induced cells recognized by cytotoxic T cells. I. Minimal requirements for lysis of ectromelia-infected P-815 cells. Scand J Immunol. 1976;5(1-2):23–30. doi: 10.1111/j.1365-3083.1976.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Akira D., Takasugi M. Loss of specific natural cell-mediated cytotoxicity with absorption of natural antibodies from serum. Int J Cancer. 1977 Jun 15;19(6):747–755. doi: 10.1002/ijc.2910190603. [DOI] [PubMed] [Google Scholar]
- Andersson T., Stejskal V., Harfast B. An in vitro method for study of human lymphocyte cytotoxicity against mumps-virus-infected target cells. J Immunol. 1975 Jan;114(1 Pt 1):237–243. [PubMed] [Google Scholar]
- Appel M. J. Pathogenesis of canine distemper. Am J Vet Res. 1969 Jul;30(7):1167–1182. [PubMed] [Google Scholar]
- Appel M., Robson D. S. A microneutralization test for canine distemper virus. Am J Vet Res. 1973 Nov;34(11):1459–1463. [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. L., McCarthy R. E. Relationship between measles and canine distemper viruses determined by delayed type hypersensitivity reactions in dogs. Nature. 1974 Mar 22;248(446):344–345. doi: 10.1038/248344a0. [DOI] [PubMed] [Google Scholar]
- Bubbers J. E., Chen S., Lilly F. Nonrandom inclusion of H-2K and H-2D antigens in Friend virus particles from mice of various strains. J Exp Med. 1978 Feb 1;147(2):340–351. doi: 10.1084/jem.147.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chiba Y., Dzierba J. L., Morag A., Ogra P. L. Cell-mediated immune response to mumps virus infection in man. J Immunol. 1976 Jan;116(1):12–15. [PubMed] [Google Scholar]
- Dausset J., Rapaport F. T., Cannon F. D., Ferrebee J. W. Histocompatibility studies in a closely bred colony of dogs. 3. Genetic definition of the DL-A system of canine histocompatibility, with particular reference to the comparative immunogenicity of the major transplantable organs. J Exp Med. 1971 Nov 1;134(5):1222–1237. doi: 10.1084/jem.134.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmeiss E., Soeberg B., Svejgaard A. Human cell-mediated cytotoxicity against modified target cells is restricted by HLA. Nature. 1977 Dec 8;270(5637):526–528. doi: 10.1038/270526a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Effros R. B., Bennink J., Doherty P. C. Characteristics of secondary cytotoxic T-cell responses in mice infected with influenza A viruses. Cell Immunol. 1978 Mar 15;36(2):345–353. doi: 10.1016/0008-8749(78)90278-2. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Chess L., Levine H., Schlossman S. F. Antibody-dependent cellular cytotoxicity by allosensitized human T cells. J Exp Med. 1978 Feb 1;147(2):605–610. doi: 10.1084/jem.147.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan P. W., Lachmann P. J. Demonstration of T-cell and K-cell cytotoxicity against measles-infected cells in normal subjects, multiple sclerosis and subacute sclerosing panencephalitis. Clin Exp Immunol. 1977 Oct;30(1):22–31. [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Neyhard N., Chess L. Cell-mediated lympholysis of trinitrophenyl-derivatized autologous human cells: in vitro triggering by nonspecific signals. J Immunol. 1978 Feb;120(2):630–637. [PubMed] [Google Scholar]
- Gardner I. D., Bowern N. A., Blanden R. V. Cell-medicated cytotoxicity against ectromelia virus-infected target cells. III. Role of the H-2 gene complex. Eur J Immunol. 1975 Feb;5(2):122–127. doi: 10.1002/eji.1830050210. [DOI] [PubMed] [Google Scholar]
- Gething M., Koszinowski U., Waterfield M. Fusion of Sendai virus with the target cell membrane is required for T cell cytotoxicity. Nature. 1978 Aug 17;274(5672):689–691. doi: 10.1038/274689a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Ho C. K., Babiuk L. A. Immune mechanisms against canine distemper. I. Identification of K cell against canine distemper virus infected target cells in vitro. Immunology. 1979 May;37(1):231–239. [PMC free article] [PubMed] [Google Scholar]
- Ho C. K., Babiuk L. A., Rouse B. T. Immune effector cell activity in canines: failure to demonstrate genetic restriction in direct antiviral cytotoxicity. Infect Immun. 1978 Jan;19(1):18–25. doi: 10.1128/iai.19.1.18-25.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härfast B., Andersson T., Perlmann P. Human lymphocyte cytotoxicity against mumps virus-infected target cells. Requirement for non-T cells. J Immunol. 1975 Jun;114(6):1820–1823. [PubMed] [Google Scholar]
- Imagawa D. T. Relationships among measles, canine distemper and rinderpest viruses. Prog Med Virol. 1968;10:160–193. [PubMed] [Google Scholar]
- Joseph B. S., Cooper N. R., Oldstone M. B. Immunologic injury of cultured cells infected with measles virus. I. role of IfG antibody and the alternative complement pathway. J Exp Med. 1975 Apr 1;141(4):761–774. [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kibler R., Meulen V. T. Antibody-mediated cytotoxicity after measles virus infection. J Immunol. 1975 Jan;114(1 Pt 1):93–98. [PubMed] [Google Scholar]
- Krakowka S., Olsen R., Confer A., Koestner A., McCullough B. Serologic response to canine distemper viral antigens in gnotobiotic dogs infected with canine distemper virus. J Infect Dis. 1975 Oct;132(4):384–392. doi: 10.1093/infdis/132.4.384. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Wallace A. L., Koestner A. Syncytia inhibition by immune lymphocytes: in vitro test for immunity to canine distemper. J Clin Microbiol. 1978 Mar;7(3):292–297. doi: 10.1128/jcm.7.3.292-297.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowka S., Wallace A. L. Lymphocyte-associated immune responses to canine distemper and measles viruses in distemper-infected gnotobiotic dogs. Am J Vet Res. 1979 May;40(5):669–672. [PubMed] [Google Scholar]
- Kreth H. W., Meulen V. Cell-mediated cytotoxicity against measles virus in SSPE. I. Enhancement by antibody. J Immunol. 1977 Jan;118(1):291–295. [PubMed] [Google Scholar]
- Kreth H. W., ter Meulen V., Eckert G. Demonstration of HLA restricted killer cells in patients with acute measles. Med Microbiol Immunol. 1979 Jan 24;165(4):203–214. doi: 10.1007/BF02152920. [DOI] [PubMed] [Google Scholar]
- Kreth W. H., Käckell M. Y., ter Meulen V. Demonstration of in vitro lymphocyte-mediated cytotoxicity against measles virus in SSPE. J Immunol. 1975 Mar;114(3):1042–1046. [PubMed] [Google Scholar]
- Labowskie R., Edelman R., Rustigian R., Bellanti J. A. Studies of cell-mediated immunity to measles virus by in vitro lymphocyte-mediated cytotoxicity. J Infect Dis. 1974 Mar;129(3):233–239. doi: 10.1093/infdis/129.3.233. [DOI] [PubMed] [Google Scholar]
- McMichael A. HLA restriction of human cytotoxic T lymphocytes specific for influenza virus. Poor recognition of virus associated with HLA A2. J Exp Med. 1978 Dec 1;148(6):1458–1467. doi: 10.1084/jem.148.6.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollen N., Cannon F. D., Ferrebee J. W., St John D. Lymphocyte typing in allografted beagles. Transplantation. 1968 Nov;6(8):939–940. doi: 10.1097/00007890-196811000-00009. [DOI] [PubMed] [Google Scholar]
- Moller-Larsen A., Heron I., Haahr S. Cell-mediated cytotoxicity to herpes-infected cells in humans: dependence on antibodies. Infect Immun. 1977 Apr;16(1):43–47. doi: 10.1128/iai.16.1.43-47.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R. In vitro and in vivo investigations on antibody-dependent cellular cytotoxicity. Curr Top Microbiol Immunol. 1978;80:65–96. doi: 10.1007/978-3-642-66956-9_3. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Tishon A., Oldstone M. B. Immunologic injury in measles virus infection. III. Presence and characterization of human cytotoxic lymphocytes. J Immunol. 1977 Jan;118(1):282–290. [PubMed] [Google Scholar]
- Perrin L. H., Zinkernagel R. M., Oldstone M. B. Immune response in humans after vaccination with vaccinia virus: generation of a virus-specific cytotoxic activity by human peripheral lymphocytes. J Exp Med. 1977 Oct 1;146(4):949–969. doi: 10.1084/jem.146.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Trinchieri G., Solter D., Knowles B. B. Mapping of H-2 genes associated with T cell-mediated cytotoxic responses to SV40-tumour-associated specific antigens. Nature. 1978 Aug 17;274(5672):691–693. doi: 10.1038/274691a0. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Vincent M. M., Hensen S. A., Walser J., Crawford M., Bellanti J. A. 51Chromium-release microassay technique for cell-mediated immunity to mumps virus: correlation with humoral and delayed-type skin hypersensitivity responses. J Infect Dis. 1976 Dec;134(6):546–551. doi: 10.1093/infdis/134.6.546. [DOI] [PubMed] [Google Scholar]
- Steele R. W., Hensen S. A., Vincent M. M., Fuccillo D. A., Bellanti J. A. A 51 Cr microassay technique for cell-mediated immunity to viruses. J Immunol. 1973 Jun;110(6):1502–1510. [PubMed] [Google Scholar]
- Takasugi J., Koide Y., Takasugi M. Reconstitution of natural cell-mediated cytotoxicity with specific antibodies. Eur J Immunol. 1977 Dec;7(12):887–892. doi: 10.1002/eji.1830071213. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Human natural cell-mediated cytotoxicity against fetal fibroblasts. I. General characteristics of the cytotoxic activity. Cell Immunol. 1977 Oct;33(2):340–352. doi: 10.1016/0008-8749(77)90163-0. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. T lymphocyte interaction with viruses and virus-infected tissues. Prog Med Virol. 1975;19:120–160. [PubMed] [Google Scholar]
- Zander A. R., Boopalam N., Epstein R. B. Surface markers on canine lymphocytes. Transplant Proc. 1975 Sep;7(3):369–373. [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977 Mar 1;145(3):644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Major transplantation antigens, viruses, and specificity of surveillance T cells. Contemp Top Immunobiol. 1977;7:179–220. doi: 10.1007/978-1-4684-3054-7_5. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Oldstone M. B. Cells that express viral antigens but lack H-2 determinants are not lysed by immune thymus-derived lymphocytes but are lysed by other antiviral immune attack mechanisms. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3666–3670. doi: 10.1073/pnas.73.10.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]


